The Incidence, Survival, and HPV Impact of Second Primary Cancer following Primary Oropharyngeal Squamous Cell Carcinoma: A 20-Year Retrospective and Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. HPV Analysis
2.3. SPC Definition
- Basal cell carcinoma of the skin was excluded;
- The tumour had to be biopsy verified and identified through The Danish Pathology Databank;
- Cancers in the oropharynx were considered a recurrence within the first five years after primary diagnosis. OPSCC later than five years after primary diagnosis was considered a SPC unless specifically stated as a recurrence in medical journals;
- Cancers in the head and neck area that were evaluated to be a different localization than the oropharynx by a multidisciplinary team or evaluated to be histologically or cytologically different were considered a SPC unless specifically stated as a recurrence in medical files;
- Synchronous and metachronous cancers were pooled, and therefore, no time limit was used.
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Cohort at the Time of Diagnosis
3.2. Factors Associated with the Risk of SPC
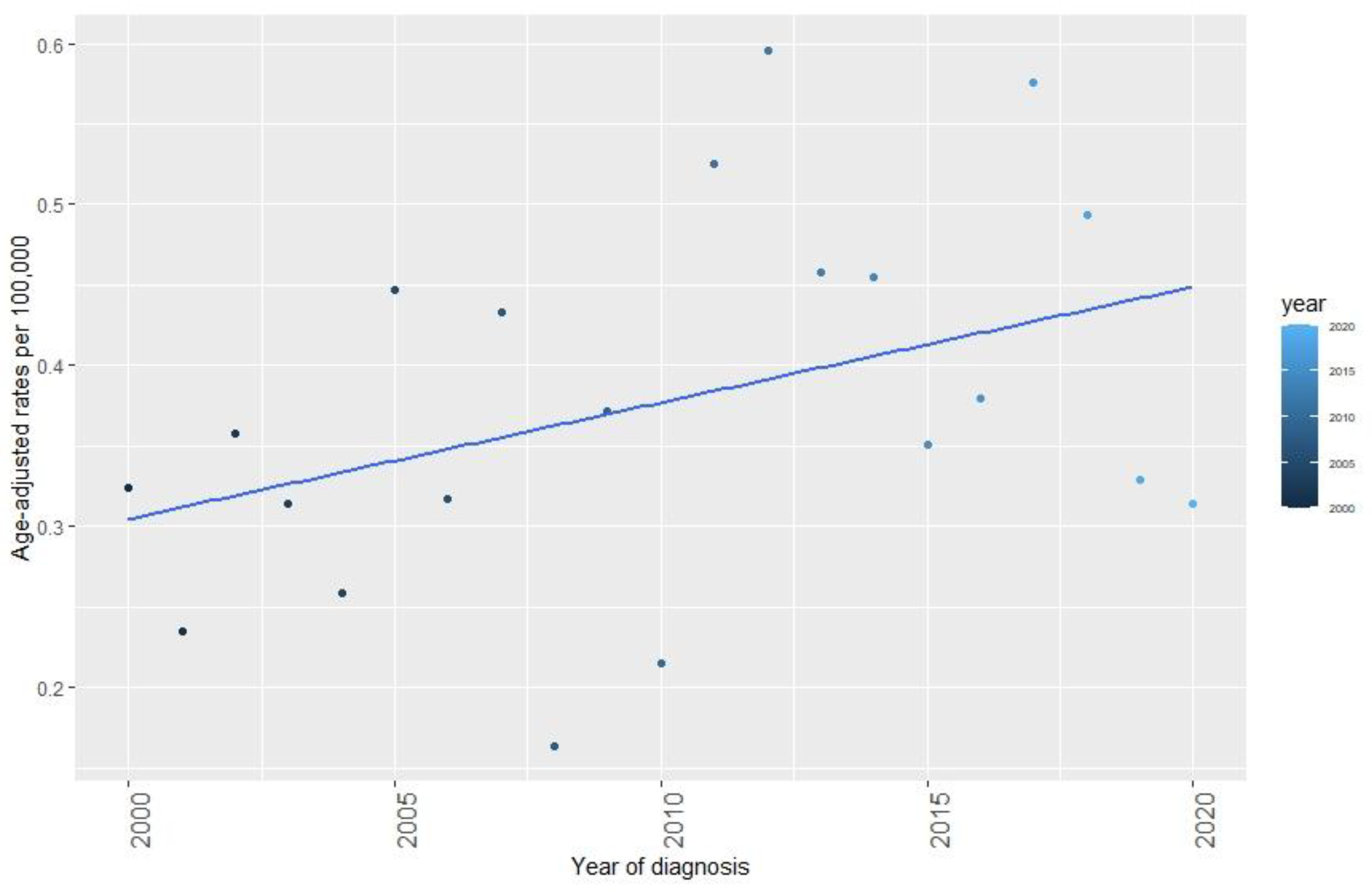
3.3. Incidence Trends and Characteristics of SPC
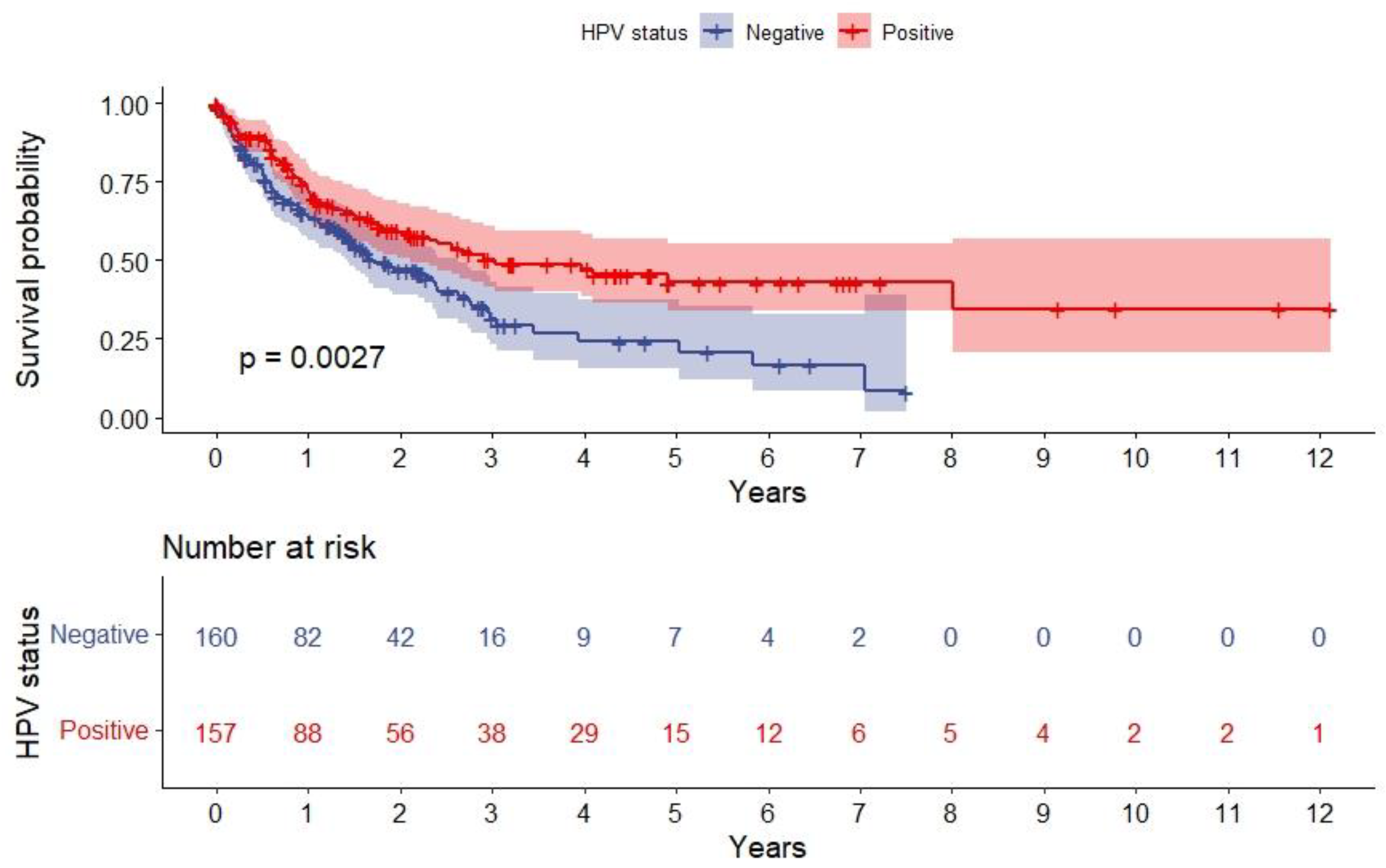
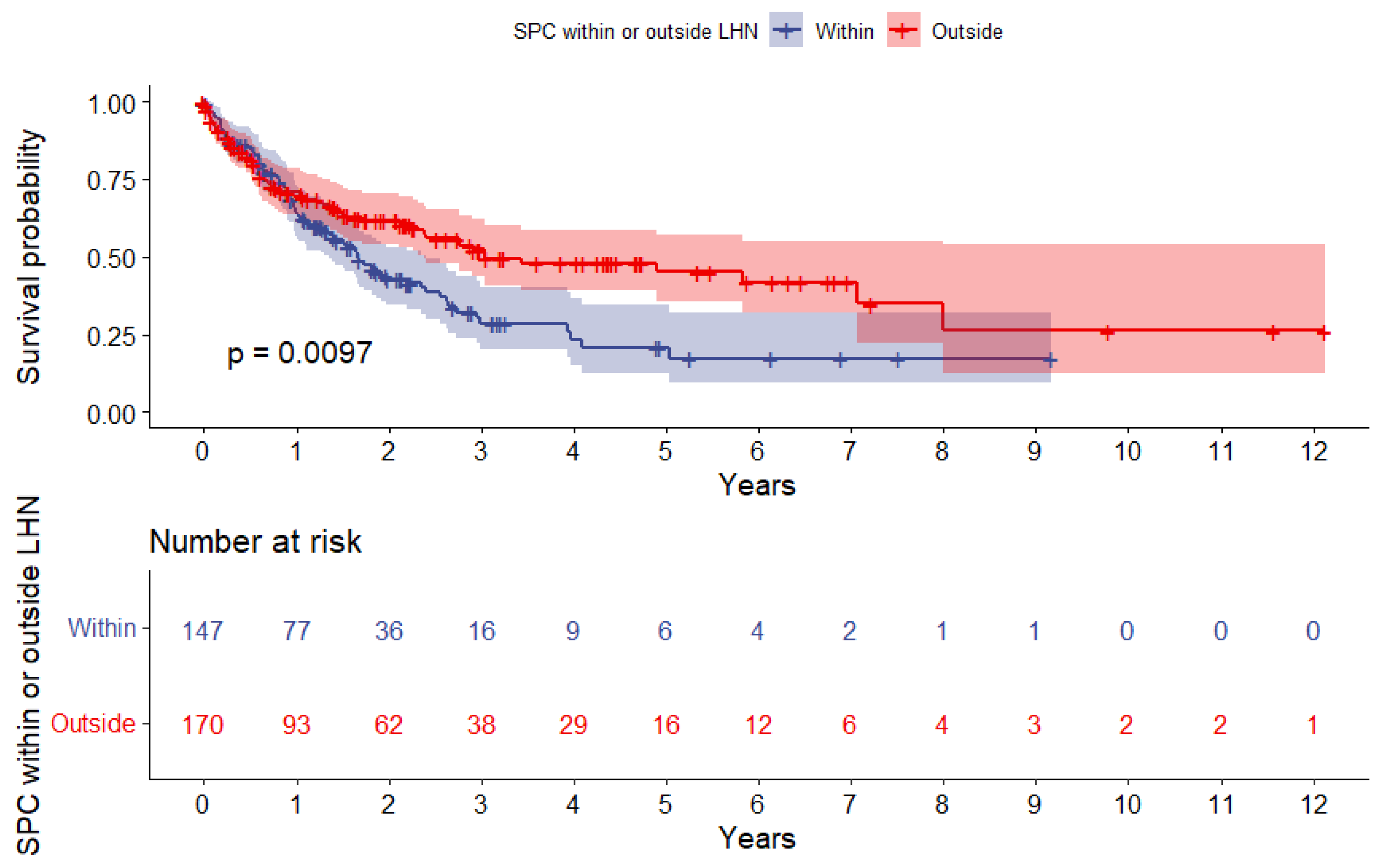
3.4. Survival Analysis after SPC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, M.; Nielsen, A.; Munk, C.; Kjaer, S.K. Trends in head and neck cancer incidence in Denmark, 1978–2007: Focus on human papillomavirus associated sites. Int. J. Cancer 2010, 129, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Suk, R.; Mahale, P.; Sonawane, K.; Sikora, A.G.; Chhatwal, J.; Schmeler, K.M.; Sigel, K.; Cantor, S.B.; Chiao, E.Y.; Deshmukh, A.A. Trends in Risks for Second Primary Cancers Associated With Index Human Papillomavirus–Associated Cancers. JAMA Netw. Open 2018, 1, e181999. [Google Scholar] [CrossRef] [PubMed]
- Garnaes, E.; Kiss, K.; Andersen, L.; Therkildsen, M.H.; Franzmann, M.B.; Filtenborg-Barnkob, B.; Hoegdall, E.; Krenk, L.; Josiassen, M.; Lajer, C.B.; et al. A high and increasing HPV prevalence in tonsillar cancers in Eastern Denmark, 2000-2010: The largest registry-based study to date. Int. J. Cancer 2014, 136, 2196–2203. [Google Scholar] [CrossRef]
- Garnaes, E.; Kiss, K.; Andersen, E.; Therkildsen, M.H.; Franzmann, M.B.; Filtenborgbarnkob, B.; Hoegdall, E.; Lajer, C.; Specht, L.; Joenson, L.; et al. Increasing incidence of base of tongue cancers from 2000 to 2010 due to HPV: The largest demographic study of 210 Danish patients. Br. J. Cancer 2015, 113, 131–134. [Google Scholar] [CrossRef]
- Carlander, A.-L.F.; Gronhoj Larsen, C.; Jensen, D.H.; Garnæs, E.; Kiss, K.; Andersen, L.; Olsen, C.H.; Franzmann, M.; Høgdall, E.; Kjær, S.K.; et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: A Danish population-based study from 2011 to 2014. Eur. J. Cancer 2017, 70, 75–82. [Google Scholar] [CrossRef]
- Attner, P.; Du, J.; Näsman, A.; Hammarstedt-Nordenvall, L.; Ramqvist, T.; Lindholm, J.; Marklund, L.; Dalianis, T.; Munck-Wikland, E. The role of human papillomavirus in the increased incidence of base of tongue cancer. Int. J. Cancer 2010, 126, 2879–2884. [Google Scholar] [CrossRef]
- Zamani, M.; Grønhøj, C.; Jensen, D.H.; Carlander, A.F.; Agander, T.; Kiss, K.; Olsen, C.; Baandrup, L.; Nielsen, F.C.; Andersen, E.; et al. The current epidemic of HPV-associated oropharyngeal cancer: An 18-year Danish population-based study with 2169 patients. Eur. J. Cancer 2020, 134, 52–59. [Google Scholar] [CrossRef]
- Garset-Zamani, M.; Carlander, A.F.; Jakobsen, K.K.; Friborg, J.; Kiss, K.; Marvig, R.L.; Olsen, C.; Nielsen, F.C.; Andersen, E.; Grønhøj, C.; et al. Impact of specific high-risk human papillomavirus genotypes on survival in oropharyngeal cancer. Int. J. Cancer 2021, 150, 1174–1183. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2016; Volume 91, pp. 386–396. [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Hayes, D.N.; Van Waes, C.; Seiwert, T.Y. Genetic Landscape of Human Papillomavirus–Associated Head and Neck Cancer and Comparison to Tobacco-Related Tumors. J. Clin. Oncol. 2015, 33, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S., Jr.; Khan, R.A.; Masand, R.P.; Chernock, R.D.; Zhang, Q.; Al-Naief, N.S.; Muller, S.; McHugh, J.B.; Prasad, M.L.; Brandwein-Gensler, M. Recognition of nonkeratinizing morphology in oropharyngeal squamous cell carcinoma–a prospective cohort and interobserver variability study. Histopathology 2012, 60, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Attner, P.; Du, J.; Näsman, A.; Hammarstedt-Nordenvall, L.; Ramqvist, T.; Lindholm, J.; Marklund, L.; Dalianis, T.; Munck-Wikland, E. Human papillomavirus and survival in patients with base of tongue cancer. Int. J. Cancer 2010, 128, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Garnaes, E.; Frederiksen, K.; Kiss, K.; Andersen, L.; Therkildsen, M.H.; Franzmann, M.B.; Specht, L.; Andersen, E.; Norrild, B.; Kjaer, S.K.; et al. Double positivity for HPV DNA/p16 in tonsillar and base of tongue cancer improves prognostication: Insights from a large population-based study. Int. J. Cancer 2016, 139, 2598–2605. [Google Scholar] [CrossRef] [PubMed]
- León, X.; Venegas, M.D.P.; Orús, C.; Kolañczak, K.; García, J.; Quer, M. Metachronous second primary tumours in the aerodigestive tract in patients with early stage head and neck squamous cell carcinomas. Eur. Arch. Oto-Rhino-Laryngol. Head Neck 2005, 262, 905–909. [Google Scholar] [CrossRef]
- Baxi, S.S.; Pinheiro, L.C.; Patil, S.M.; Pfister, D.G.; Oeffinger, K.C.; Elkin, E.B. Causes of death in long-term survivors of head and neck cancer. Cancer 2014, 120, 1507–1513. [Google Scholar] [CrossRef]
- Nørregaard, C.; Grønhøj, C.; Jensen, D.; Friborg, J.; Andersen, E.; von Buchwald, C. Cause-specific mortality in HPV+ and HPV− oropharyngeal cancer patients: Insights from a population-based cohort. Cancer Med. 2017, 7, 87–94. [Google Scholar] [CrossRef]
- Saber, C.N.; Grønhøj, C.; Jensen, D.H.; Nørregaard, C.; Carlander, A.; Garnæs, E.; Kiss, K.; Specht, L.; von Buchwald, C. Synchronous, bilateral tonsillar carcinomas: Patient characteristics and human papillomavirus genotypes. Oral Oncol. 2017, 74, 105–110. [Google Scholar] [CrossRef]
- Jain, K.S.; Sikora, A.G.; Baxi, S.S.; Morris, L.G. Synchronous cancers in patients with head and neck cancer: Risks in the era of human papillomavirus-associated oropharyngeal cancer. Cancer 2013, 119, 1832–1837. [Google Scholar] [CrossRef]
- Milliet, F.; Bozec, A.; Schiappa, R.; Viotti, J.; Modesto, A.; Dassonville, O.; Poissonnet, G.; Guelfucci, B.; Bizeau, A.; Vergez, S.; et al. Synchronous primary neoplasia in patients with oropharyngeal cancer: Impact of tumor HPV status. A GETTEC multicentric study. Oral Oncol. 2020, 112, 105041. [Google Scholar] [CrossRef]
- Bollig, C.A.; Lee, D.S.; Mazul, A.L.; Stepan, K.; Puram, S.V.; Massa, S.T.; Zenga, J.; Faden, D.L.; Doering, M.M.; Jackson, R.S.; et al. Systematic Review of Second Primary Oropharyngeal Cancers in Patients With p16+ Oropharyngeal Cancer. Otolaryngol. Neck Surg. 2020, 164, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Holstead, R.; Rasul, R.; Golden, A.; Kamdar, D.; Ghaly, M.; Teckie, S.; Frank, D.; Fantasia, J.; Seetharamu, N. Identifying patterns of failure and secondary primary malignancies in HPV-related oropharyngeal squamous cell carcinomas. Futur. Oncol. 2020, 16, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.C.; Biron, V.L.; Puttagunta, L.; Seikaly, H. HPV Status and second primary tumours in Oropharyngeal Squamous Cell Carcinoma. J. Otolaryngol. Head Neck Surg. 2013, 42, 36. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- rStudio Team. RStudio: Integrated Development Environment for R; RStudio, P.: Boston, MA, USA, 2021; Available online: http://www.rstudio.com/ (accessed on 29 September 2022).
- eSundhed. Available online: https://www.esundhed.dk/ (accessed on 5 May 2021).
- Statistics Denmark. Available online: https://www.statistikbanken.dk/ (accessed on 11 September 2022).
- Kræftens Bekæmpelse. Available online: https://www.cancer.dk/ (accessed on 5 July 2021).
- León, X.; Venegas, M.D.P.; Orús, C.; López, M.; García, J.; Quer, M.; Lorenzo, J.G. Influence of the persistence of tobacco and alcohol use in the appearance of second neoplasm in patients with a head and neck cancer. A case–control study. Cancer Causes Control. 2008, 20, 645–652. [Google Scholar] [CrossRef]
- BBosshart, S.; Morand, G.; Broglie, M. Frequency and Localization of Second Primary Tumors in Patients with Oropharyngeal Carcinoma—The Influence of the Human Papilloma Virus. Cancers 2021, 13, 1755. [Google Scholar] [CrossRef]
- Hashibe, M.; Ritz, B.; Le, A.D.; Li, G.; Sankaranarayanan, R.; Zhang, Z.-F. Radiotherapy for oral cancer as a risk factor for second primary cancers. Cancer Lett. 2005, 220, 185–195. [Google Scholar] [CrossRef]
- Grønhøj, C.; Jakobsen, K.K.; Jensen, D.H.; Rasmussen, J.; Andersen, E.; Friborg, J.; von Buchwald, C. Pattern of and survival following loco-regional and distant recurrence in patients with HPV+ and HPV− oropharyngeal squamous cell carcinoma: A population-based study. Oral Oncol. 2018, 83, 127–133. [Google Scholar] [CrossRef]
- Oguejiofor, K.; Galletta-Williams, H.; Dovedi, S.J.; Roberts, D.L.; Stern, P.L.; West, C.M. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget 2017, 8, 14416–14427. [Google Scholar] [CrossRef]
- Lechien, J.R.; Seminerio, I.; Descamps, G.; Mat, Q.; Mouawad, F.; Hans, S.; Julieron, M.; Dequanter, D.; Vanderhaegen, T.; Journe, F.; et al. Impact of HPV Infection on the Immune System in Oropharyngeal and Non-Oropharyngeal Squamous Cell Carcinoma: A Systematic Review. Cells 2019, 8, 1061. [Google Scholar] [CrossRef] [PubMed]
- Grønhøj, C.; Jakobsen, K.K.; Kjær, E.; Friborg, J.; von Buchwald, C. Comorbidity in HPV+ and HPV− oropharyngeal cancer patients: A population-based, case-control study. Oral Oncol. 2019, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sharma, A.; Osazuwa-Peters, N.; Simpson, M.C.; Schootman, M.; Piccirillo, J.F.; Huh, W.K.; Boakye, E.A. Risk of subsequent malignant neoplasms after an index potentially-human papillomavirus (HPV)-associated cancers. Cancer Epidemiol. 2019, 64, 101649. [Google Scholar] [CrossRef] [PubMed]
| Total | SPC- (n = 2267, 87.7%) | SPC+ (n = 317, 12.3%) | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| Median age at diagnosis, (range) years | 61 | (30–92) | 60 | (30–92) | 64 | (37–86) | <0.001 * |
| Sex, n (%) Female Male | 2584 697 1887 | (100%) (27.0%) (73.0%) | 609 1658 | (26.9%) (73.1%) | 88 229 | (27.8%) (72.2%) | 0.736 |
| UICC8 T-classification, n (%) 1 2 3 4 | 2575a 635 1065 529 346 | (100%) (24.7%) (41.4%) (20.5%) (13.4%) | 559 926 468 306 | (24.7%) (41.0%) (20.7%) (13.5%) | 76 139 61 40 | (24.1%) (44.0%) (19.3%) (12.7%) | 0.896 |
| UICC8 N-classification, n (%) 0 1 2 3 | 2584 589 1306 580 109 | (100%) (22.8%) (50.5%) (22.4%) (4.2%) | 488 1161 522 96 | (21.5%) (51.2%) (23.0%) (4.2%) | 101 145 58 13 | (31.9%) (45.7%) (18.3%) (4.1%) | <0.001 * |
| UICC8 M-classification, n (%) 0 1 | 2569a 2551 18 | (100%) (99.3%) (0.7%) | 2237 16 | (99.3%) (0.7%) | 314 2 | (99.4%) (0.6%) | 1.000 |
| UICC8 Stage, n (%) I II III IV | 2518a 1127 489 427 475 | (100%) (44.8%) (19.4%) (17.0%) (18.9%) | 1006 414 371 415 | (45.6%) (18.8%) (16.8%) (18.8%) | 121 75 56 60 | (38.8%) (24.0%) (17.9%) (19.2%) | 0.077 |
| Alcohol consumption **, n (%) No abuse Previous or current abuse | 2583a 1059 1524 | (100%) (41.0%) (59.0%) | 951 1315 | (42.0%) (58.0%) | 108 209 | (34.1%) (65.9%) | 0.007 * |
| Smoking status, n (%) Never smoker Former smoker Current smoker | 2555a 566 1001 988 | (100%) (22.2%) (39.2%) (38.7%) | 513 862 867 | (22.9%) (38.4%) (38.7%) | 53 139 121 | (16.9%) (44.4%) (38.7%) | 0.032 * |
| Histopathological type, n (%) Non-keratinizing Keratinizing/hybrid/other | 2514a 1378 1136 | (100%) (54.8%) (45.2%) | 1240 966 | (56.2%) (43.8%) | 138 170 | (44.8%) (55.2%) | 0.002 * |
| HPV status (HPV DNA/p16), n (%) HPV-negative HPV-positive | 2584 1052 1532 | (100%) (40.7%) (59.3%) | 892 1375 | (39.3%) (60.7%) | 160 157 | (50.5%) (49.5%) | <0.001 * |
| Primary tumour location, n (%) Palatine tonsils Base of tongue Other | 2584 1308 762 514 | (100%) (50.6%) (29.5%) (19.9%) | 1149 688 430 | (50.7%) (30.3%) (19.0%) | 159 74 84 | (50.2%) (23.3%) (26.5%) | 0.002 * |
| Performance score, n (%) 0 1–4 | 2248a 1697 551 | (100%) (75.5%) (24.5%) | 1499 475 | (75.9%) (24.1%) | 198 76 | (72.3%) (27.7%) | 0.211 |
| Prior cancer, n (%) No Yes | 2575a 2308 267 | (100%) (89.6%) (10.4%) | 2035 226 | (90.0%) (10.0%) | 273 41 | (86.9%) (13.1%) | 0.095 |
| Chemotherapy type, n (%) Cisplatin Other *** | 1194b 1094 100 | (100%) (91.6%) (8.4%) | 986 94 | (91.3%) (8.7%) | 108 6 | (94.7%) (5.3%) | 0.207 |
| Risk Factors | Univariable HR [95% CI] | p-Value | Multivariable HR [95% CI] | p-Value |
|---|---|---|---|---|
| Age | 1.05 [1.04–1.06] | <0.001 * | 1.04 [1.03–1.06] | <0.001 * |
| Sex Female Male | Ref. 1.03 [0.80–1.31] | - 0.836 | Ref. 1.15 [0.89–1.48] | - 0.287 |
| Sublocation Palatine tonsils Base of tongue Other | Ref. 0.91 [0.69–1.21] 1.82 [1.39–2.37] | - 0.524 <0.001 * | Ref. 0.80 [0.61–1.07] 1.02 [0.75–1.39] | - 0.132 0.887 |
| UICC8-stage I II III IV | Ref. 1.76 [1.32–2.35] 1.84 [1.34–2.53] 2.47 [1.80–3.39] | - <0.001 * <0.001 * <0.001 * | Ref. 1.80 [1.20–2.69] 1.60 [0.99–2.59] 1.94 [0.98–3.87] | - 0.004 * 0.057 0.058 |
| UICC8 T-classification T1 T2 T3 T4 | Ref. 1.02 [0.77–1.35] 1.19 [0.85–1.66] 1.60 [1.09–2.35] | - 0.900 0.323 0.017 * | Ref. 0.86 [0.64–1.15] 0.67 [0.44–1.04] 0.91 [0.53–1.54] | - 0.302 0.072 0.722 |
| UICC8 N-classification N0 N1 N2 N3 | Ref. 0.52 [0.40–0.66] 0.74 [0.53–1.02] 0.81 [0.45–1.44] | - <0.001 * 0.068 0.465 | Ref. 0.80 [0.60–1.07] 0.61 [0.38–0.97] 0.77 [0.37–1.57] | - 0.129 0.036 * 0.466 |
| Smoking status Never smoker Former smoker Current smoker | Ref. 1.63 [1.19–2.24] 1.89 [1.37–2.62] | - 0.002 * <0.001 * | Ref. 1.03 [0.94–1.80] 1.10 [0.77–1.59] | - 0.112 0.594 |
| HPV status (HPV DNA/p16) HPV-negative HPV-positive | Ref. 0.411 [0.33–0.52] | - <0.001 * | Ref. 0.56 [0.40–0.80] | - 0.001 * |
| HPV- | HPV+ | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Second primary cancer localization, n (%) Lung Head and neck Gastrointestinal tract Skin ** Gender-specific *** Haematologic cancer Breast Liver or pancreas Urologic cancer CNS or brain Non-oropharyngeal HPV- Associated **** Other | 317 95 52 47 29 24 21 18 11 11 4 3 2 | (100%) (30.0%) (16.4%) (14.8%) (9.1%) (7.6%) (6.6%) (5.7%) (3.5%) (3.5%) (1.3%) (0.9%) (0.6%) | 160 57 31 29 12 1 5 10 6 4 1 2 2 | (50.5%) (35.6%) (19.4%) (18.1%) (7.5%) (0.6%) (3.1%) (6.2%) (3.8%) (2.5%) (0.6%) (1.3%) (1.3%) | 157 38 21 18 17 23 16 8 5 7 3 1 0 | (49.5%) (24.2%) (13.4%) (11.5%) (10.8%) (14.6%) (10.2%) (5.1%) (3.2%) (4.5%) (1.9%) (0.6%) (0.0%) | |
| Localizations stratified by LHN, n (%) Within LHN Outside LHN | 88 72 | (55.0%) (45.0%) | 59 98 | (37.6%) (62.4%) | 0.003 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, L.; Jakobsen, K.K.; Carlander, A.-L.F.; Garset-Zamani, M.; Friborg, J.; Kiss, K.; Marvig, R.L.; Olsen, C.; Nielsen, F.C.; Andersen, E.; et al. The Incidence, Survival, and HPV Impact of Second Primary Cancer following Primary Oropharyngeal Squamous Cell Carcinoma: A 20-Year Retrospective and Population-Based Study. Viruses 2023, 15, 34. https://doi.org/10.3390/v15010034
Andersen L, Jakobsen KK, Carlander A-LF, Garset-Zamani M, Friborg J, Kiss K, Marvig RL, Olsen C, Nielsen FC, Andersen E, et al. The Incidence, Survival, and HPV Impact of Second Primary Cancer following Primary Oropharyngeal Squamous Cell Carcinoma: A 20-Year Retrospective and Population-Based Study. Viruses. 2023; 15(1):34. https://doi.org/10.3390/v15010034
Chicago/Turabian StyleAndersen, Lasse, Kathrine Kronberg Jakobsen, Amanda-Louise Fenger Carlander, Martin Garset-Zamani, Jeppe Friborg, Katalin Kiss, Rasmus L. Marvig, Caroline Olsen, Finn Cilius Nielsen, Elo Andersen, and et al. 2023. "The Incidence, Survival, and HPV Impact of Second Primary Cancer following Primary Oropharyngeal Squamous Cell Carcinoma: A 20-Year Retrospective and Population-Based Study" Viruses 15, no. 1: 34. https://doi.org/10.3390/v15010034
APA StyleAndersen, L., Jakobsen, K. K., Carlander, A.-L. F., Garset-Zamani, M., Friborg, J., Kiss, K., Marvig, R. L., Olsen, C., Nielsen, F. C., Andersen, E., Grønhøj, C., & Buchwald, C. v. (2023). The Incidence, Survival, and HPV Impact of Second Primary Cancer following Primary Oropharyngeal Squamous Cell Carcinoma: A 20-Year Retrospective and Population-Based Study. Viruses, 15(1), 34. https://doi.org/10.3390/v15010034







