Establishment of a Direct PCR Assay for Simultaneous Differential Diagnosis of African Swine Fever and Classical Swine Fever Using Crude Tissue Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Clinical Samples of Field Cases
2.3. Clinical Samples of Experimentally Infected Animals
2.4. Preparation of Test Samples for Multiplex rRT-PCR
2.5. Multiplex rRT-PCR Amplification
2.6. Conventional Real-Time PCR (rPCR) and RT-PCR
3. Results
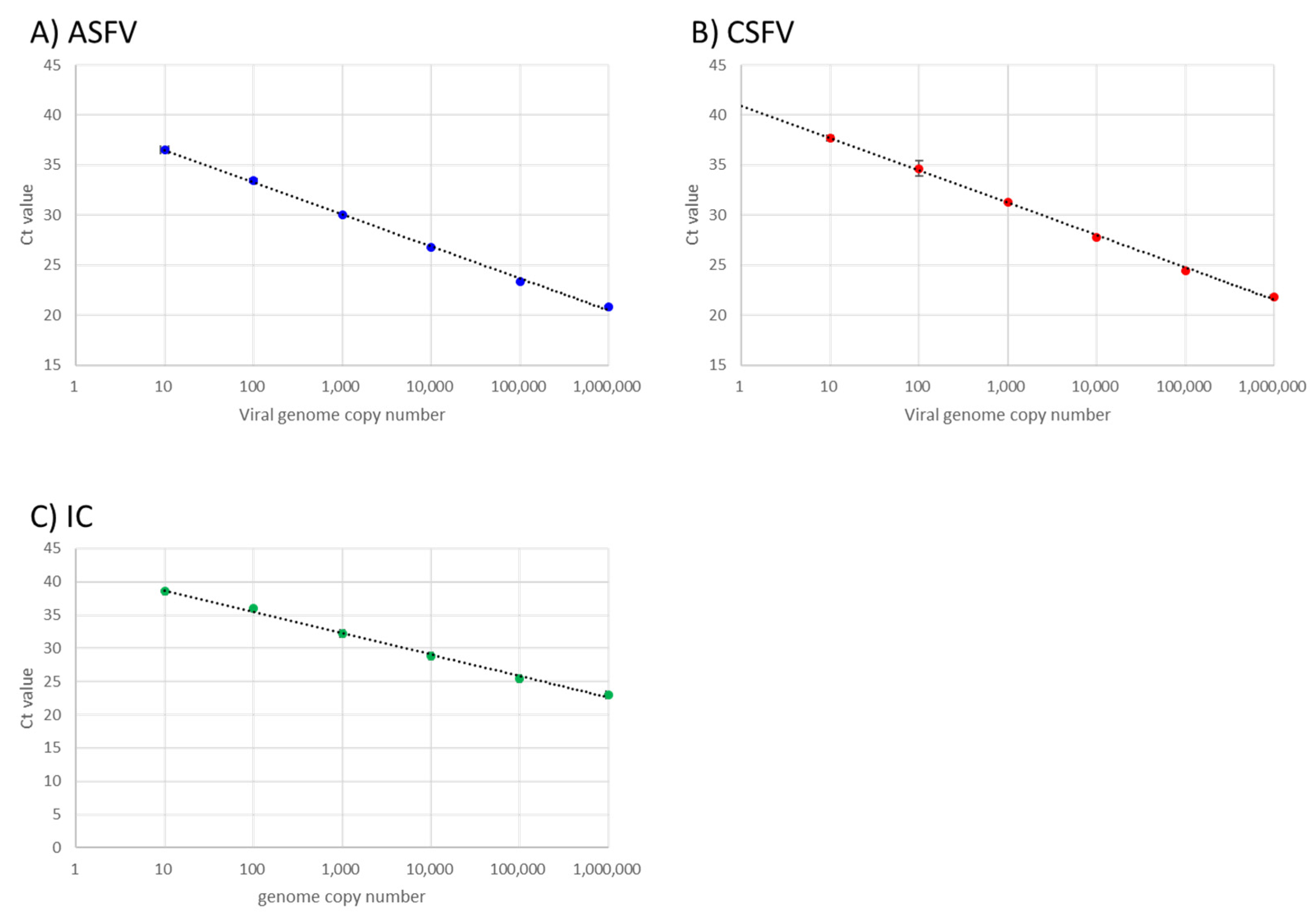
3.1. Analytical Sensitivity and Linearity of the Optimised Multiplex Real-Time RT-PCR Assay
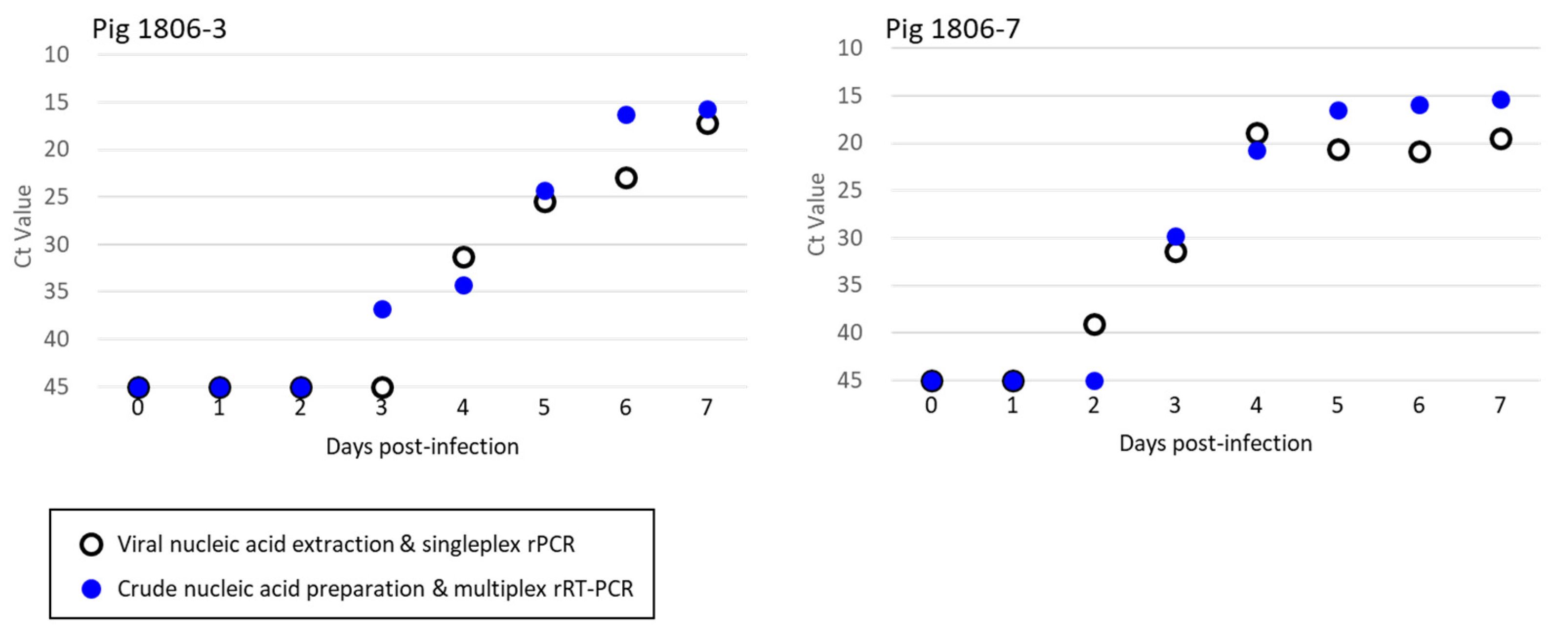
3.2. Analytical Sensitivities of ASFV and CSFV from Crude Nucleic Acid and Purified Nucleic Acid
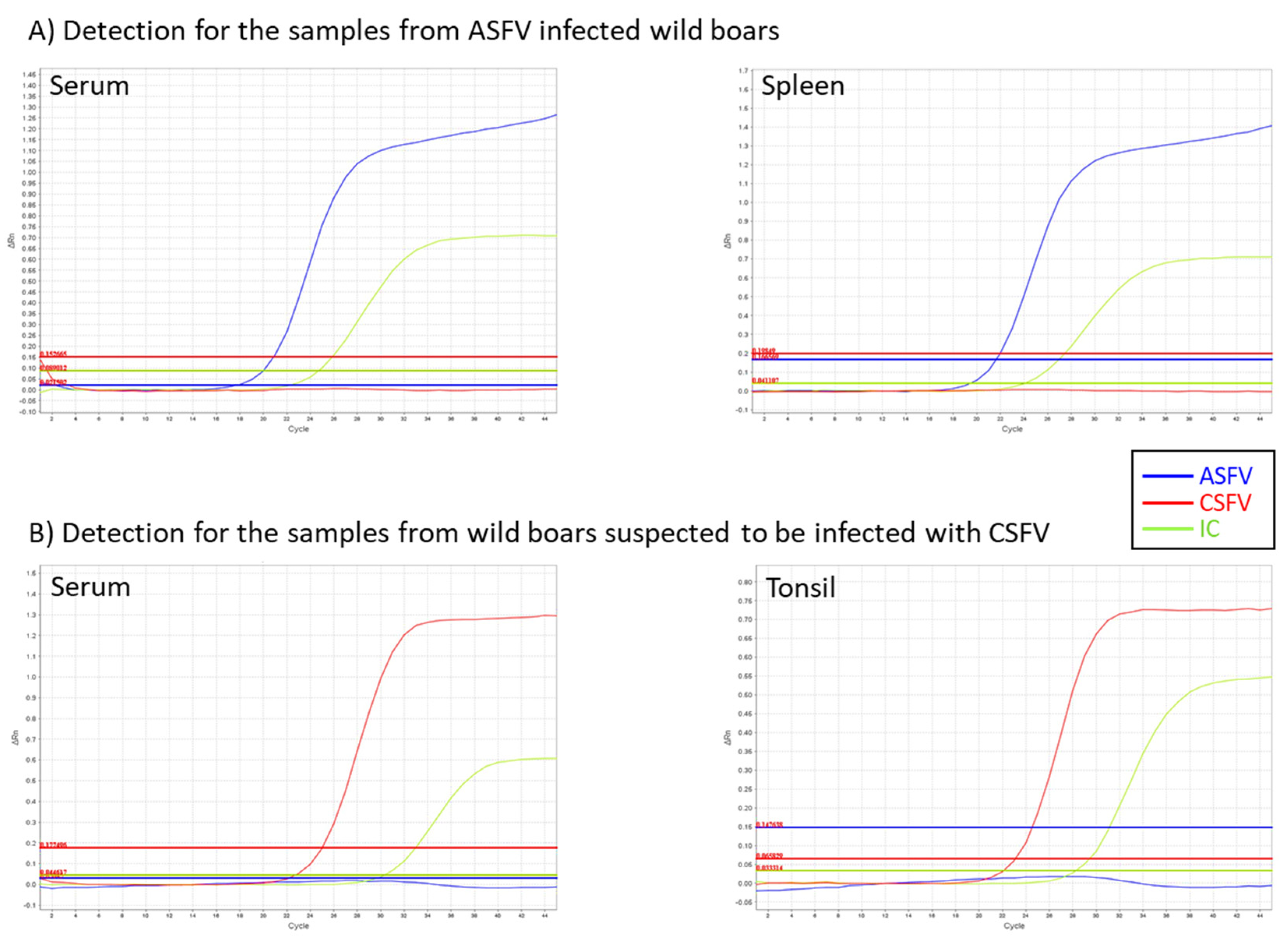
3.3. Detection of ASFV in the Specimens of Experimentally Infected Pigs and Wild Boar
3.4. Detection of CSFV in Experimentally Infected Pigs and Pig-Boar Hybrids
3.5. Validation Study Using Clinical Samples Obtained from Experimental and Field Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African Swine Fever in Domestic Pigs and European Wild Boar. Virus Res. 2013, 173, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Staubach, C.; Henke, J.; Carlson, J.; Beer, M. Classical Swine Fever-an Updated Review. Viruses 2017, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Gomez-Villamandos, J.C.; Carrasco, L. An Update on the Epidemiology and Pathology of African Swine Fever. J. Comp. Pathol. 2015, 152, 9–21. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [Green Version]
- Postel, A.; Nishi, T.; Kameyama, K.I.; Meyer, D.; Suckstorff, O.; Fukai, K.; Becher, P. Reemergence of Classical Swine Fever, Japan, 2018. Emerg. Infect. Dis. 2019, 25, 1228–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African Swine Fever Status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Sawai, K.; Nishi, T.; Fukai, K.; Kato, T.; Hayama, Y.; Yamamoto, T. Phylogenetic and Phylodynamic Analysis of a Classical Swine Fever Virus Outbreak in Japan (2018–2020). Transbound. Emerg. Dis. 2021; accepted. [Google Scholar] [CrossRef]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. Ictv Report Consortium. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- World Organization for Animal Health. African Swine Fever. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Chapter 3.8.1. 2019. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.08.01_ASF.pdf (accessed on 15 December 2021).
- Liu, J.; Fan, X.Z.; Wang, Q.; Xu, L.; Zhao, Q.Z.; Huang, W.; Zhou, Y.C.; Tang, B.; Chen, L.; Zou, X.Q.; et al. Dynamic Distribution and Tissue Tropism of Classical Swine Fever Virus in Experimentally Infected Pigs. Virol. J. 2011, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Masujin, K.; Kameyama, K.I.; Yamazoe, R.; Kubo, T.; Iwata, K.; Tamura, A.; Hibi, H.; Shiratori, T.; Koizumi, S.; et al. Experimental Infection of Pigs with Different Doses of the African Swine Fever Virus Armenia 07 Strain by Intramuscular Injection and Direct Contact. J. Vet. Med. Sci. 2021, 82, 1835–1845. [Google Scholar] [CrossRef]
- Fernández-Pinero, J.; Gallardo, C.; Elizalde, M.; Robles, A.; Gómez, C.; Bishop, R.; Heath, L.; Couacy-Hymann, E.; Fasina, F.O.; Pelayo, V.; et al. Molecular Diagnosis of African Swine Fever by a New Real-Time PCR Using Universal Probe Library. Transbound. Emerg. Dis. 2013, 60, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, E.; Huang, L.; Zhang, X.; Zhang, J.; Shen, D.; Zhang, Z.; Wang, Z.; Huo, H.; Wang, W.; Huangfu, H.; et al. Genotype I African Swine Fever Viruses Emerged in Domestic Pigs in China and Caused Chronic Infection. Emerg. Microbes Infect. 2021, 10, 2183–2193. [Google Scholar] [CrossRef] [PubMed]
- Duvigneau, J.C.; Hartl, R.T.; Groiss, S.; Gemeiner, M. Quantitative Simultaneous Multiplex Real-Time PCR for the Detection of Porcine Cytokines. J. Immunol. Methods 2005, 306, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Tautz, N.; Tews, B.A.; Meyers, G. The Molecular Biology of Pestiviruses. Adv. Virus Res. 2015, 93, 47–160. [Google Scholar] [CrossRef]
- Liess, B.; Moennig, V. Ruminant Pestivirus Infection in Pigs. Rev. Sci. Tech. 1990, 9, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Vilcek, S.; Herring, A.J.; Herring, J.A.; Nettleton, P.F.; Lowings, J.P.; Paton, D.J. Pestiviruses Isolated from Pigs, Cattle and Sheep Can Be Allocated Into at Least Three Genogroups Using Polymerase Chain Reaction and Restriction Endonuclease Analysis. Arch. Virol. 1994, 136, 309–323. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a Real-Time RT-PCR Assay for Sensitive and Specific Detection of Classical Swine Fever. J. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef]
- Komaniwa, H.; Fukusho, A.; Shimizu, Y. Micro method for performing titration and neutralization test of hog cholera virus using established porcine kidney cell strain. Natl. Inst. Anim. Health Q. 1981, 21, 153–158. [Google Scholar]
- Masujin, K.; Kitamura, T.; Kameyama, K.; Okadera, K.; Nishi, T.; Takenouchi, T.; Kitani, H.; Kokuho, T. An Immortalized Porcine Macrophage Cell Line Competent for the Isolation of African Swine Fever Virus. Sci. Rep. 2021, 11, 4759. [Google Scholar] [CrossRef]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a Taqman PCR Assay with Internal Amplification Control for the Detection of African Swine Fever Virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef]
- Fukai, K.; Nishi, T.; Yamada, M.; Ikezawa, M. Toward Better Control of Classical Swine Fever in Wild Boars: Susceptibility of Boar-Pig Hybrids to a Recent Japanese Isolate and Effectiveness of a Bait Vaccine. Vet. Res. 2020, 51, 96. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.I.; Nishi, T.; Yamada, M.; Masujin, K.; Morioka, K.; Kokuho, T.; Fukai, K. Experimental Infection of Pigs with a Classical Swine Fever Virus Isolated in Japan for the First Time in 26 Years. J. Vet. Med. Sci. 2019, 81, 1277–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Multiplex rRT-PCR * | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Inoculated | Date of Sampling | Singleplex | CSF | ASF | IC | ||||
| Animal | ID | Virus Strain | Sample | rPCR | Cy5 | FAM | VIC | Decision | |
| Pig | 1806-3 | Armenia/07 | 0 dpi | serum | − | − | − | 32.2 | negative |
| 1 dpi | serum | − | − | − | 33.7 | negative | |||
| 2 dpi | serum | − | − | − | 34.0 | negative | |||
| 3 dpi | serum | − | − | 36.7 | 33.3 | ASFV | |||
| 4 dpi | serum | 31.3 | − | 34.3 | 31.3 | ASFV | |||
| 5 dpi | serum | 25.4 | − | 24.3 | 32.6 | ASFV | |||
| 6 dpi | serum | 23.0 | − | 16.3 | 23.4 | ASFV | |||
| 7 dpi | serum | 17.2 | − | 15.7 | 23.7 | ASFV | |||
| Pig | 1806-7 | Armenia/07 | 0 dpi | serum | − | − | − | 34.7 | negative |
| 1 dpi | serum | − | − | − | 33.5 | negative | |||
| 2 dpi | serum | 39.0 | − | − | 34.3 | negative | |||
| 3 dpi | serum | 31.4 | − | 29.8 | 34.2 | ASFV | |||
| 4 dpi | serum | 18.9 | − | 20.8 | 28.8 | ASFV | |||
| 5 dpi | serum | 20.6 | − | 16.5 | 24.0 | ASFV | |||
| 6 dpi | serum | 20.8 | − | 15.9 | 24.0 | ASFV | |||
| 7 dpi | serum | 19.5 | − | 15.4 | 24.2 | ASFV | |||
| WB | 2002-1 | Armenia/07 | 0 dpi | serum | − | − | − | 34.8 | negative |
| 2 dpi | serum | 23.7 | − | 28.1 | 32.4 | ASFV | |||
| WB | 2002-2 | Armenia/07 | 0 dpi | serum | − | − | − | 35.6 | negative |
| 2 dpi | serum | 31.2 | − | 35.9 | 35.1 | ASFV | |||
| 4 dpi | serum | 17.9 | − | 21.0 | 24.3 | ASFV | |||
| WB | 2002-3 | Armenia/07 | 0 dpc | serum | − | − | − | 34.4 | negative |
| 7 dpc | serum | 31.2 | − | 38.0 | 35.0 | ASFV | |||
| 9 dpc | serum | 17.8 | − | 20.1 | 24.0 | ASFV | |||
| 13 dpc | serum | 20.8 | − | 22.3 | 27.9 | ASFV | |||
| WB | 2002-4 | Armenia/07 | 0 dpc | serum | − | − | − | 36.3 | negative |
| 7 dpc | serum | − | − | − | 35.4 | negative | |||
| 9 dpc | serum | − | − | − | 34.1 | negative | |||
| 11 dpc | serum | − | − | − | 34.2 | negative | |||
| 14 dpc | serum | 21.9 | − | 26.3 | 30.4 | ASFV | |||
| WB | 2002-5 | Armenia/07 | 0 dpc | serum | − | − | − | 35.6 | negative |
| 7 dpc | serum | − | − | − | 34.9 | negative | |||
| 9 dpc | serum | 25.6 | − | 28.9 | 32.6 | ASFV | |||
| Pig | 1705-1 | Armenia/07 | 5 dpi | spleen | 23.1 | − | 23.4 | 25.7 | ASFV |
| Pig | 1705-2 | Armenia/07 | 6 dpi | spleen | 23.3 | − | 23.7 | 25.2 | ASFV |
| Pig | 1705-3 | Kenya/05 | 6 dpi | spleen | 24.0 | − | 23.9 | 25.2 | ASFV |
| Pig | 1705-4 | Kenya/05 | 6 dpi | spleen | 24.2 | − | 24.8 | 24.3 | ASFV |
| Pig | 1705-5 | Espana/75 | 6 dpi | spleen | 22.6 | − | 22.0 | 24.4 | ASFV |
| Pig | 1705-6 | Espana/75 | 5 dpi | spleen | 23.8 | − | 21.7 | 24.1 | ASFV |
| Pig | 2105-9 | AQS-C-1-22 | 8 dpi | tonsil | 27.7 | − | 29.5 | 25.9 | ASFV |
| spleen | 21.6 | − | 24.9 | 24.8 | ASFV | ||||
| kidney | 27.4 | − | 27.6 | 19.1 | ASFV | ||||
| mesenteric LN | 27.2 | − | 29.0 | 22.4 | ASFV | ||||
| Pig | 2105-10 | AQS-C-1-22 | 9 dpi | tonsil | 16.9 | − | 22.7 | 24.0 | ASFV |
| spleen | 19.5 | − | 23.0 | 25.5 | ASFV | ||||
| kidney | 22.9 | − | 25.9 | 23.4 | ASFV | ||||
| mesenteric LN | 20.9 | − | 25.8 | 25.1 | ASFV | ||||
| WB | 2101-1 | Armenia/07 | 9 dpi | tonsil | 21.2 | − | 22.1 | 25.6 | ASFV |
| spleen | 21.4 | − | 22.3 | 27.5 | ASFV | ||||
| kidney | 25.8 | − | 25.5 | 28.0 | ASFV | ||||
| WB | 2101-2 | Armenia/07 | 13 dpi | tonsil | 20.5 | − | 22.9 | 26.6 | ASFV |
| spleen | 17.0 | − | 20.4 | 26.5 | ASFV | ||||
| kidney | 24.3 | − | 24.8 | 26.4 | ASFV | ||||
| WB | 2101-3 | Armenia/07 | 7 dpi | tonsil | 21.4 | − | 21.8 | 24.4 | ASFV |
| spleen | 19.9 | − | 20.8 | 26.4 | ASFV | ||||
| kidney | 22.2 | − | 24.6 | 25.9 | ASFV | ||||
| Multiplex rRT-PCR b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Inoculated | Date of Sampling | RT-PCR a (5′-UTR) | CSF | ASF | IC | |||
| Animal | ID | Virus Strain | Cy5 | FAM | VIC | Decision | ||
| Pig | 1810-1 | JPN/1/2018 | 0 dpi | − | − | − | 35.1 | negative |
| 1 dpi | − | − | − | 34.8 | negative | |||
| 2 dpi | − | − | − | 34.7 | negative | |||
| 3 dpi | − | − | − | 35.9 | negative | |||
| 4 dpi | + | 36.9 | − | 35.8 | CSFV | |||
| 5 dpi | + | 32.4 | − | 35.3 | CSFV | |||
| 6 dpi | + | 29.3 | − | 35.1 | CSFV | |||
| 7 dpi | + | 26.3 | − | 35.1 | CSFV | |||
| Pig | 1810-2 | JPN/1/2018 | 0 dpi | − | − | − | 34.9 | negative |
| 1 dpi | − | − | − | 35.8 | negative | |||
| 2 dpi | − | − | − | 33.4 | negative | |||
| 3 dpi | − | 36.8 | − | 36.0 | CSFV | |||
| 4 dpi | + | 34.9 | − | 35.8 | CSFV | |||
| 5 dpi | + | 32.3 | − | 35.1 | CSFV | |||
| 6 dpi | + | 29.6 | − | 35.1 | CSFV | |||
| 7 dpi | + | 27.3 | − | 35.0 | CSFV | |||
| pig-boar hybrid | 1905-4 | JPN/27/2019 | 0 dpi | − | − | − | 35.6 | negative |
| 1 dpi | − | − | − | 35.7 | negative | |||
| 2 dpi | − | − | − | 36.1 | negative | |||
| 3 dpi | − | − | − | 34.1 | negative | |||
| 4 dpi | − | 33.9 | − | 38.3 | CSFV | |||
| 5 dpi | + | 31.7 | − | 35.6 | CSFV | |||
| 6 dpi | + | 29.0 | − | 34.5 | CSFV | |||
| 7 dpi | + | 26.7 | − | 36.0 | CSFV | |||
| 8 dpi | + | 24.2 | − | 37.2 | CSFV | |||
| 10 dpi | + | 22.9 | − | 34.7 | CSFV | |||
| 14 dpi | + | 21.1 | − | 37.3 | CSFV | |||
| 28 dpi | + | 27.8 | − | 37.2 | CSFV | |||
| pig-boar hybrid | 1905-5 | JPN/27/2019 | 0 dpi | − | − | − | 33.9 | negative |
| 1 dpi | − | − | − | 35.6 | negative | |||
| 2 dpi | − | − | − | 35.3 | negative | |||
| 3 dpi | − | 37.4 | − | 36.0 | CSFV | |||
| 4 dpi | − | 35.2 | − | 35.8 | CSFV | |||
| 5 dpi | + | 32.6 | − | 34.3 | CSFV | |||
| 6 dpi | + | 29.2 | − | 36.3 | CSFV | |||
| 7 dpi | + | 26.2 | − | 37.5 | CSFV | |||
| 14 dpi | + | 24.7 | − | 36.9 | CSFV | |||
| Crude NA Preparation & Multiplex rRT-PCR | |||||||||
| Serum | ASFV Positive | ASFV Negative | |||||||
| WB | Pig | WB | Pig | Sensitivity | Specificity | ||||
| Viral NA extraction & ASFV rPCR | Positive | 8 | 9 | 0 | 1 | 94.4% | (17/18) | 97.1% | (34/35) |
| Negative | 0 | 1 | 29 | 5 | |||||
| CSFV Positive | CSFV Negative | ||||||||
| WB | Pig | WB | Pig | Sensitivity | Specificity | ||||
| Viral NA extraction & pestivirus RT-PCR | Positive | 36 | 31 | 4 | 0 | 94.4% | (67/71) | 91.9% | (34/37) |
| Negative | 2 | 1 | 27 | 7 | |||||
| Crude NA preparation & multiplex rRT-PCR | |||||||||
| Tissue homogenate | ASFV Positive | ASFV Negative | |||||||
| WB | Pig | WB | Pig | Sensitivity | Specificity | ||||
| Viral NA extraction & ASFV rPCR | Positive | 9 | 14 | 0 | 0 | 100% | (23/23) | 100% | (12/12) |
| Negative | 0 | 0 | 0 | 12 | |||||
| CSFV Positive | CSFV Negative | ||||||||
| WB | Pig | WB | Pig | Sensitivity | Specificity | ||||
| Viral NA extraction & pestivirus RT-PCR | Positive | 14 | 10 | 0 | 0 | 100% | (24/24) | 100% | (12/12) |
| Negative | 0 | 0 | 0 | 12 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishi, T.; Okadera, K.; Fukai, K.; Yoshizaki, M.; Nakasuji, A.; Yoneyama, S.; Kokuho, T. Establishment of a Direct PCR Assay for Simultaneous Differential Diagnosis of African Swine Fever and Classical Swine Fever Using Crude Tissue Samples. Viruses 2022, 14, 498. https://doi.org/10.3390/v14030498
Nishi T, Okadera K, Fukai K, Yoshizaki M, Nakasuji A, Yoneyama S, Kokuho T. Establishment of a Direct PCR Assay for Simultaneous Differential Diagnosis of African Swine Fever and Classical Swine Fever Using Crude Tissue Samples. Viruses. 2022; 14(3):498. https://doi.org/10.3390/v14030498
Chicago/Turabian StyleNishi, Tatsuya, Kota Okadera, Katsuhiko Fukai, Miwa Yoshizaki, Ai Nakasuji, Syuji Yoneyama, and Takehiro Kokuho. 2022. "Establishment of a Direct PCR Assay for Simultaneous Differential Diagnosis of African Swine Fever and Classical Swine Fever Using Crude Tissue Samples" Viruses 14, no. 3: 498. https://doi.org/10.3390/v14030498





