Advanced Nanomedicine for High-Risk HPV-Driven Head and Neck Cancer
Abstract
:1. Introduction
2. Cancer Biology
3. Vaccine
4. Gene Silencing and Editing
4.1. RNA Interference
4.2. CRISPR/Cas9
4.3. p53
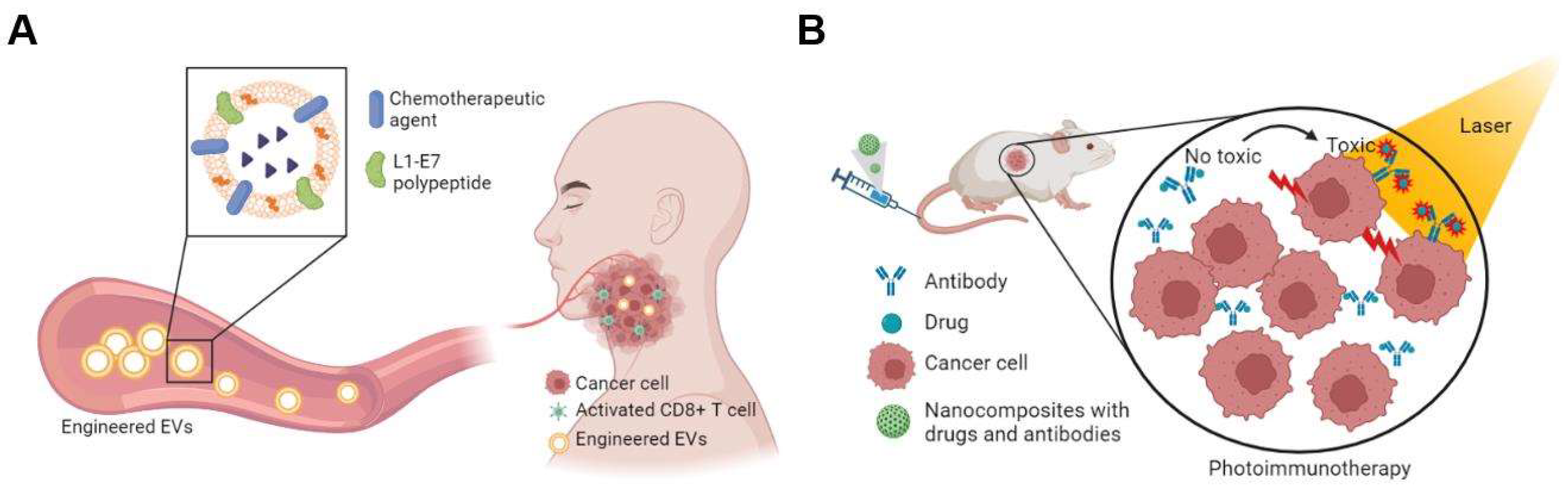
5. Extracellular Vesicles
6. Targeting PI3K/AKT Pathway
7. Combination Therapies
| Antigen | Type | Combination | Adjuvant | Delivery Platform | Cancer Model | References |
|---|---|---|---|---|---|---|
| HPV-16 E7 | Peptide | Anti-41BB | CpG-B 1826 oligonucleotide | Poly (propylene sulfide) nanoparticle | TC-1 tumor-bearing mice | [133] |
| HPV-16 E7 | Peptide | R-DOTAP cationic lipid nanoparticle | TC-1 tumor-bearing mice | [56] | ||
| HPV-16 E7 | mRNA | PD-L1 antibody | DOTMA/DOPE liposome | TC-1 & C3 tumor-bearing mice | [41] | |
| HPV-16 E7 | DCs | Poly (I:C) | PLGA nanoparticles | TC-1 tumor-bearing mice | [134] | |
| HPV-16 E6/E7 | Protein | Anti PD-L1, Cisplatin | Poly (I:C), R848, CpG ODNs | PLGA nanoparticles | TC-1 tumor-bearing mice, cynomolgus monkey | [44] |
| HPV-16 E6/E7 | RNA | DOTMA/DOPE liposome | TC-1 tumor-bearing mice | [54] | ||
| HPV-16 E7 | Peptide | MPLA, CpG | Synthetic high-density lipoprotein nanodisc | TC-1 tumor-bearing mice | [57] | |
| HPV-16 E7 | Peptide | Polyethyleneimine, GM-CSF, CPG-ODN | Mesoporous silica micro-rod | TC-1 tumor-bearing mice | [60] | |
| HPV-16 E7 | Peptide | Q11 peptide assembled nanofiber | TC-1 tumor-bearing mice | [64] | ||
| HPV-16 E7 | Peptide | Poly (I: C), CpG-ODN | Hyaluronic acid-modified cationic lipid-PLGA hybrid nanoparticles | TC-1 tumor-bearing mice | [135] | |
| HPV-16 E7 | RNA | Heterocyclic lipid nanoparticle | TC-1 tumor-bearing mice | [42] | ||
| HPV-16 E7 | DNA/Protein | Supercharged green fluorescent protein | TC-1 tumor-bearing mice | [136] | ||
| HPV-16 E7 | Protein | Anti-CD40 | Pam3CSK4, Poly (I: C) | PLGA nanoparticle | TC-1 tumor-bearing mice | [137] |
| HPV-16 E7 | Peptide | GM-CSF | HIV tat peptide | TC-1 tumor-bearing mice | [65] | |
| HPV-16 E7 | Peptide | MPLA | PEG-PE micelle | TC-1 tumor-bearing mice | [138] | |
| HPV-16 E7 | Protein | Bacterial outer membrane vesicles | TC-1 tumor-bearing mice | [43] | ||
| HPV-16 E6/E7 | Peptide | Anti-PD-L1 | Nanosatellite | Mouse HNSCC (PCI-13, UMSCC22b, UMSCC47, and FaDu cells) | [63] | |
| HPV-16 E7 | DNA | Branched amphiphilic peptide capsules | TC-1 tumor-bearing mice | [139] | ||
| HPV-16 E7 | Peptide | Virus-like particles | TC-1 tumor-bearing mice | [66] | ||
| HPV-16 E7 | Peptide | Liposome | TC-1 tumor-bearing mice | [140] | ||
| HPV-16 E7 | Peptide | GM-CSF, CpG-ODN | Mesoporous silica rods | Mouse HNSCC (MOC2-E6E7 cells) | [61] | |
| HPV-16 E7 | Peptide | R837 | Crosslinked BSA- E7 | TC-1 tumor-bearing mice | [68] | |
| HPV-16 E7 | mRNA | Local Radiotherapy | DOTMA/DOPE liposome | TC-1 tumor-bearing mice | [55] | |
| HPV-16 E6/E7 | Peptide | PHAD-3D6A, QS-21 | CPQ liposome | TC-1 tumor-bearing mice | [141] | |
| HPV-16 E7 | Peptide | Surgery | CpG-B 1826 oligonucleotide | Poly (propylene sulfide) nanoparticle | mEERL95 tumor-bearing mice | [69] |
| HPV-16 E7 | Peptide | manganese-doped silica nanoparticles | TC-1 tumor-bearing mice | [62] | ||
| HPV-16 E7 | Peptide | CpG-ODN | Mannose-Modified Liposome | TC-1 tumor-bearing mice | [142] | |
| HPV-16 E6/E7 | Peptide | Bintrafusp alfa (M7824), NHS- IL12 | R-DOTAP containing lipid nanoparticle | TC-1 and mEER tumor-bearing mice | [47] | |
| HPV -16 L1/E6/E7 | DNA | Archaeosome | TC-1 tumor-bearing mice | [143] | ||
| HPV -16 E7 | Peptide | Anti-PD-1 (G4C2) | CpG-1826 | Spycatcher modified Ferritin nanoparticle | TC-1 tumor-bearing mice | [144] |
8. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ji, B.; Wei, M.; Yang, B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Shu, X.; Xu, Q.; Zhu, C.; Kaufmann, A.; Zheng, Z.; Albers, A.; Qian, X. Current Status of Human Papillomavirus-Related Head and Neck Cancer: From Viral Genome to Patient Care. Virol. Sin. 2021, 36, 1284–1302. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Thangavelu, K.; Kürten, C.; Galland, L.; Höing, B.; Deuss, E.; Mattheis, S.; Lang, S.; Deuschl, C.; Forsting, M.; et al. The accuracy of clinical neck staging for p16-positive and negative oropharyngeal cancer and its therapeutic implications. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. Affil. Ger. Soc. Oto-Rhino-Laryngol. Head Neck Surg. 2022, 279, 5339–5345. [Google Scholar] [CrossRef] [PubMed]
- Caudell, J.; Gillison, M.; Maghami, E.; Spencer, S.; Pfister, D.; Adkins, D.; Birkeland, A.; Brizel, D.; Busse, P.; Cmelak, A.; et al. NCCN Guidelines® Insights: Head and Neck Cancers, Version 1.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Albers, A.; Qian, X.; Kaufmann, A.; Coordes, A. Meta analysis: HPV and p16 pattern determines survival in patients with HNSCC and identifies potential new biologic subtype. Sci. Rep. 2017, 7, 16715. [Google Scholar] [CrossRef] [Green Version]
- Rischin, D.; Mehanna, H.; Young, R.; Bressel, M.; Dunn, J.; Corry, J.; Soni, P.; Fulton-Lieuw, T.; Iqbal, G.; Kenny, L.; et al. Prognostic stratification of HPV-associated oropharyngeal cancer based on CD103 immune cell abundance in patients treated on TROG 12.01 and De-ESCALaTE randomized trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 804–813. [Google Scholar] [CrossRef]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Fang, M.; Zhu, J.; Dong, H.; Cao, J.; Yan, L.; Leonard, F.; Oppel, F.; Sudhoff, H.; Kaufmann, A.; et al. Insights into Nanomedicine for Immunotherapeutics in Squamous Cell Carcinoma of the head and neck. Int. J. Biol. Sci. 2020, 16, 2506–2517. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Z.; Fu, T.; Zheng, L.; Wu, H.; He, L.; Huang, H.; Yang, C.; Wang, R.; Qian, X.; et al. Engineering Aptamers with Selectively Enhanced Biostability in the Tumor Microenvironment. Angew. Chem. 2022, 61, e202201220. [Google Scholar] [CrossRef]
- Pierce, R. Translational nanomedicine—Through the therapeutic window. Nanomedicine 2015, 10, 3249–3260. [Google Scholar] [CrossRef]
- Aragon-Sanabria, V.; Aditya, A.; Zhang, L.; Chen, F.; Yoo, B.; Cao, T.; Madajewski, B.; Lee, R.; Turker, M.; Ma, K.; et al. Ultrasmall Nanoparticle Delivery of Doxorubicin Improves Therapeutic Index for High-Grade Glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 2938–2952. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, J.; Lin, W.; Fu, Z.; Ji, X.; Yu, B.; Lu, M.; Cui, W.; Deng, L.; Engle, J.; et al. Open-Shell Nanosensitizers for Glutathione Responsive Cancer Sonodynamic Therapy. Adv. Mater. 2022, 34, e2110283. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal-Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nano-Micro Lett. 2020, 12, 103. [Google Scholar] [CrossRef]
- Bhatia, S.; Chen, X.; Dobrovolskaia, M.; Lammers, T. Cancer nanomedicine. Nat. Rev. Cancer 2022, 22, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Montesion, M.; Alexander, B.; Ramkissoon, S.; Elvin, J.; Ross, J.; Williams, K.; Glomski, K.; Bledsoe, J.; Tse, J.; et al. CYLD mutation characterizes a subset of HPV-positive head and neck squamous cell carcinomas with distinctive genomics and frequent cylindroma-like histologic features. Mod. Pathol. Off. J. United States Can. Acad. Pathol. Inc. 2021, 34, 358–370. [Google Scholar] [CrossRef]
- Faden, D.; Kuhs, K.; Lin, M.; Langenbucher, A.; Pinheiro, M.; Yeager, M.; Cullen, M.; Boland, J.; Steinberg, M.; Bass, S.; et al. APOBEC Mutagenesis Is Concordant between Tumor and Viral Genomes in HPV-Positive Head and Neck Squamous Cell Carcinoma. Viruses 2021, 13, 1666. [Google Scholar] [CrossRef]
- Henderson, S.; Chakravarthy, A.; Su, X.; Boshoff, C.; Fenton, T. APOBEC-mediated cytosine deamination links PIK3CA helical domain mutations to human papillomavirus-driven tumor development. Cell Rep. 2014, 7, 1833–1841. [Google Scholar] [CrossRef] [Green Version]
- Faden, D.; Ding, F.; Lin, Y.; Zhai, S.; Kuo, F.; Chan, T.; Morris, L.; Ferris, R. APOBEC mutagenesis is tightly linked to the immune landscape and immunotherapy biomarkers in head and neck squamous cell carcinoma. Oral Oncol. 2019, 96, 140–147. [Google Scholar] [CrossRef]
- Zhou, C.; Tuong, Z.; Frazer, I. Papillomavirus Immune Evasion Strategies Target the Infected Cell and the Local Immune System. Front. Oncol. 2019, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Cicchini, L.; Westrich, J.; Xu, T.; Vermeer, D.; Berger, J.; Clambey, E.; Lee, D.; Song, J.; Lambert, P.; Greer, R.; et al. Suppression of Antitumor Immune Responses by Human Papillomavirus through Epigenetic Downregulation of CXCL14. mBio 2016, 7, e00270-16. [Google Scholar] [CrossRef]
- Ward, M.; Thirdborough, S.; Mellows, T.; Riley, C.; Harris, S.; Suchak, K.; Webb, A.; Hampton, C.; Patel, N.; Randall, C.; et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br. J. Cancer 2014, 110, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corredor, G.; Toro, P.; Koyuncu, C.; Lu, C.; Buzzy, C.; Bera, K.; Fu, P.; Mehrad, M.; Ely, K.; Mokhtari, M.; et al. An Imaging Biomarker of Tumor-Infiltrating Lymphocytes to Risk-Stratify Patients with HPV-Associated Oropharyngeal Cancer. J. Natl. Cancer Inst. 2022, 114, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Cillo, A.; Kürten, C.; Tabib, T.; Qi, Z.; Onkar, S.; Wang, T.; Liu, A.; Duvvuri, U.; Kim, S.; Soose, R.; et al. Immune Landscape of Viral- and Carcinogen-Driven Head and Neck Cancer. Immunity 2020, 52, 183–199.e189. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.; Ferguson, P.; Dodds, T.; Jones, D.; Li, M.; Yang, J.; Scolyer, R. Significant association of PD-L1 expression with human papillomavirus positivity and its prognostic impact in oropharyngeal cancer. Oral Oncol. 2019, 92, 33–39. [Google Scholar] [CrossRef]
- Eberhardt, C.; Kissick, H.; Patel, M.; Cardenas, M.; Prokhnevska, N.; Obeng, R.; Nasti, T.; Griffith, C.; Im, S.; Wang, X.; et al. Functional HPV-specific PD-1 stem-like CD8 T cells in head and neck cancer. Nature 2021, 597, 279–284. [Google Scholar] [CrossRef]
- Wieland, A.; Patel, M.; Cardenas, M.; Eberhardt, C.; Hudson, W.; Obeng, R.; Griffith, C.; Wang, X.; Chen, Z.; Kissick, H.; et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021, 597, 274–278. [Google Scholar] [CrossRef]
- Zhu, G.; Amin, N.; Herberg, M.; Maroun, C.; Wang, H.; Guller, M.; Gourin, C.; Rooper, L.; Vosler, P.; Tan, M.; et al. Association of Tumor Site With the Prognosis and Immunogenomic Landscape of Human Papillomavirus-Related Head and Neck and Cervical Cancers. JAMA Otolaryngol. Head Neck Surg. 2022, 148, 70–79. [Google Scholar] [CrossRef]
- Clara, J.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef]
- Zhang, M.; Kumar, B.; Piao, L.; Xie, X.; Schmitt, A.; Arradaza, N.; Cippola, M.; Old, M.; Agrawal, A.; Ozer, E.; et al. Elevated intrinsic cancer stem cell population in human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 2014, 120, 992–1001. [Google Scholar] [CrossRef] [Green Version]
- Qian, X.; Wagner, S.; Ma, C.; Klussmann, J.; Hummel, M.; Kaufmann, A.; Albers, A. ALDH1-positive cancer stem-like cells are enriched in nodal metastases of oropharyngeal squamous cell carcinoma independent of HPV status. Oncol. Rep. 2013, 29, 1777–1784. [Google Scholar] [CrossRef]
- Swanson, M.; Kokot, N.; Sinha, U. The Role of HPV in Head and Neck Cancer Stem Cell Formation and Tumorigenesis. Cancers 2016, 8, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyagi, A.; Vishnoi, K.; Mahata, S.; Verma, G.; Srivastava, Y.; Masaldan, S.; Roy, B.; Bharti, A.; Das, B. Cervical Cancer Stem Cells Selectively Overexpress HPV Oncoprotein E6 that Controls Stemness and Self-Renewal through Upregulation of HES1. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4170–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Moon, J.; Kim, C.; No, J.; Eun, Y.; Chang Lim, Y. Integrin alpha 6 as a stemness driver is a novel promising target for HPV (+) head and neck squamous cell carcinoma. Exp. Cell Res. 2021, 407, 112815. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, P.; Das, B. HPV oral-tongue cancer stem cells: A potential target for relapse-free therapy. Transl. Oncol. 2021, 14, 100919. [Google Scholar] [CrossRef] [PubMed]
- Reid, P.; Wilson, P.; Li, Y.; Marcu, L.; Staudacher, A.; Brown, M.; Bezak, E. In vitro investigation of head and neck cancer stem cell proportions and their changes following X-ray irradiation as a function of HPV status. PLoS ONE 2017, 12, e0186186. [Google Scholar] [CrossRef] [PubMed]
- Kajitani, N.; Schwartz, S. The role of RNA-binding proteins in the processing of mRNAs produced by carcinogenic papillomaviruses. Semin. Cancer Biol. 2022, 86, 482–496. [Google Scholar] [CrossRef]
- Mirza, S.; Kalluchi, A.; Raza, M.; Saleem, I.; Mohapatra, B.; Pal, D.; Ouellette, M.; Qiu, F.; Yu, L.; Lobanov, A.; et al. Ecdysoneless Protein Regulates Viral and Cellular mRNA Splicing to Promote Cervical Oncogenesis. Mol. Cancer Res. 2022, 20, 305–318. [Google Scholar] [CrossRef]
- Beyaert, S.; Machiels, J.; Schmitz, S. Vaccine-Based Immunotherapy for Head and Neck Cancers. Cancers 2021, 13, 6041. [Google Scholar] [CrossRef]
- Peng, S.; Ferrall, L.; Gaillard, S.; Wang, C.; Chi, W.; Huang, C.; Roden, R.; Wu, T.; Chang, Y.; Hung, C. Development of DNA Vaccine Targeting E6 and E7 Proteins of Human Papillomavirus 16 (HPV16) and HPV18 for Immunotherapy in Combination with Recombinant Vaccinia Boost and PD-1 Antibody. mBio 2021, 12, e03224-20. [Google Scholar] [CrossRef]
- Choi, Y.; Hur, S.; Kim, T.; Hong, S.; Lee, J.; Cho, C.; Park, K.; Woo, J.; Sung, Y.; Suh, Y.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef]
- Grunwitz, C.; Salomon, N.; Vascotto, F.; Selmi, A.; Bukur, T.; Diken, M.; Kreiter, S.; Türeci, Ö.; Sahin, U. HPV16 RNA-LPX vaccine mediates complete regression of aggressively growing HPV-positive mouse tumors and establishes protective T cell memory. Oncoimmunology 2019, 8, e1629259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, W.; Li, K.; Yao, Y.; Yang, X.; Bai, H.; Sun, W.; Liu, C.; Ma, Y. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int. J. Nanomed. 2017, 12, 6813–6825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ilyinskii, P.O.; Kovalev, G.I.; O’Neil, C.P.; Roy, C.J.; Michaud, A.M.; Drefs, N.M.; Pechenkin, M.A.; Fu, F.N.; Johnston, L.P.M.; Ovchinnikov, D.A.; et al. Synthetic vaccine particles for durable cytolytic T lymphocyte responses and anti-tumor immunotherapy. PLoS ONE 2018, 13, e0197694. [Google Scholar] [CrossRef] [Green Version]
- Massarelli, E.; William, W.; Johnson, F.; Kies, M.; Ferrarotto, R.; Guo, M.; Feng, L.; Lee, J.J.; Tran, H.; Kim, Y.U.; et al. Combining Immune Checkpoint Blockade and Tumor-Specific Vaccine for Patients With Incurable Human Papillomavirus 16-Related Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Karkada, M.; Quinton, T.; Blackman, R.; Mansour, M. Tumor Inhibition by DepoVax-Based Cancer Vaccine Is Accompanied by Reduced Regulatory/Suppressor Cell Proliferation and Tumor Infiltration. ISRN Oncol. 2013, 2013, 753427. [Google Scholar] [CrossRef] [PubMed]
- Rumfield, C.S.; Pellom, S.T.; Morillon Ii, Y.M.; Schlom, J.; Jochems, C. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J. Immunother. Cancer 2020, 8, e000612. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.G.; Rajapakshe, K.; Rodriguez Canales, J.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and nivolumab for HPV-16 cancer: Updated clinical efficacy and immune correlates of response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef]
- Prasad, S.; Cody, V.; Saucier-Sawyer, J.; Fadel, T.; Edelson, R.; Birchall, M.; Hanlon, D. Optimization of stability, encapsulation, release, and cross-priming of tumor antigen-containing PLGA nanoparticles. Pharm. Res. 2012, 29, 2565–2577. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Luo, Q.; Xie, H.; Huang, X.; Ni, Y.; Mou, Y.; Hu, Q. Therapeutic antitumor efficacy of tumor-derived autophagosome (DRibble) vaccine on head and neck cancer. Int. J. Nanomed. 2015, 10, 1921–1930. [Google Scholar] [CrossRef]
- Aggarwal, C.; Cohen, R.B.; Morrow, M.P.; Kraynyak, K.A.; Sylvester, A.J.; Knoblock, D.M.; Bauml, J.M.; Weinstein, G.S.; Lin, A.; Boyer, J.; et al. Immunotherapy Targeting HPV16/18 Generates Potent Immune Responses in HPV-Associated Head and Neck Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 110–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, M. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Fobian, S.; Cheng, Z.; Ten Hagen, T. Smart Lipid-Based Nanosystems for Therapeutic Immune Induction against Cancers: Perspectives and Outlooks. Pharmaceutics 2021, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Salomon, N.; Selmi, A.; Grunwitz, C.; Kong, A.; Stanganello, E.; Neumaier, J.; Petschenka, J.; Diken, M.; Kreiter, S.; Tureci, O.; et al. Local radiotherapy and E7 RNA-LPX vaccination show enhanced therapeutic efficacy in preclinical models of HPV16 (+) cancer. Cancer Immunol. Immunother. 2021, 71, 1975–1988. [Google Scholar] [CrossRef]
- Gandhapudi, S.K.; Ward, M.; Bush, J.P.C.; Bedu-Addo, F.; Conn, G.; Woodward, J.G. Antigen Priming with Enantiospecific Cationic Lipid Nanoparticles Induces Potent Antitumor CTL Responses through Novel Induction of a Type I IFN Response. J. Immunol. 2019, 202, 3524–3536. [Google Scholar] [CrossRef]
- Kuai, R.; Sun, X.; Yuan, W.; Ochyl, L.J.; Xu, Y.; Hassani Najafabadi, A.; Scheetz, L.; Yu, M.Z.; Balwani, I.; Schwendeman, A.; et al. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J. Control. Release Off. J. Control. Release Soc. 2018, 282, 131–139. [Google Scholar] [CrossRef]
- Hassani Najafabadi, A.; Zhang, J.; Aikins, M.; Najaf Abadi, Z.; Liao, F.; Qin, Y.; Okeke, E.; Scheetz, L.; Nam, J.; Xu, Y.; et al. Cancer Immunotherapy via Targeting Cancer Stem Cells Using Vaccine Nanodiscs. Nano Lett. 2020, 20, 7783–7792. [Google Scholar] [CrossRef]
- Qian, X.; Ma, C.; Nie, X.; Lu, J.; Lenarz, M.; Kaufmann, A.; Albers, A. Biology and immunology of cancer stem (-like) cells in head and neck cancer. Crit. Rev. Oncol. Hematol. 2015, 95, 337–345. [Google Scholar] [CrossRef]
- Li, A.W.; Sobral, M.C.; Badrinath, S.; Choi, Y.; Graveline, A.; Stafford, A.G.; Weaver, J.C.; Dellacherie, M.O.; Shih, T.Y.; Ali, O.A.; et al. A facile approach to enhance antigen response for personalized cancer vaccination. Nat. Mater. 2018, 17, 528–534. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Piotrowski, S.L.; Huang, C.; Newton, J.M.; Golfman, L.S.; Hanoteau, A.; Koshy, S.T.; Li, A.W.; Pulikkathara, M.X.; Zhang, B.; et al. Anti-tumor immunity induced by ectopic expression of viral antigens is transient and limited by immune escape. Oncoimmunology 2019, 8, e1568809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, J.; Teoh, S.M.; Kuo, P.; Tolley, L.; Bashaw, A.A.; Tuong, Z.K.; Liu, Y.; Chen, Z.; Wells, J.W.; Yu, C.; et al. Manganese-Doped Silica-Based Nanoparticles Promote the Efficacy of Antigen-Specific Immunotherapy. J. Immunol. 2021, 206, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.S.; Sansanaphongpricha, K.; Xie, Y.; Donnelly, C.R.; Luo, X.; Heath, B.R.; Zhao, X.; Bellile, E.; Hu, H.; Chen, H.; et al. Mitigating SOX2-potentiated Immune Escape of Head and Neck Squamous Cell Carcinoma with a STING-inducing Nanosatellite Vaccine. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4242–4255. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhang, Q.; Bai, H.; Huang, W.; Shu, C.; Ye, C.; Sun, W.; Ma, Y. Self-Assembled Nanofibers Elicit Potent HPV16 E7-Specific Cellular Immunity And Abolish Established TC-1 Graft Tumor. Int. J. Nanomed. 2019, 14, 8209–8219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Yin, R.; Tian, Y.; Huang, Z.; Shi, J.; Fu, X.; Wang, L.; Wu, Y.; Hao, F.; Ni, B. A novel self-assembled nanoparticle vaccine with HIV-1 Tat(4)(9)(-)(5)(7)/HPV16 E7(4)(9)(-)(5)(7) fusion peptide and GM-CSF DNA elicits potent and prolonged CD8 (+) T cell-dependent anti-tumor immunity in mice. Vaccine 2012, 30, 1071–1082. [Google Scholar] [CrossRef]
- Chu, X.; Li, Y.; Long, Q.; Xia, Y.; Yao, Y.; Sun, W.; Huang, W.; Yang, X.; Liu, C.; Ma, Y. Chimeric HBcAg virus-like particles presenting a HPV 16 E7 epitope significantly suppressed tumor progression through preventive or therapeutic immunization in a TC-1-grafted mouse model. Int. J. Nanomed. 2016, 11, 2417–2429. [Google Scholar] [CrossRef] [Green Version]
- Grippin, A.; Wummer, B.; Wildes, T.; Dyson, K.; Trivedi, V.; Yang, C.; Sebastian, M.; Mendez-Gomez, H.; Padala, S.; Grubb, M.; et al. Dendritic Cell-Activating Magnetic Nanoparticles Enable Early Prediction of Antitumor Response with Magnetic Resonance Imaging. ACS Nano 2019, 13, 13884–13898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, K.; Huang, Y.; Zhang, H.; Zhou, L.; Li, A.; Sun, Y. Photosensitizer-induced HPV16 E7 nanovaccines for cervical cancer immunotherapy. Biomaterials 2022, 282, 121411. [Google Scholar] [CrossRef]
- Domingos-Pereira, S.; Roh, V.; Hiou-Feige, A.; Galliverti, G.; Simon, C.; Tolstonog, G.V.; Nardelli-Haefliger, D. Vaccination with a nanoparticle E7 vaccine can prevent tumor recurrence following surgery in a human papillomavirus head and neck cancer model. Oncoimmunology 2021, 10, 1912473. [Google Scholar] [CrossRef]
- Wefers, C.; Schreibelt, G.; Massuger, L.; de Vries, I.; Torensma, R. Immune Curbing of Cancer Stem Cells by CTLs Directed to NANOG. Front. Immunol. 2018, 9, 1412. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.; Hu, Z.; Keskin, D.; Shukla, S.; Sun, J.; Bozym, D.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerich, L.; Binder, A.; Brody, J. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Mol. Oncol. 2015, 9, 1966–1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Qiu, M.; Ye, Z.; Nyalile, T.; Li, Y.; Glass, Z.; Zhao, X.; Yang, L.; Chen, J.; Xu, Q. In situ cancer vaccination using lipidoid nanoparticles. Sci. Adv. 2021, 7, eabf1244. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, H.; Ye, Q.; Tao, F.; Wheeldon, I.; Yuan, A.; Hu, Y.; Wu, J. Systemic immune responses to irradiated tumours via the transport of antigens to the tumour periphery by injected flagellate bacteria. Nat. Biomed. Eng. 2022, 6, 44–53. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Dai, Y.; Fan, W.; Niu, G.; Yang, Z.; Chen, X. Burst release of encapsulated annexin A5 in tumours boosts cytotoxic T-cell responses by blocking the phagocytosis of apoptotic cells. Nat. Biomed. Eng. 2020, 4, 1102–1116. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Snijders, P.J.; Keune, W.J.; Meijer, C.J.; Ruijter-Schippers, H.J.; Leemans, C.R.; Brakenhoff, R.H. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J. Natl. Cancer Inst. 2004, 96, 998–1006. [Google Scholar] [CrossRef] [Green Version]
- Tomar, S.; Graves, C.A.; Altomare, D.; Kowli, S.; Kassler, S.; Sutkowski, N.; Gillespie, M.B.; Creek, K.E.; Pirisi, L. Human papillomavirus status and gene expression profiles of oropharyngeal and oral cancers from European American and African American patients. Head Neck 2016, 38 (Suppl. S1), E694–E704. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, P.M.; Yu, Z.; Haffty, B.G.; Kowalski, D.; Harigopal, M.; Brandsma, J.; Sasaki, C.; Joe, J.; Camp, R.L.; Rimm, D.L.; et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J. Clin. Oncol. 2006, 24, 736–747. [Google Scholar] [CrossRef]
- Abboodi, F.; Buckhaults, P.; Altomare, D.; Liu, C.; Hosseinipour, M.; Banister, C.E.; Creek, K.E.; Pirisi, L. HPV-inactive cell populations arise from HPV16-transformed human keratinocytes after p53 knockout. Virology 2021, 554, 9–16. [Google Scholar] [CrossRef]
- Rampias, T.; Sasaki, C.; Weinberger, P.; Psyrri, A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J. Natl. Cancer Inst. 2009, 101, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Adhim, Z.; Otsuki, N.; Kitamoto, J.; Morishita, N.; Kawabata, M.; Shirakawa, T.; Nibu, K. Gene silencing with siRNA targeting E6/E7 as a therapeutic intervention against head and neck cancer-containing HPV16 cell lines. Acta Oto-Laryngol. 2013, 133, 761–771. [Google Scholar] [CrossRef]
- Li, C.; Johnson, D. Liberation of functional p53 by proteasome inhibition in human papilloma virus-positive head and neck squamous cell carcinoma cells promotes apoptosis and cell cycle arrest. Cell Cycle 2013, 12, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Kampel, L.; Goldsmith, M.; Ramishetti, S.; Veiga, N.; Rosenblum, D.; Gutkin, A.; Chatterjee, S.; Penn, M.; Lerman, G.; Peer, D.; et al. Therapeutic inhibitory RNA in head and neck cancer via functional targeted lipid nanoparticles. J. Control. Release Off. J. Control. Release Soc. 2021, 337, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, P.; Yu, S.; Thomson, S.; Birkenshaw, A.; Leavitt, B.; Ross, C. Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components. Mol. Pharm. 2022, 19, 1669–1686. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef]
- Kennedy, E.; Kornepati, A.; Goldstein, M.; Bogerd, H.; Poling, B.; Whisnant, A.; Kastan, M.; Cullen, B. Inactivation of the human papillomavirus E6 or E7 gene in cervical carcinoma cells by using a bacterial CRISPR/Cas RNA-guided endonuclease. J. Virol. 2014, 88, 11965–11972. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Yu, L.; Zhu, D.; Ding, W.; Wang, X.; Zhang, C.; Wang, L.; Jiang, X.; Shen, H.; He, D.; et al. Disruption of HPV16-E7 by CRISPR/Cas system induces apoptosis and growth inhibition in HPV16 positive human cervical cancer cells. BioMed Res. Int. 2014, 2014, 612823. [Google Scholar] [CrossRef] [Green Version]
- Zhen, S.; Hua, L.; Takahashi, Y.; Narita, S.; Liu, Y.; Li, Y. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by CRISPR/Cas9. Biochem. Biophys. Res. Commun. 2014, 450, 1422–1426. [Google Scholar] [CrossRef]
- Zhen, S.; Lu, J.; Wang, L.; Sun, X.; Zhang, J.; Li, X.; Luo, W.; Zhao, L. In Vitro and In Vivo Synergistic Therapeutic Effect of Cisplatin with Human Papillomavirus16 E6/E7 CRISPR/Cas9 on Cervical Cancer Cell Line. Transl. Oncol. 2016, 9, 498–504. [Google Scholar] [CrossRef]
- Zhen, S.; Lu, J.; Liu, Y.; Chen, W.; Li, X. Synergistic antitumor effect on cervical cancer by rational combination of PD1 blockade and CRISPR-Cas9-mediated HPV knockout. Cancer Gene Ther. 2020, 27, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Bortnik, V.; Wu, M.; Julcher, B.; Salinas, A.; Nikolic, I.; Simpson, K.; McMillan, N.; Idris, A. Loss of HPV type 16 E7 restores cGAS-STING responses in human papilloma virus-positive oropharyngeal squamous cell carcinomas cells. J. Microbiol. Immunol. Infect. Wei Mian Yu Gan Ran Za Zhi 2021, 54, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, T.; Saga, Y.; Urabe, M.; Uchibori, R.; Matsubara, S.; Fujiwara, H.; Mizukami, H. CRISPR/Cas9-mediated cervical cancer treatment targeting human papillomavirus E6. Oncol. Lett. 2019, 17, 2197–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrke-Schulz, E.; Heinemann, S.; Schulte, L.; Schiwon, M.; Ehrhardt, A. Adenoviral Vectors Armed with PAPILLOMAVIRUs Oncogene Specific CRISPR/Cas9 Kill Human-Papillomavirus-Induced Cervical Cancer Cells. Cancers 2020, 12, 1934. [Google Scholar] [CrossRef] [PubMed]
- Jubair, L.; Fallaha, S.; McMillan, N. Systemic Delivery of CRISPR/Cas9 Targeting HPV Oncogenes Is Effective at Eliminating Established Tumors. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Jubair, L.; Lam, A.; Fallaha, S.; McMillan, N. CRISPR/Cas9-loaded stealth liposomes effectively cleared established HPV16-driven tumours in syngeneic mice. PLoS ONE 2021, 16, e0223288. [Google Scholar] [CrossRef]
- Gao, X.; Jin, Z.; Tan, X.; Zhang, C.; Zou, C.; Zhang, W.; Ding, J.; Das, B.; Severinov, K.; Hitzeroth, I.; et al. Hyperbranched poly(β-amino ester) based polyplex nanopaticles for delivery of CRISPR/Cas9 system and treatment of HPV infection associated cervical cancer. J. Control. Release Off. J. Control. Release Soc. 2020, 321, 654–668. [Google Scholar] [CrossRef]
- Xiong, J.; Tan, S.; Yu, L.; Shen, H.; Qu, S.; Zhang, C.; Ren, C.; Zhu, D.; Wang, H. E7-Targeted Nanotherapeutics for Key HPV Afflicted Cervical Lesions by Employing CRISPR/Cas9 and Poly (Beta-Amino Ester). Int. J. Nanomed. 2021, 16, 7609–7622. [Google Scholar] [CrossRef]
- Zhu, D.; Shen, H.; Tan, S.; Hu, Z.; Wang, L.; Yu, L.; Tian, X.; Ding, W.; Ren, C.; Gao, C.; et al. Nanoparticles Based on Poly (β-Amino Ester) and HPV16-Targeting CRISPR/shRNA as Potential Drugs for HPV16-Related Cervical Malignancy. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 2443–2455. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Liu, J.; Jiang, Y.; Zhang, M.; Mao, L.; Wang, M. Cell-Selective Messenger RNA Delivery and CRISPR/Cas9 Genome Editing by Modulating the Interface of Phenylboronic Acid-Derived Lipid Nanoparticles and Cellular Surface Sialic Acid. ACS Appl. Mater. Interfaces 2019, 11, 46585–46590. [Google Scholar] [CrossRef]
- Zhen, S.; Liu, Y.; Lu, J.; Tuo, X.; Yang, X.; Chen, H.; Chen, W.; Li, X. Human Papillomavirus Oncogene Manipulation Using Clustered Regularly Interspersed Short Palindromic Repeats/Cas9 Delivered by pH-Sensitive Cationic Liposomes. Hum. Gene Ther. 2020, 31, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Lao, Y.; Li, M.; Gao, M.; Shao, D.; Chi, C.; Huang, D.; Chakraborty, S.; Ho, T.; Jiang, W.; Wang, H.; et al. Natronobacterium gregoryiHPV Oncogene Manipulation Using Nonvirally Delivered CRISPR/Cas9 or Argonaute. Adv. Sci. 2018, 5, 1700540. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Liu, J.; Fan, W.; Li, R.; Cui, Z.; Jin, Z.; Huang, Z.; Xie, H.; Li, L.; Huang, Z.; et al. E6Gene knock-out chain reaction enables high disruption efficiency of HPV18/genes in cervical cancer cells. Mol. Ther. Oncolytics 2022, 24, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, H.; Wang, T.; He, D.; Tian, R.; Cui, Z.; Tian, X.; Gao, Q.; Ma, X.; Yang, J.; et al. In vitro and in vivo growth inhibition of human cervical cancer cells via human papillomavirus E6/E7 mRNAs’ cleavage by CRISPR/Cas13a system. Antivir. Res. 2020, 178, 104794. [Google Scholar] [CrossRef]
- Idres, Y.; McMillan, N.; Idris, A. Hyperactivating p53 in Human Papillomavirus-Driven Cancers: A Potential Therapeutic Intervention. Mol. Diagn. Ther. 2022, 26, 301–308. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, L.; Li, D.; Liu, J.; Li, X.; Li, W.; Xu, X.; Zhang, M.J.; Chandler, L.A.; Lin, H.; et al. The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic. Hum. Gene Ther. 2018, 29, 160–179. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.J.; Zhang, S.W.; Chen, C.B.; Xiao, S.W.; Sun, Y.; Liu, C.Q.; Su, X.; Li, D.M.; Xu, G.; Xu, B.; et al. Effect of recombinant adenovirus-p53 combined with radiotherapy on long-term prognosis of advanced nasopharyngeal carcinoma. J. Clin. Oncol. 2009, 27, 799–804. [Google Scholar] [CrossRef] [Green Version]
- Pirisinu, M.; Pham, T.; Zhang, D.; Hong, T.; Nguyen, L.; Le, M. Extracellular vesicles as natural therapeutic agents and innate drug delivery systems for cancer treatment: Recent advances, current obstacles, and challenges for clinical translation. Semin. Cancer Biol. 2022, 80, 340–355. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef]
- Acevedo-Sánchez, V.; Rodríguez-Hernández, R.; Aguilar-Ruíz, S.; Torres-Aguilar, H.; Romero-Tlalolini, M. Extracellular Vesicles in Cervical Cancer and HPV Infection. Membranes 2021, 11, 453. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, S.; Tong, F.; Wang, Y.; Wei, L. HPV HNSCC-derived exosomal miR-9-5p inhibits TGF-β signaling-mediated fibroblast phenotypic transformation through NOX4. Cancer Sci. 2022, 113, 1475–1487. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Mao, X.; Zhang, S.; Xie, H.; Yan, B.; Wang, B.; Sun, J.; Wei, L. HPV + HNSCC-derived exosomal miR-9 induces macrophage M1 polarization and increases tumor radiosensitivity. Cancer Lett. 2020, 478, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Iannotta, D.; Yang, M.; Celia, C.; Di Marzio, L.; Wolfram, J. Extracellular vesicle therapeutics from plasma and adipose tissue. Nano Today 2021, 39, 101159. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.; Wolfram, J. Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat. Rev. Mater. 2021, 6, 103–106. [Google Scholar] [CrossRef]
- Di Bonito, P.; Accardi, L.; Galati, L.; Ferrantelli, F.; Federico, M. Anti-Cancer Vaccine for HPV-Associated Neoplasms: Focus on a Therapeutic HPV Vaccine Based on a Novel Tumor Antigen Delivery Method Using Endogenously Engineered Exosomes. Cancers 2019, 11, 138. [Google Scholar] [CrossRef] [Green Version]
- Ferrantelli, F.; Arenaccio, C.; Manfredi, F.; Olivetta, E.; Chiozzini, C.; Leone, P.; Percario, Z.; Ascione, A.; Flego, M.; Di Bonito, P.; et al. The Intracellular Delivery Of Anti-HPV16 E7 scFvs Through Engineered Extracellular Vesicles Inhibits The Proliferation Of HPV-Infected Cells. Int. J. Nanomed. 2019, 14, 8755–8768. [Google Scholar] [CrossRef] [Green Version]
- Abbasifarid, E.; Bolhassani, A.; Irani, S.; Sotoodehnejadnematalahi, F. Synergistic effects of exosomal crocin or curcumin compounds and HPV L1-E7 polypeptide vaccine construct on tumor eradication in C57BL/6 mouse model. PLoS ONE 2021, 16, e0258599. [Google Scholar] [CrossRef]
- Meng, D.; He, W.; Zhang, Y.; Liang, Z.; Zheng, J.; Zhang, X.; Zheng, X.; Zhan, P.; Chen, H.; Li, W.; et al. Development of PI3K inhibitors: Advances in clinical trials and new strategies (Review). Pharmacol. Res. 2021, 173, 105900. [Google Scholar] [CrossRef]
- Mizrachi, A.; Shamay, Y.; Shah, J.; Brook, S.; Soong, J.; Rajasekhar, V.K.; Humm, J.L.; Healey, J.H.; Powell, S.N.; Baselga, J.; et al. Tumour-specific PI3K inhibition via nanoparticle-targeted delivery in head and neck squamous cell carcinoma. Nat. Commun. 2017, 8, 14292. [Google Scholar] [CrossRef]
- Tian, L.; Wang, L.; Qiao, Y.; Lu, L.; Lee, P.; Chang, A.; Ravi, S.; Rogers, T.A.; Melancon, M.P. Antitumor Efficacy of Liposome-Encapsulated NVP-BEZ235 Combined with Irreversible Electroporation for Head and Neck Cancer. Molecules 2019, 24, 3560. [Google Scholar] [CrossRef]
- Yanes-Díaz, J.; Palao-Suay, R.; Aguilar, M.R.; Riestra-Ayora, J.I.; Ferruelo-Alonso, A.; Rojo Del Olmo, L.; Vázquez-Lasa, B.; Sanz-Fernández, R.; Sánchez-Rodríguez, C. Antitumor Activity of Nanoparticles Loaded with PHT-427, a Novel AKT/PDK1 Inhibitor, for the Treatment of Head and Neck Squamous Cell Carcinoma. Pharmaceutics 2021, 13, 1242. [Google Scholar] [CrossRef] [PubMed]
- Nissi, L.; Suilamo, S.; Kytö, E.; Vaittinen, S.; Irjala, H.; Minn, H. Recurrence of head and neck squamous cell carcinoma in relation to high-risk treatment volume. Clin. Transl. Radiat. Oncol. 2021, 27, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mazumdar, T.; Xu, W.; Powell, R.; Stephan, C.; Shen, L.; Shah, P.; Pickering, C.; Myers, J.; Wang, J.; et al. Combined TRIP13 and Aurora kinase inhibition induces apoptosis in human papillomavirus-driven cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 4479–4493. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Baijal, G.; Somashekar, R.; Iyer, S.; Nayak, V. One Pot Synthesis of PEGylated Bimetallic Gold-Silver Nanoparticles for Imaging and Radiosensitization of Oral Cancers. Int. J. Nanomed. 2021, 16, 7103–7121. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Gota, V.; Vishwakarma, K.; Pai, V.; Chaudhari, P.; Mohanty, B.; Thorat, R.; Yadav, S.; Gurjar, M.; Goda, J.; et al. Loco-regional radiosensitizing nanoparticles-in-gel augments head and neck cancer chemoradiotherapy. J. Control. Release Off. J. Control. Release Soc. 2022, 343, 288–302. [Google Scholar] [CrossRef]
- Celli, J.; Spring, B.; Rizvi, I.; Evans, C.; Samkoe, K.; Verma, S.; Pogue, B.; Hasan, T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Tang, C.; Xu, W.; Ran, J.; Wei, Z.; Wang, Y.; Zou, H.; Cheng, W.; Cai, Y.; Han, W. Hypoxia-Targeting Multifunctional Nanoparticles for Sensitized Chemotherapy and Phototherapy in Head and Neck Squamous Cell Carcinoma. Int. J. Nanomed. 2020, 15, 347–361. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, X.; Liu, X.; Yu, J.; Bai, X.; Wu, X.; Guo, X.; Liu, Z.; Liu, X. Combination of phototherapy with immune checkpoint blockade: Theory and practice in cancer. Front. Immunol. 2022, 13, 955920. [Google Scholar] [CrossRef]
- Cognetti, D.; Johnson, J.; Curry, J.; Kochuparambil, S.; McDonald, D.; Mott, F.; Fidler, M.; Stenson, K.; Vasan, N.; Razaq, M.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef]
- Bhandari, C.; Fakhry, J.; Eroy, M.; Song, J.; Samkoe, K.; Hasan, T.; Hoyt, K.; Obaid, G. Towards Photodynamic Image-Guided Surgery of Head and Neck Tumors: Photodynamic Priming Improves Delivery and Diagnostic Accuracy of Cetuximab-IRDye800CW. Front. Oncol. 2022, 12, 853660. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, A.; Zhang, Z.; Zhao, Q.; Li, J.; Mei, Y.; Yin, Y.; Wang, W. Multifunctional inorganic nanomaterials for cancer photoimmunotherapy. Cancer Commun. 2022, 42, 141–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, L.; Wang, C.; Han, Y.; Lu, Y.; Liu, J.; Hu, X.; Yao, T.; Lin, Y.; Liang, S.; et al. Tumor-Targeted Drug and CpG Delivery System for Phototherapy and Docetaxel-Enhanced Immunotherapy with Polarization toward M1-Type Macrophages on Triple Negative Breast Cancers. Adv. Mater. 2019, 31, e1904997. [Google Scholar] [CrossRef] [PubMed]
- Galliverti, G.; Tichet, M.; Domingos-Pereira, S.; Hauert, S.; Nardelli-Haefliger, D.; Swartz, M.; Hanahan, D.; Wullschleger, S. Nanoparticle Conjugation of Human Papillomavirus 16 E7-long Peptides Enhances Therapeutic Vaccine Efficacy against Solid Tumors in Mice. Cancer Immunol. Res. 2018, 6, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Han, H.D.; Byeon, Y.; Kang, T.H.; Jung, I.D.; Lee, J.W.; Shin, B.C.; Lee, Y.J.; Sood, A.K.; Park, Y.M. Toll-like receptor 3-induced immune response by poly(d,l-lactide-co-glycolide) nanoparticles for dendritic cell-based cancer immunotherapy. Int. J. Nanomed. 2016, 11, 5729–5742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Chu, X.; Yan, M.; Qi, J.; Liu, H.; Gao, F.; Gao, R.; Ma, G.; Ma, Y. Encapsulation of Poly I:C and the natural phosphodiester CpG ODN enhanced the efficacy of a hyaluronic acid-modified cationic lipid-PLGA hybrid nanoparticle vaccine in TC-1-grafted tumors. Int. J. Pharm. 2018, 553, 327–337. [Google Scholar] [CrossRef]
- Motevalli, F.; Bolhassani, A.; Hesami, S.; Shahbazi, S. Supercharged green fluorescent protein delivers HPV16E7 DNA and protein into mammalian cells in vitro and in vivo. Immunol. Lett. 2018, 194, 29–39. [Google Scholar] [CrossRef]
- Rosalia, R.A.; Cruz, L.J.; van Duikeren, S.; Tromp, A.T.; Silva, A.L.; Jiskoot, W.; de Gruijl, T.; Lowik, C.; Oostendorp, J.; van der Burg, S.H.; et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015, 40, 88–97. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Qin, Y.; Liang, W. Delivered antigen peptides to resident CD8alpha (+) DCs in lymph node by micelle-based vaccine augment antigen-specific CD8 (+) effector T cell response. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V 2020, 147, 76–86. [Google Scholar] [CrossRef]
- Avila, L.A.; Aps, L.; Ploscariu, N.; Sukthankar, P.; Guo, R.; Wilkinson, K.E.; Games, P.; Szoszkiewicz, R.; Alves, R.P.S.; Diniz, M.O.; et al. Gene delivery and immunomodulatory effects of plasmid DNA associated with Branched Amphiphilic Peptide Capsules. J. Control. Release Off. J. Control. Release Soc. 2016, 241, 15–24. [Google Scholar] [CrossRef]
- Cui, Z.; Han, S.J.; Vangasseri, D.P.; Huang, L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol. Pharm. 2005, 2, 22–28. [Google Scholar] [CrossRef]
- He, X.; Zhou, S.; Quinn, B.; Jahagirdar, D.; Ortega, J.; Abrams, S.I.; Lovell, J.F. HPV-Associated Tumor Eradication by Vaccination with Synthetic Short Peptides and Particle-Forming Liposomes. Small 2021, 17, e2007165. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H.; Yang, Y.; Jia, W.; Su, T.; Che, Y.; Feng, Y.; Yuan, X.; Wang, X. Mannose-Modified Liposome Co-Delivery of Human Papillomavirus Type 16 E7 Peptide and CpG Oligodeoxynucleotide Adjuvant Enhances Antitumor Activity Against Established Large TC-1 Grafted Tumors in Mice. Int. J. Nanomed. 2020, 15, 9571–9586. [Google Scholar] [CrossRef]
- Karimi, H.; Soleimanjahi, H.; Abdoli, A.; Banijamali, R.S. Combination therapy using human papillomavirus L1/E6/E7 genes and archaeosome: A nanovaccine confer immuneadjuvanting effects to fight cervical cancer. Sci. Rep. 2020, 10, 5787. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Liu, Z.; Zhou, X.; Guo, Z.; Zhang, J.; Zhu, P.; Yao, S.; Zhu, M. Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zandberg, D.P.; Rollins, S.; Goloubeva, O.; Morales, R.E.; Tan, M.; Taylor, R.; Wolf, J.S.; Schumaker, L.M.; Cullen, K.J.; Zimrin, A.; et al. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN). Cancer Immunol. Immunother. 2015, 64, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Reuschenbach, M.; Pauligk, C.; Karbach, J.; Rafiyan, M.R.; Kloor, M.; Prigge, E.S.; Sauer, M.; Al-Batran, S.E.; Kaufmann, A.M.; Schneider, A.; et al. A phase 1/2a study to test the safety and immunogenicity of a p16(INK4a) peptide vaccine in patients with advanced human papillomavirus-associated cancers. Cancer 2016, 122, 1425–1433. [Google Scholar] [CrossRef]
- Chandra, J.; Woo, W.P.; Finlayson, N.; Liu, H.Y.; McGrath, M.; Ladwa, R.; Brauer, M.; Xu, Y.; Hanson, S.; Panizza, B.; et al. A phase 1, single centre, open label, escalating dose study to assess the safety, tolerability and immunogenicity of a therapeutic human papillomavirus (HPV) DNA vaccine (AMV002) for HPV-associated head and neck cancer (HNC). Cancer Immunol. Immunother. 2021, 70, 743–753. [Google Scholar] [CrossRef]
- Nagarsheth, N.B.; Norberg, S.M.; Sinkoe, A.L.; Adhikary, S.; Meyer, T.J.; Lack, J.B.; Warner, A.C.; Schweitzer, C.; Doran, S.L.; Korrapati, S.; et al. TCR-engineered T cells targeting E7 for patients with metastatic HPV-associated epithelial cancers. Nat. Med. 2021, 27, 419–425. [Google Scholar] [CrossRef]
- Schuler, P.J.; Harasymczuk, M.; Visus, C.; Deleo, A.; Trivedi, S.; Lei, Y.; Argiris, A.; Gooding, W.; Butterfield, L.H.; Whiteside, T.L.; et al. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 2433–2444. [Google Scholar] [CrossRef]

| Vaccine | Type | Combination | Phase | Antigen | Disease | Delivery Platform | Status (as of 1 September 2022) | Identifier |
|---|---|---|---|---|---|---|---|---|
| PDS0101 | Peptide | M7824 (Targeting both PD-L1 and TGFβ), NHS-IL12 (immunocytokine) | I/II | HPV16 E6/E7 | locally advanced or metastatic HPV associated cancer | R-DOTAP containing lipid nanoparticle | Recruiting | NCT04287868 |
| PDS0101 | Peptide | Pembrolizumab | II | HPV16 E6/E7 | HPV16+ Recurrent and/or Metastatic HNSCC | R-DOTAP containing lipid nanoparticle | Recruiting | NCT04260126 |
| PDS0101 | Peptide | Pembrolizumab | I/II | HPV16 E6/E7 | Locally Advanced HPV Associated Oropharynx Cancer | R-DOTAP containing lipid nanoparticle | Recruiting | NCT05232851 |
| BNT113 | mRNA | Anti-CD40 | I/II | HPV 16 E6/E7 | Advanced HPV16+ cancer | RNA-lipoplex | Recruiting | NCT03418480 |
| BNT113 | mRNA | Pembrolizumab | II | HPV 16 E6/E7 | Unresectable recurrent or metastatic HPV16+ and PD-L1+ HNSCC | RNA-lipoplex | Recruiting | NCT04534205 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Chen, Y.; Jin, Y.; Wang, Z.; Dong, H.; Kaufmann, A.M.; Albers, A.E.; Qian, X. Advanced Nanomedicine for High-Risk HPV-Driven Head and Neck Cancer. Viruses 2022, 14, 2824. https://doi.org/10.3390/v14122824
Xu Q, Chen Y, Jin Y, Wang Z, Dong H, Kaufmann AM, Albers AE, Qian X. Advanced Nanomedicine for High-Risk HPV-Driven Head and Neck Cancer. Viruses. 2022; 14(12):2824. https://doi.org/10.3390/v14122824
Chicago/Turabian StyleXu, Qiang, Ye Chen, Yuan Jin, Zhiyu Wang, Haoru Dong, Andreas M. Kaufmann, Andreas E. Albers, and Xu Qian. 2022. "Advanced Nanomedicine for High-Risk HPV-Driven Head and Neck Cancer" Viruses 14, no. 12: 2824. https://doi.org/10.3390/v14122824
APA StyleXu, Q., Chen, Y., Jin, Y., Wang, Z., Dong, H., Kaufmann, A. M., Albers, A. E., & Qian, X. (2022). Advanced Nanomedicine for High-Risk HPV-Driven Head and Neck Cancer. Viruses, 14(12), 2824. https://doi.org/10.3390/v14122824









