Genome Characterization and Phylogenetic Analysis of a Novel Endornavirus That Infects Fungal Pathogen Sclerotinia sclerotiorum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Biological Characterization
2.2. Total RNA Extraction and Sequencing
2.3. RT-PCR and RACE Cloning of Viral Genome
2.4. Sequence Analysis
3. Results
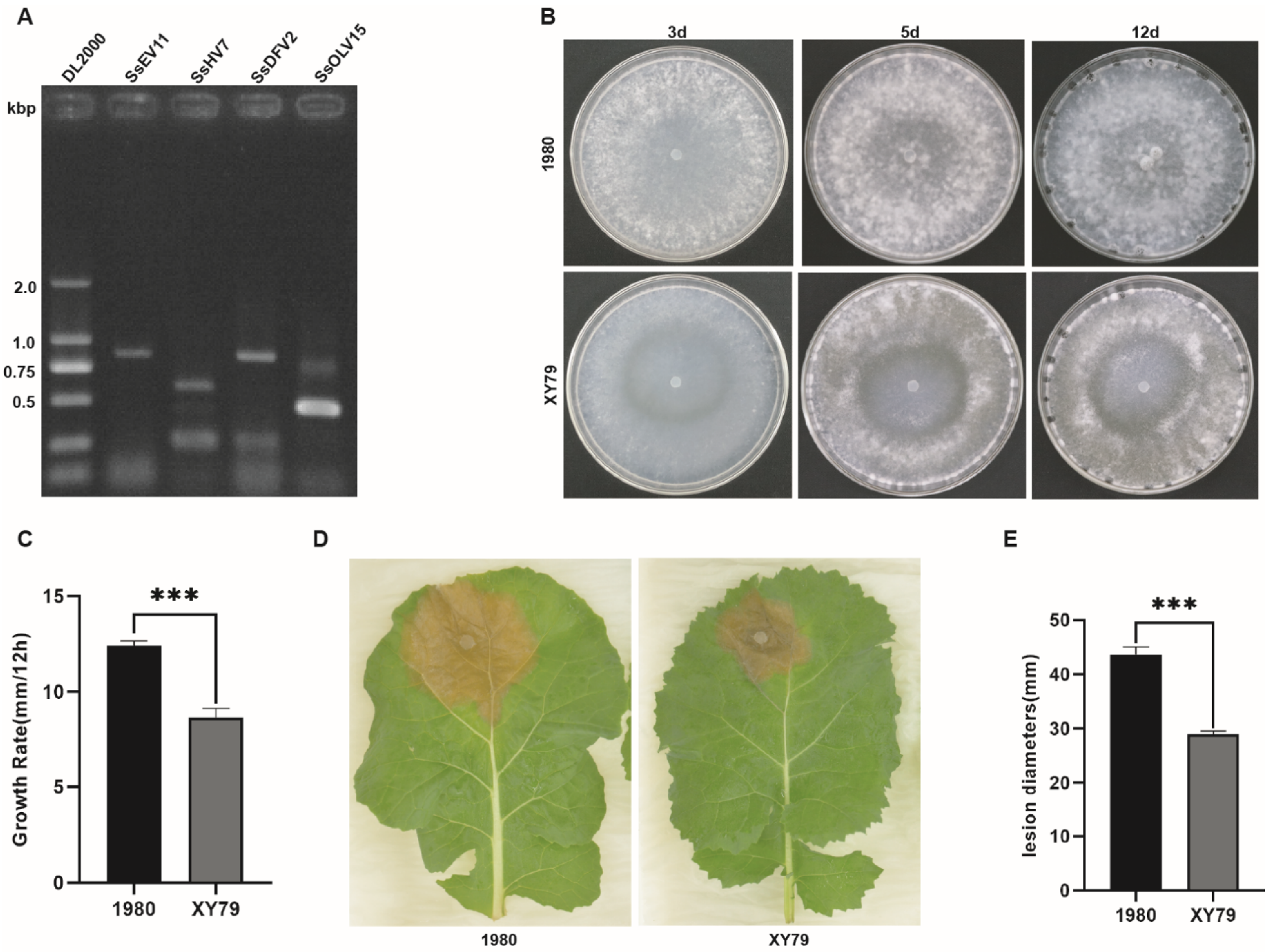
3.1. Virus Diversity and Biological Characteristics of Strain XY79
3.2. Genome Characteristics of SsEV11
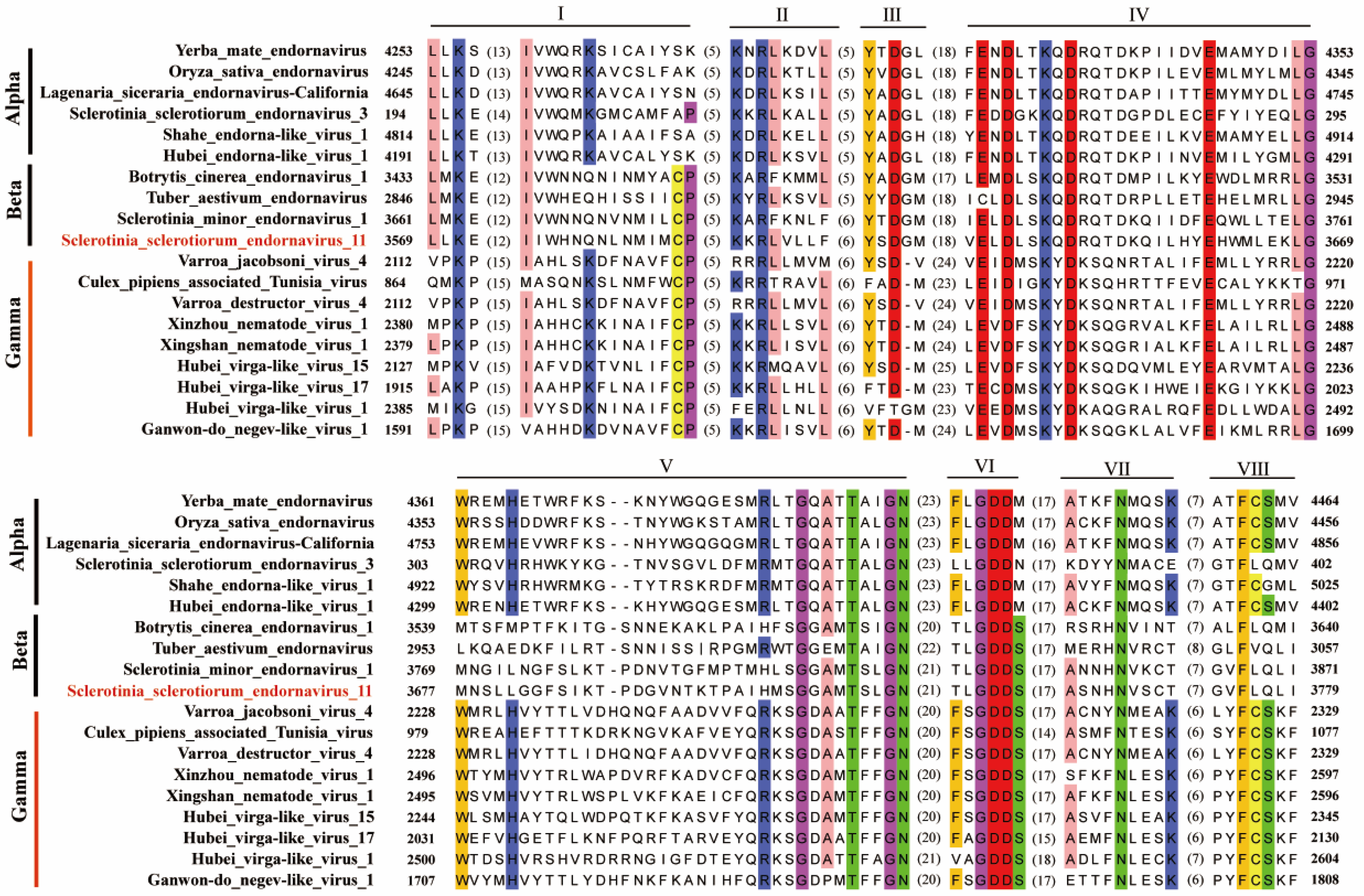
3.3. Alignment Analysis of SsEV11
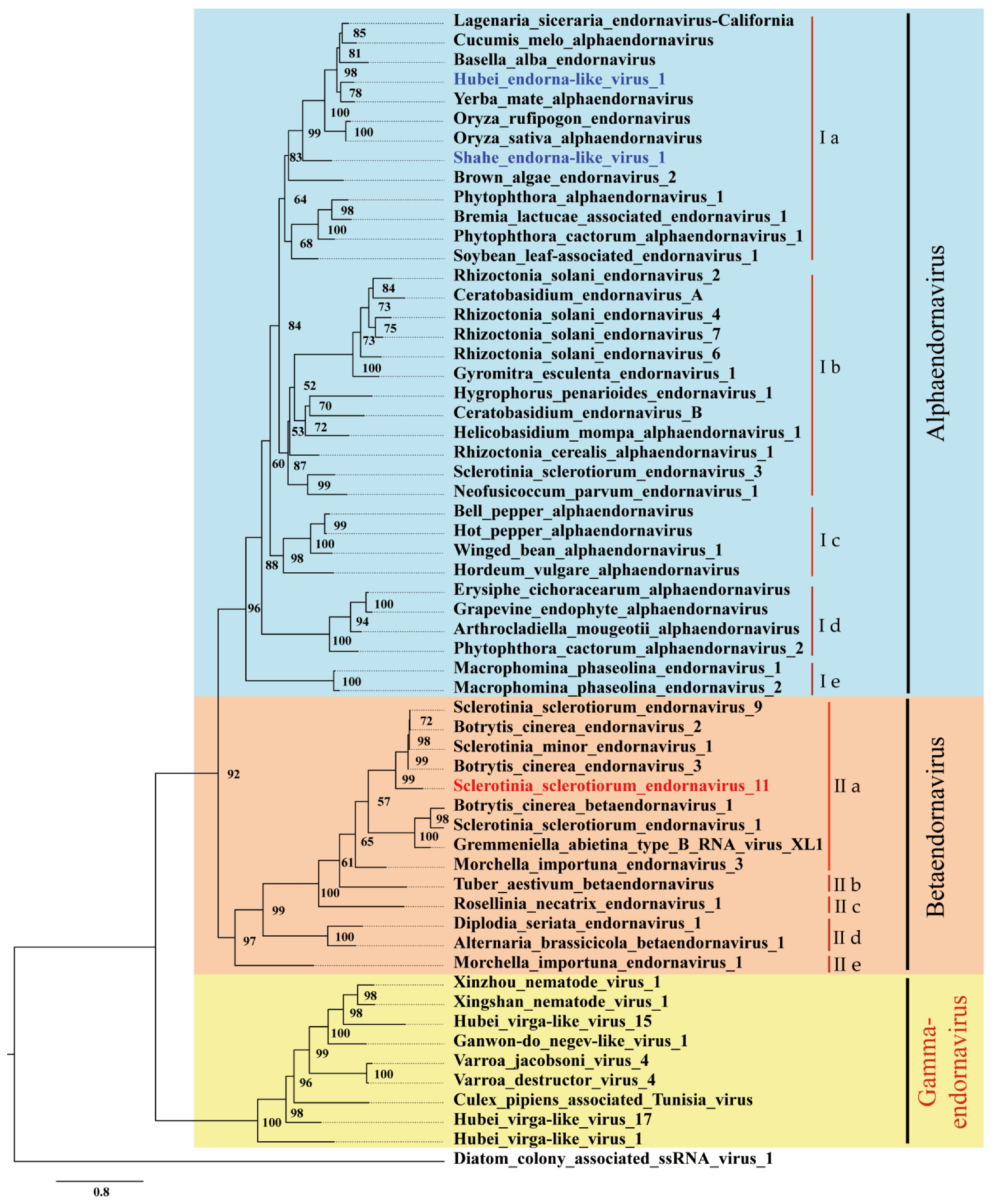
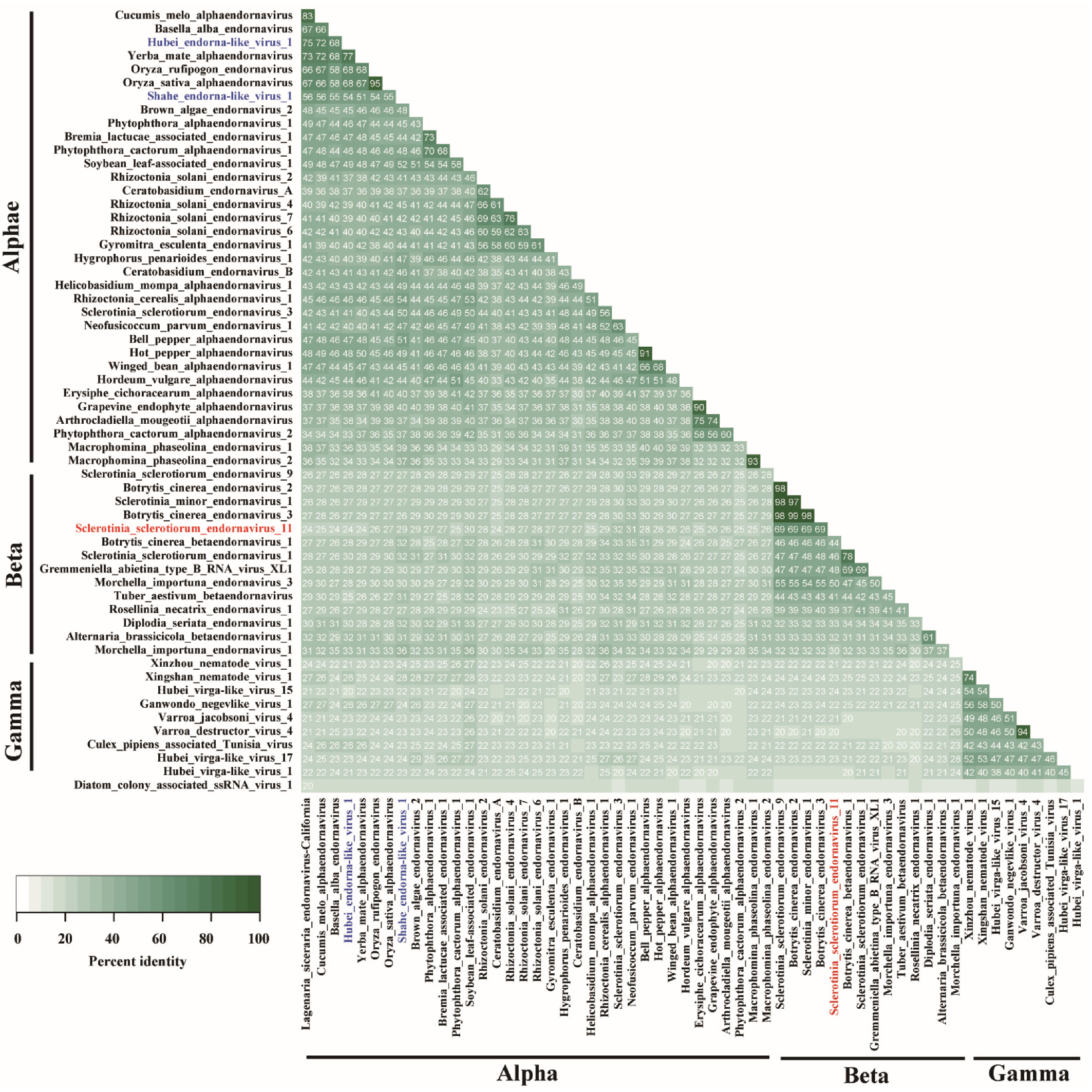
3.4. Phylogenetic Analysis Exhibits Multiple Lineages of Endornaviruses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009). Arch. Virol. 2010, 155, 133–146. [Google Scholar] [CrossRef]
- Valverde, R.A.; Khalifa, M.E.; Okada, R.; Fukuhara, T.; Sabanadzovic, S. ICTV Virus Taxonomy Profile: Endornaviridae. J. Gen. Virol. 2019, 100, 1204–1205. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.W.L.; Li, H.; Sivasithamparam, K.; Dixon, K.W.; Jones, M.G.K.; Wylie, S.J. Novel Endorna-like viruses, including three with two open reading frames, challenge the membership criteria and taxonomy of the Endornaviridae. Virology 2016, 499, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, H.; Horiuchi, H.; Nitta, T.; Fukuhara, T. Unusual inheritance of evolutionarily-related double-stranded RNAs in interspecific hybrid between rice plants Oryza sativa and Oryza rufipogon. Plant Mol. Biol. 1999, 39, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, M.J.; Koga, R.; Moriyama, H.; Pfeiffer, P.; Fukuhara, T. Phylogenetic analysis of some large double-stranded RNA replicons from plants suggests they evolved from a defective single-stranded RNA virus. J. Gen. Virol. 2000, 81, 227–233. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Pearson, M.N. Molecular characterisation of an endornavirus infecting the phytopathogen Sclerotinia sclerotiorum. Virus Res. 2014, 189, 303–309. [Google Scholar] [CrossRef]
- Wakarchuk, D.A.; Hamilton, R.I. Cellular double-stranded RNA in Phaseolus vulgaris. Plant Mol. Biol. 1985, 5, 55–63. [Google Scholar] [CrossRef]
- Lefebvre, A.; Scalla, R.; Pfeiffer, P. The double-stranded RNA associated with the ‘447’ cytoplasmic male sterility in Vicia faba is packaged together with its replicase in cytoplasmic membranous vesicles. Plant Mol. Biol. 1990, 14, 477–490. [Google Scholar] [CrossRef]
- Valverde, R.A.; Nameth, S.; Abdallha, O.; Almusa, O.; Desjardins, P.; Dodds, A. Indigenous double-stranded RNA from pepper (Capsicum annuum). Plant Sci. 1990, 67, 195–201. [Google Scholar] [CrossRef]
- Zabalgogeazcoa, I.A.; Gildow, F.E. Double-stranded ribonucleic-acid in ‘Barsoy’ barley. Plant Sci. 1992, 83, 187–194. [Google Scholar] [CrossRef]
- Fukuhara, T.; Moriyama, H.; Pak, J.Y.; Hyakutake, H.; Nitta, T. Enigmatic double-stranded RNA in Japonica rice. Plant Mol. Biol. 1993, 21, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Hacker, C.V.; Brasier, C.M.; Buck, K.W. A double-stranded RNA from a Phytophthora species is related to the plant endornaviruses and contains a putative UDP glycosyltransferase gene. J. Gen. Virol. 2005, 86, 1561–1570. [Google Scholar] [CrossRef] [PubMed]
- Tuomivirta, T.T.; Hantula, J. Three unrelated viruses occur in a single isolate of Gremmeniella abietina var. abietina type A. Virus Res. 2005, 110, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.; Sela, N.; Erez, T.; Nestel, D.; Pettis, J.; Neumann, P.; Chejanovsky, N. New viruses from the ectoparasite mite Varroa destructor infesting Apis mellifera and Apis cerana. Viruses 2019, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, J.M.K.; Simbiken, N.; Dale, C.; Armstrong, J.; Anderson, D.L. Tolerance of honey bees to Varroa mite in the absence of deformed wing virus. Viruses 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Bigot, D.; Atyame, C.M.; Weill, M.; Justy, F.; Herniou, E.A.; Gayral, P. Discovery of Culex pipiens associated tunisia virus: A new ssRNA(+) virus representing a new insect associated virus family. Virus Evol. 2018, 4, vex040. [Google Scholar] [CrossRef] [Green Version]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Xie, J.; Fu, Y.; Cheng, J.; Li, B.; Chen, T.; Zhao, Y.; Gao, Z.; Yang, P.; Barbetti, M.J.; et al. A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef]
- Xie, J.; Xiao, X.; Fu, Y.; Liu, H.; Cheng, J.; Ghabrial, S.A.; Li, G.; Jiang, D. A novel mycovirus closely related to hypoviruses that infects the plant pathogenic fungus Sclerotinia sclerotiorum. Virology 2011, 418, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Li, B.; Fu, Y.; Jiang, D.; Ghabrial, S.A.; Li, G.; Peng, Y.; Xie, J.; Cheng, J.; Huang, J.; et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc. Natl. Acad. Sci. USA 2010, 107, 8387–8392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Li, B.; Fu, Y.P.; Xie, J.T.; Cheng, J.S.; Ghabrial, S.A.; Li, G.Q.; Yi, X.H.; Jiang, D.H. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc. Natl. Acad. Sci. USA 2013, 110, 1452–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, F.; Xie, J.; Cheng, S.; You, M.P.; Barbetti, M.J.; Jia, J.; Wang, Q.; Cheng, J.; Fu, Y.; Chen, T.; et al. Virome characterization of a collection of S. sclerotiorum from Australia. Front. Microbiol. 2018, 8, 2540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Mu, F.; Xie, J.; Cheng, J.; Fu, Y.; Jiang, D. A single ssRNA segment encoding RdRp is sufficient for replication, infection, and transmission of ourmia-like virus in fungi. Front. Microbiol. 2020, 11, 379. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Xie, J.; Cheng, J.; Fu, Y.; Li, G.; Yi, X.; Jiang, D. Fungal negative-stranded RNA virus that is related to bornaviruses and nyaviruses. Proc. Natl. Acad. Sci. USA 2014, 111, 12205–12210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Fu, Y.; Jiang, D.; Li, G.; Xie, J.; Cheng, J.; Peng, Y.; Ghabrial, S.A.; Yi, X. Widespread horizontal gene transfer from double-stranded RNA viruses to eukaryotic nuclear genomes. J. Virol. 2010, 84, 11876–11887. [Google Scholar] [CrossRef] [Green Version]
- Marzano, S.L.; Nelson, B.D.; Ajayi-Oyetunde, O.; Bradley, C.A.; Hughes, T.J.; Hartman, G.L.; Eastburn, D.M.; Domier, L.L. Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J. Virol. 2016, 90, 6846–6863. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Fu, Y.; Jiang, D.; Mu, F.; Cheng, J.; Lin, Y.; Li, B.; Marzano, S.L.; Xie, J. Interannual dynamics, diversity and evolution of the virome in Sclerotinia sclerotiorum from a single crop field. Virus Evol. 2021, 7, veab032. [Google Scholar] [CrossRef]
- Mu, F.; Li, B.; Cheng, S.; Jia, J.; Jiang, D.; Fu, Y.; Cheng, J.; Lin, Y.; Chen, T.; Xie, J. Nine viruses from eight lineages exhibiting new evolutionary modes that co-infect a hypovirulent phytopathogenic fungus. PLoS Pathog. 2021, 17, e1009823. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Esmael, A.; Duan, J.; Bian, X.; Jia, J.; Xie, J.; Cheng, J.; Fu, Y.; Jiang, D.; et al. Four novel botourmiaviruses co-infecting an isolate of the rice blast fungus Magnaporthe oryzae. Viruses 2020, 12, 1383. [Google Scholar] [CrossRef]
- Potgieter, A.C.; Page, N.A.; Liebenberg, J.; Wright, I.M.; Landt, O.; van Dijk, A.A. Improved strategies for sequence-independent amplification and sequencing of viral double-stranded RNA genomes. J. Gen. Virol. 2009, 90, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Wang, W. The Molecular Detection of Corynespora Cassiicola on Cucumber by PCR Assay Using DNAman Software and NCBI. Computer and Computing Technologies in Agriculture; China Agricultural University: Beijing, China, 2015; Volume 27, p. 9. [Google Scholar]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Osaki, H.; Nakamura, H.; Sasaki, A.; Matsumoto, N.; Yoshida, K. An endornavirus from a hypovirulent strain of the violet root rot fungus, Helicobasidium mompa. Virus Res. 2006, 118, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Shu, C.; Zhang, M.; Yang, M.; Zhou, E. Molecular characterization of a novel Endornavirus conferring hypovirulence in rice sheath blight fungus Rhizoctonia solani AG-1 IA strain GD-2. Viruses 2019, 11, 178. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Wu, M.; Zhang, J.; Chen, W.; Li, G.; Yang, L. Sclerotinia minor endornavirus 1, a novel pathogenicity debilitation-associated mycovirus with a wide spectrum of horizontal transmissibility. Viruses 2018, 10, 589. [Google Scholar] [CrossRef] [Green Version]
- Tuomivirta, T.T.; Kaitera, J.; Hantula, J. A novel putative virus of Gremmeniella abietina type B (Ascomycota: Helotiaceae) has a composite genome with endornavirus affinities. J. Gen. Virol. 2009, 90, 2299–2305. [Google Scholar] [CrossRef]
- Stielow, B.; Klenk, H.P.; Menzel, W. Complete genome sequence of the first endornavirus from the ascocarp of the ectomycorrhizal fungus Tuber aestivum Vittad. Arch. Virol. 2011, 156, 343–345. [Google Scholar] [CrossRef]
- Donaire, L.; Ayllon, M.A. Deep sequencing of mycovirus-derived small RNAs from Botrytis species. Mol. Plant Pathol. 2017, 18, 1127–1137. [Google Scholar] [CrossRef]
- Yaegashi, H.; Kanematsu, S. Natural infection of the soil-borne fungus Rosellinia necatrix with novel mycoviruses under greenhouse conditions. Virus Res. 2016, 219, 83–91. [Google Scholar] [CrossRef]
- Nerva, L.; Turina, M.; Zanzotto, A.; Gardiman, M.; Gaiotti, F.; Gambino, G.; Chitarra, W. Isolation, molecular characterization and virome analysis of culturable wood fungal endophytes in esca symptomatic and asymptomatic grapevine plants. Environ. Microbiol. 2019, 21, 2886–2904. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.H.; Zhong, J.; Zhang, R.J.; Chen, C.Y.; Gao, B.D.; Zhu, H.J. Genome sequence of a novel endornavirus from the phytopathogenic fungus Alternaria brassicicola. Arch. Virol. 2015, 160, 1827–1830. [Google Scholar] [CrossRef]
- Gilbert, K.B.; Holcomb, E.E.; Allscheid, R.L.; Carrington, J.C. Hiding in plain sight: New virus genomes discovered via a systematic analysis of fungal public transcriptomes. PLoS ONE 2019, 14, e0219207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marais, A.; Faure, C.; Comont, G.; Candresse, T.; Stempien, E.; Corio-Costet, M.F. Characterization of the mycovirome of the phytopathogenic fungus, Neofusicoccum parvum. Viruses 2021, 13, 375. [Google Scholar] [CrossRef]
- Chiba, Y.; Tomaru, Y.; Shimabukuro, H.; Kimura, K.; Hirai, M.; Takaki, Y.; Hagiwara, D.; Nunoura, T.; Urayama, S.I. Viral RNA genomes identified from marine macroalgae and a diatom. Microbes Environ. 2020, 35, ME20016. [Google Scholar] [CrossRef]
- Debat, H.J.; Grabiele, M.; Aguilera, P.M.; Bubillo, R.; Zapata, P.D.; Marti, D.A.; Ducasse, D.A. The complete genome of a putative endornavirus identified in yerba mate (Ilex paraguariensis St. Hil.). Virus Genes 2014, 49, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Picarelli, M.; Forgia, M.; Rivas, E.B.; Nerva, L.; Chiapello, M.; Turina, M.; Colariccio, A. Extreme diversity of mycoviruses present in isolates of Rhizoctonia solani AG2-2 LP from Zoysia japonica from Brazil. Front. Cell Infect. Microbiol. 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Kim, K.H.; Zhao, F.; Yoo, R.H.; Igori, D.; Lee, S.H.; Moon, J.S. Complete genome sequence of a novel endornavirus isolated from hot pepper. Arch. Virol. 2015, 160, 3153–3156. [Google Scholar] [CrossRef]
- Hao, F.; Zhou, Z.; Wu, M.; Li, G. Molecular characterization of a novel endornavirus from the phytopathogenic fungus Botrytis cinerea. Arch. Virol. 2017, 162, 313–316. [Google Scholar] [CrossRef]
- Escalante, C.; Valverde, R.A. Morphological and physiological characteristics of endornavirus-infected and endornavirus-free near-isogenic lines of bell pepper (Capsicum annuum). Sci. Hortic-Amst. 2019, 250, 104–112. [Google Scholar] [CrossRef]
- Herschlag, R.; Okada, R.; Alcala-Briseno, R.I.; de Souto, E.R.; Valverde, R.A. Identification of a novel endornavirus in Geranium carolinianum and occurrence within three agroecosystems. Virus Res. 2020, 288, 198116. [Google Scholar] [CrossRef]
- Otulak-Koziel, K.; Koziel, E.; Escalante, C.; Valverde, R.A. Ultrastructural analysis of cells from Bell pepper (Capsicum annuum) infected with Bell pepper endornavirus. Front. Plant Sci. 2020, 11, 491. [Google Scholar] [CrossRef]
- Uchida, K.; Sakuta, K.; Ito, A.; Takahashi, Y.; Katayama, Y.; Omatsu, T.; Mizutani, T.; Arie, T.; Komatsu, K.; Fukuhara, T.; et al. Two novel endornaviruses co-infecting a phytophthora pathogen of asparagus officinalis modulate the developmental stages and fungicide sensitivities of the host oomycete. Front. Microbiol. 2021, 12, 633502. [Google Scholar] [CrossRef]

| Virus Name | Abbreviation | GenBank Accession No | Genome Length (nt) | Host | Sequence Identity (%) | |
|---|---|---|---|---|---|---|
| Polyprotein | RdRp | |||||
| Botrytis cinerea endornavirus 3 | BcEV3 | MN839443 | 13,582 | F | 44.66 | 68.94 |
| Botrytis cinerea endornavirus 2 | BcEV2 | MN617758 | 13,581 | F | 44.75 | 68.51 |
| Sclerotinia sclerotiorum endornavirus 9 | SsEV9 | MT646421 | 13,562 | F | 44.76 | 69.36 |
| Sclerotinia minor endornavirus 1 | SmEV1 | NC_040631 | 12,626 | F | 45.61 | 68.94 |
| Gremmeniella abietina type B RNA virus XL1 | GaBRV/XL1 | NC_007920 | 10,375 | F | 35.00 | 48.51 |
| Sclerotinia sclerotiorum endornavirus 1 | SsEV1/JZJL2 | NC_021706 | 10,770 | F | 29.00 | 45.53 |
| Botrytis cinerea endornavirus 1 | BcEV1 | NC_031752 | 11,557 | F | 29.08 | 43.40 |
| Tuber aestivum endornavirus | TaEV | NC_014904 | 9760 | F | 32.00 | 43.40 |
| Rosellinia necatrix endornavirus 1 | RnEV1 | NC_030938 | 9639 | F | 30.11 | 42.11 |
| Alternaria brassicicola endornavirus 1 | AbEV1 | NC_026136 | 10,290 | F | 27.59 | 32.91 |
| Grapevine endophyte endornavirus | GEEV | NC_019493 | 12,154 | P | 24.00 | 26.89 |
| Bell pepper endornavirus | BpEV | NC_015781 | 14,728 | P | 24.00 | 29.86 |
| Winged bean alphaendornavirus 1 | WbEV1 | NC_031336 | 14,623 | P | 23.39 | 25.45 |
| Rhizoctonia solani endornavirus—RS002 | RsEV/RS002 | KC792590 | 14,694 | F | 29.00 | 29.20 |
| Hot pepper endornavirus | HpEV | NC_027920 | 14,729 | P | 25.20 | 28.77 |
| Oryza sativa endornavirus | OsEV | D32136 | 13,952 | P | 25.00 | 28.70 |
| Oryza rufipogon endornavirus | OrEV | NC_007649 | 17,635 | P | 24.00 | 29.24 |
| Phaseolus vulgaris endornavirus 1 | PvEV1 | AB719397 | 13,908 | P | 24.00 | 23.85 |
| Phytophthora endornavirus 1 | PEV1 | AJ877914 | 13,883 | O | 26.68 | 27.44 |
| Virus Name | Host | Accession Number | Genome Length (nt) | Query Cover | Identity (%) | Positivity (%) |
|---|---|---|---|---|---|---|
| Varroa jacobsoni virus 4 | mite | QKW94174 | 8337 | 84% | 24% (51/216) | 47% (102/216) |
| Culex pipiens associated Tunisia virus | insects | AUT77208 | 6816 | 88% | 26% (55/215) | 44% (95/215) |
| Varroa destructor virus 4 | mite | QGA69815 | 8332 | 84% | 22% (45/204) | 47% (96/204) |
| Xinzhou nematode virus 1 | nematode | NC_033728 | 11,525 | 83% | 28% (57/207) | 47% (99/207) |
| Xingshan nematode virus 1 | nematode | NC_032483 | 11,374 | 76% | 29% (57/198) | 47% (94/198) |
| Hubei virga-like virus 15 | insects | NC_033211 | 10,423 | 76% | 28% (53/188) | 49% (93/188) |
| Hubei virga-like virus 17 | insects | NC_033222 | 9481 | 72% | 29% (53/180) | 45% (82/180) |
| Hubei virga-like virus 1 | insects | NC_033165 | 9141 | 66% | 26% (43/163) | 49% (81/163) |
| Ganwon-do negev-like virus 1 | mite | MT757507 | 6451 | 88% | 26% (58/221) | 43% (97/221) |
| Hubei endorna-like virus 1 | insects | NC_033204 | 13,693 | 88% | 27% (57/210) | 48% (102/210) |
| Shahe endorna-like virus 1 | insects | NC_032798 | 15,783 | 79% | 33% (64/192) | 53% (103/192) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Jiang, D.; Xie, J.; Jia, J.; Duan, J.; Cheng, J.; Fu, Y.; Chen, T.; Yu, X.; Li, B.; et al. Genome Characterization and Phylogenetic Analysis of a Novel Endornavirus That Infects Fungal Pathogen Sclerotinia sclerotiorum. Viruses 2022, 14, 456. https://doi.org/10.3390/v14030456
Luo X, Jiang D, Xie J, Jia J, Duan J, Cheng J, Fu Y, Chen T, Yu X, Li B, et al. Genome Characterization and Phylogenetic Analysis of a Novel Endornavirus That Infects Fungal Pathogen Sclerotinia sclerotiorum. Viruses. 2022; 14(3):456. https://doi.org/10.3390/v14030456
Chicago/Turabian StyleLuo, Xin, Daohong Jiang, Jiatao Xie, Jichun Jia, Jie Duan, Jiasen Cheng, Yanping Fu, Tao Chen, Xiao Yu, Bo Li, and et al. 2022. "Genome Characterization and Phylogenetic Analysis of a Novel Endornavirus That Infects Fungal Pathogen Sclerotinia sclerotiorum" Viruses 14, no. 3: 456. https://doi.org/10.3390/v14030456







