Persistence of Naturally Acquired and Functional SARS-CoV-2 Antibodies in Blood Donors One Year after Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
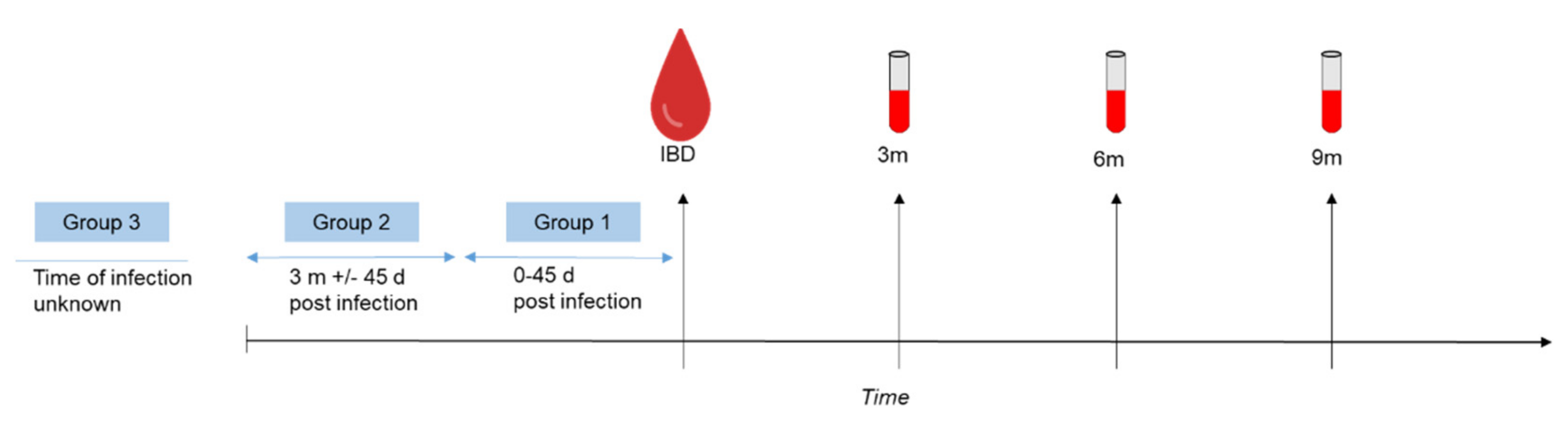
2.2. Sample Collection and Study Design
2.3. Questionnaires
2.4. Serological Testing
2.5. Data Collection and Statistical Analysis
3. Results
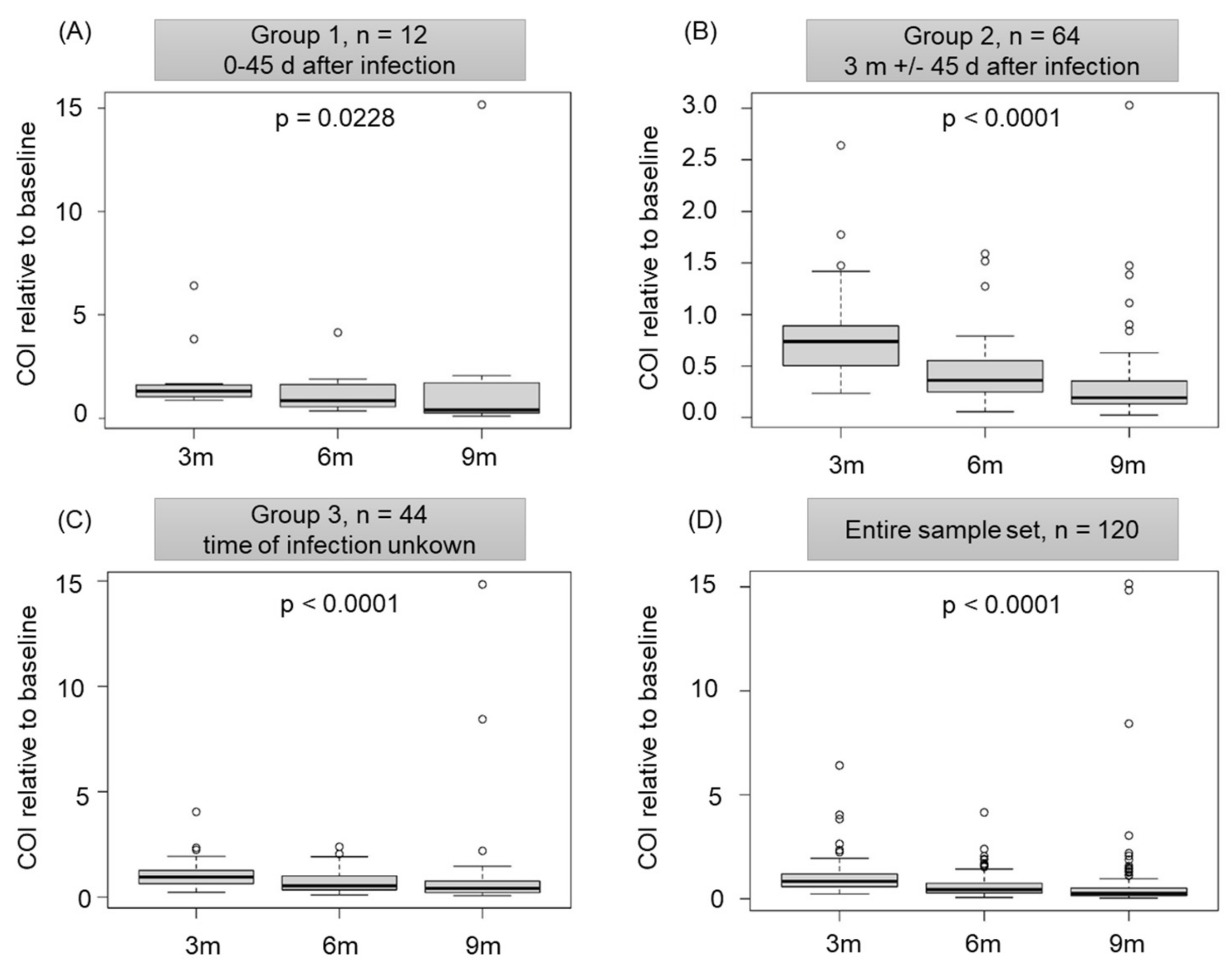
3.1. Infection-Acquired Natural SARS-CoV-2 Antibodies Are Detectable and Functional In Vitro for at Least One Year
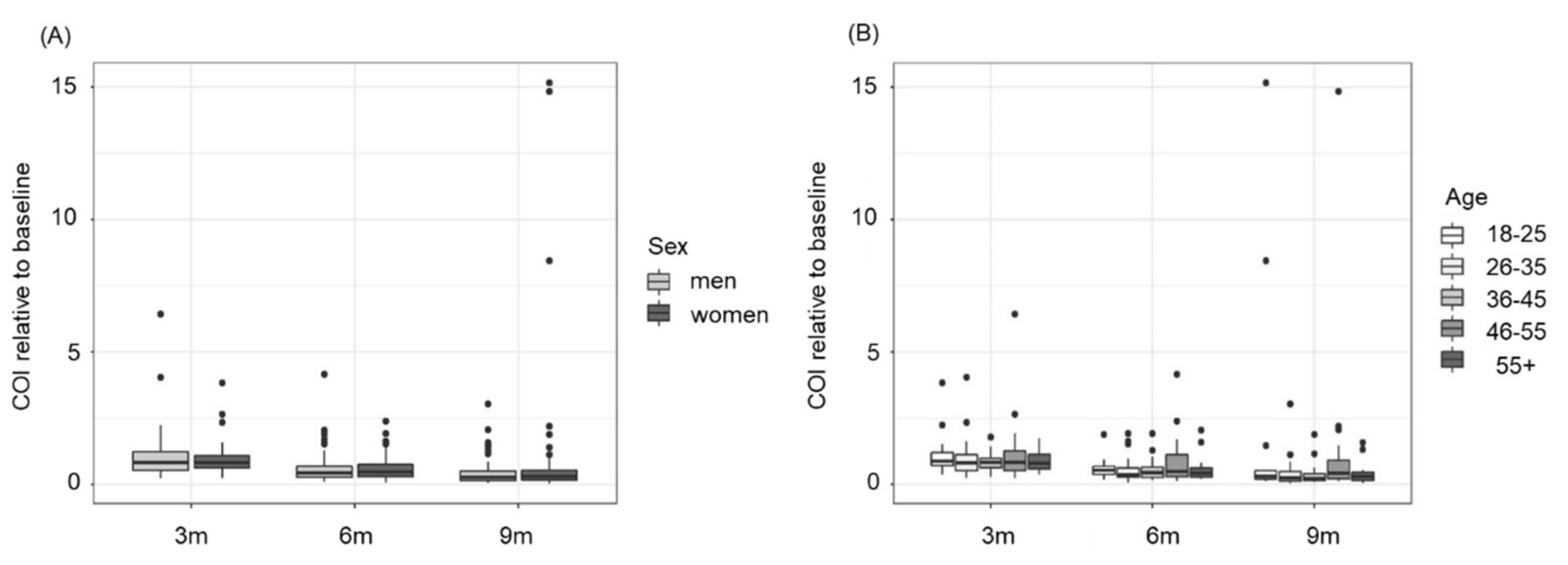
3.2. The Decline in Anti-N Antibodies Is Independent of Sex and Age
3.3. Blood Group AB Shows Significantly Lower Levels and In Vitro Functionality of SARS-CoV-2 Antibodies
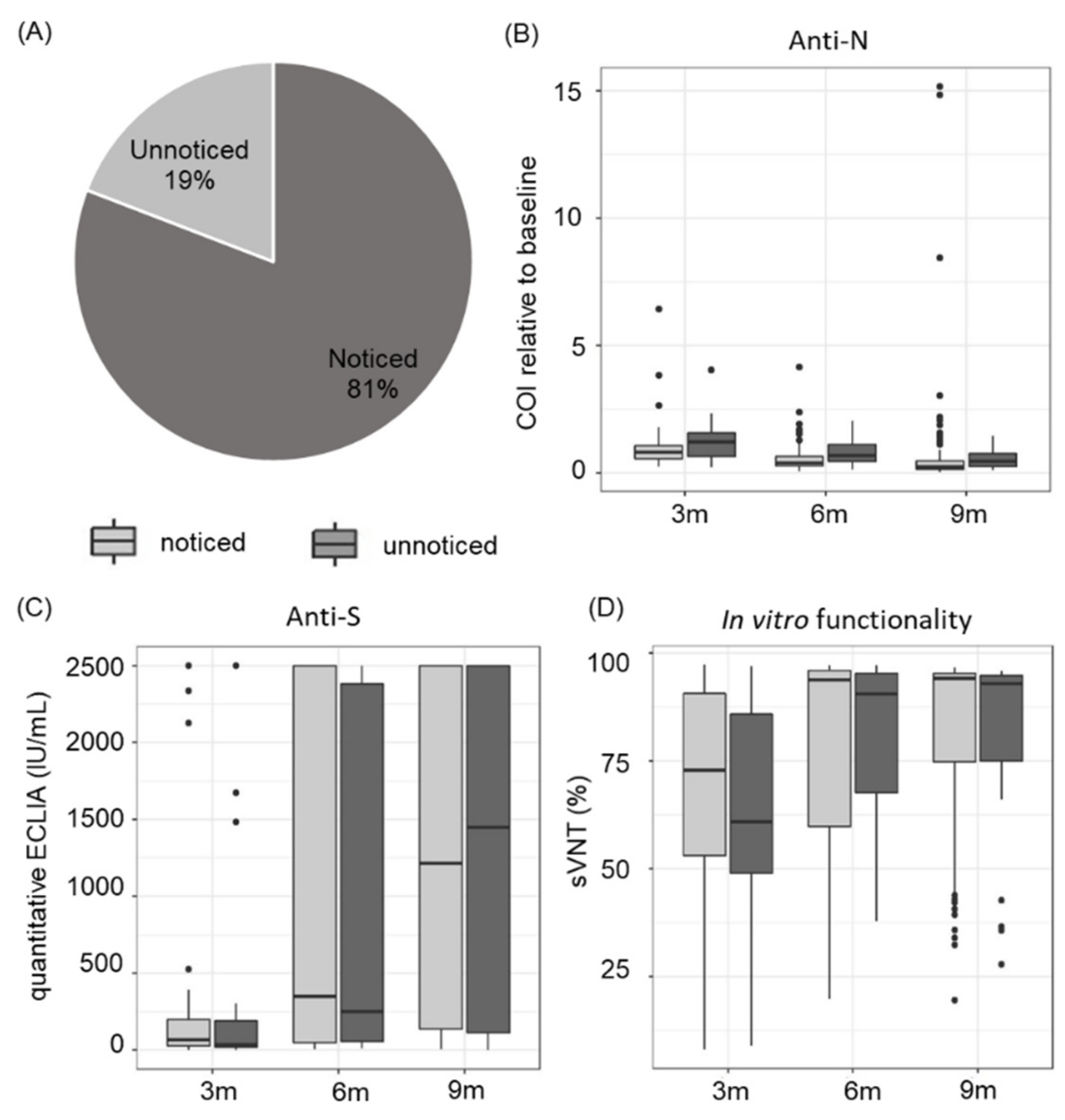
3.4. Asymptomatic and Symptomatic COVID-19 Disease Course Leads to a Similar Antibody Response
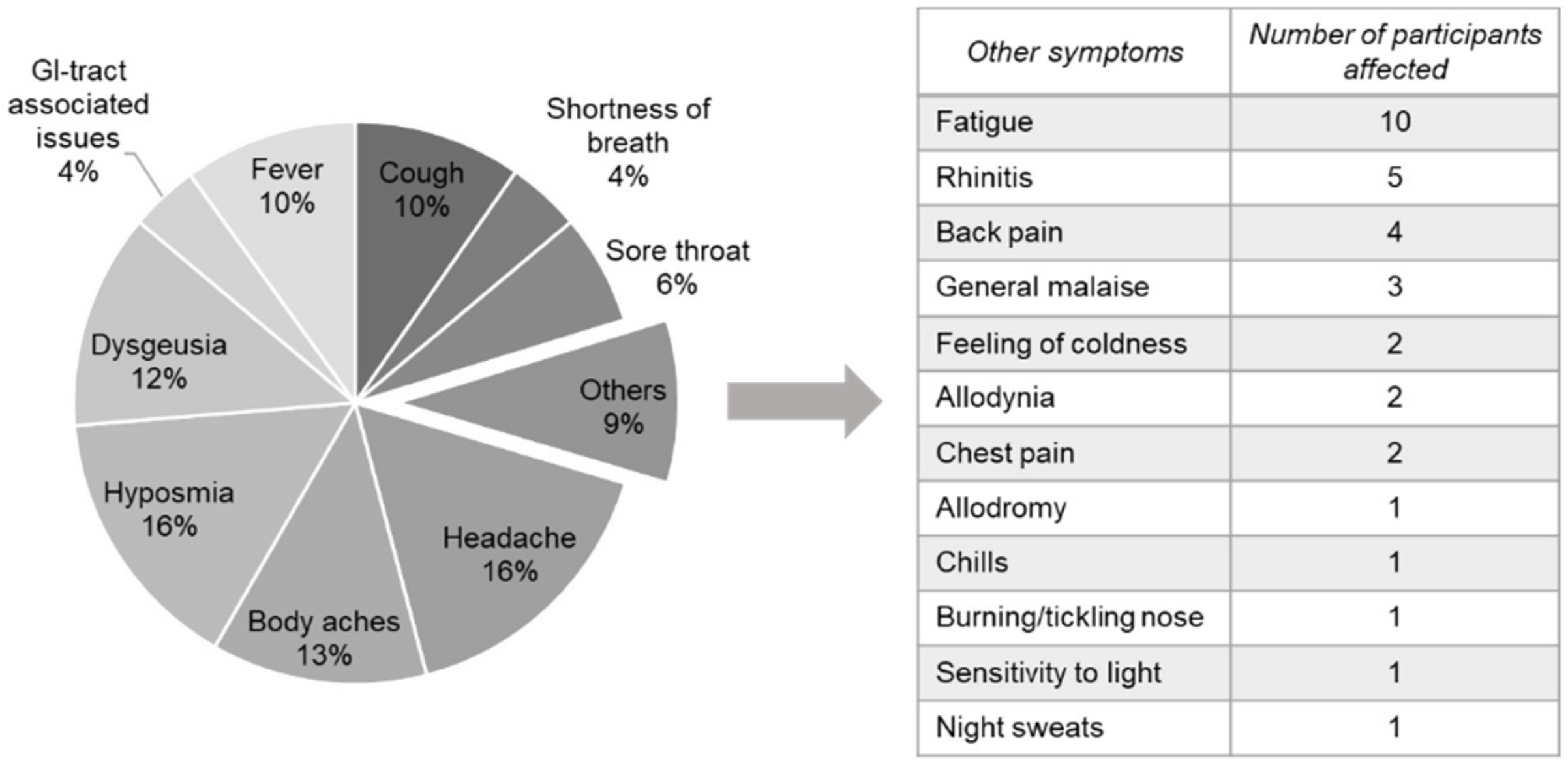
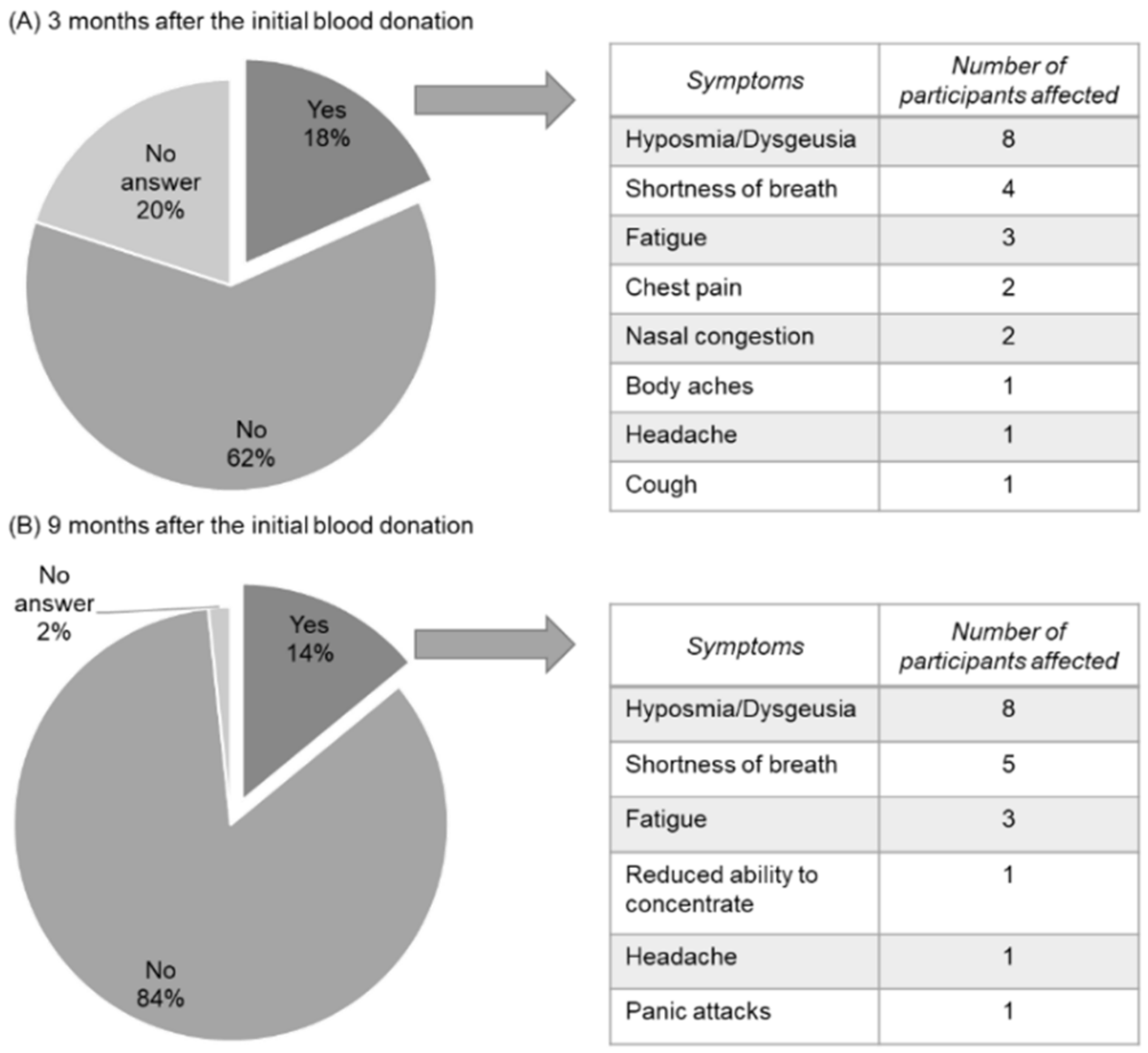
3.5. COVID-Related Symptoms Last up to 9 Months in Healthy Blood Donors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-COVID-19---11-march-2020 (accessed on 2 March 2022).
- Ramesh, S.; Govindarajulu, M.; Parise, R.S.; Neel, L.; Shankar, T.; Patel, S.; Lowery, P.; Smith, F.; Dhanasekaran, M.; Moore, T. Emerging SARS-CoV-2 variants: A review of its mutations, its implications and vaccine efficacy. Vaccines 2021, 9, 1195. [Google Scholar] [CrossRef]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- WHO. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021. Available online: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-SARS-CoV-2-variant-of-concern (accessed on 2 March 2022).
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. Neuroimmune Pharm. 2020, 15, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Bchetnia, M.; Girard, C.; Duchaine, C.; Laprise, C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): A review of the current global status. J. Infect. Public Health 2020, 13, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’Em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLOS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Dong, Y.; Dai, T.; Wang, B.; Zhang, L.; Zeng, L.H.; Huang, J.; Yan, H.; Zhang, L.; Zhou, F. The way of SARS-CoV-2 vaccine development: Success and challenges. Signal Transduct. Target. Ther. 2021, 6, 387. [Google Scholar] [CrossRef]
- Ling, Y.; Zhong, J.; Luo, J. Safety and effectiveness of SARS-CoV-2 vaccines: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 6486–6495. [Google Scholar] [CrossRef]
- Lipsitch, M.; Krammer, F.; Regev-Yochay, G.; Lustig, Y.; Balicer, R.D. SARS-CoV-2 breakthrough infections in vaccinated individuals: Measurement, causes and impact. Nat. Rev. Immunol. 2022, 22, 57–65. [Google Scholar] [CrossRef]
- Mao, Y.; Wang, W.; Ma, J.; Wu, S.; Sun, F. Reinfection rates among patients previously infected by SARS-CoV-2: Systematic review and meta-analysis. Chin. Med. J. 2021, 135, 145–152. [Google Scholar] [CrossRef]
- Jeffery-Smith, A.; Rowland, T.A.J.; Patel, M.; Whitaker, H.; Iyanger, N.; Williams, S.V.; Giddings, R.; Thompson, L.; Zavala, M.; Aiano, F.; et al. Reinfection with new variants of SARS-CoV-2 after natural infection: A prospective observational cohort in 13 care homes in England. Lancet Healthy Longev. 2021, 2, e811–e819. [Google Scholar] [CrossRef]
- Dhillon, R.A.; Qamar, M.A.; Gilani, J.A.; Irfan, O.; Waqar, U.; Sajid, M.I.; Mahmood, S.F. The mystery of COVID-19 reinfections: A global systematic review and meta-analysis. Ann. Med. Surg. 2021, 72, 103130. [Google Scholar] [CrossRef]
- Murchu, E.O.; Byrne, P.; Walsh, K.A.; Carty, P.G.; Connolly, M.; De Gascun, C.; Jordan, K.; Keoghan, M.; O’Brien, K.K.; O’Neill, M.; et al. Immune response following infection with SARS-CoV-2 and other coronaviruses: A rapid review. Rev. Med. Virol. 2020, 31, e2162. [Google Scholar] [CrossRef]
- Siracusano, G.; Brombin, C.; Pastori, C.; Cugnata, F.; Noviello, M.; Tassi, E.; Princi, D.; Cantoni, D.; Malnati, M.S.; Maugeri, N.; et al. Profiling antibody response patterns in COVID-19: Spike S1-reactive IgA signature in the evolution of SARS-CoV-2 infection. Front. Immunol. 2021, 12, 772239. [Google Scholar] [CrossRef]
- Petersen, M.S.; Hansen, C.B.; Kristiansen, M.F.; Fjallsbak, J.P.; Larsen, S.; Hansen, J.L.; Jarlhelt, I.; Perez-Alos, L.; Steig, B.A.; Christiansen, D.H.; et al. SARS-CoV-2 natural antibody response persists for at least 12 months in a nationwide study from the Faroe Islands. Open Forum Infect. Dis. 2021, 8, ofab378. [Google Scholar] [CrossRef]
- Wei, J.; Matthews, P.C.; Stoesser, N.; Maddox, T.; Lorenzi, L.; Studley, R.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat. Commun. 2021, 12, 6250. [Google Scholar] [CrossRef]
- Weidner, L.; Nunhofer, V.; Jungbauer, C.; Hoeggerl, A.D.; Gruner, L.; Grabmer, C.; Zimmermann, G.; Rohde, E.; Laner-Plamberger, S. Seroprevalence of anti-SARS-CoV-2 total antibody is higher in younger Austrian blood donors. Infection 2021, 49, 1187–1194. [Google Scholar] [CrossRef]
- Weidner, L.; Gansdorfer, S.; Unterweger, S.; Weseslindtner, L.; Drexler, C.; Farcet, M.; Witt, V.; Schistal, E.; Schlenke, P.; Kreil, T.R.; et al. Quantification of SARS-CoV-2 antibodies with eight commercially available immunoassays. J. Clin. Virol. 2020, 129, 104540. [Google Scholar] [CrossRef]
- Noguchi, K.; Gel, Y.R.; Brunner, E.; Konietschke, F. nparLD: An R software package for the nonparametric analysis of longitudinal data in factorial experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef] [Green Version]
- R-Core-Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Vaselli, N.M.; Hungerford, D.; Shenton, B.; Khashkhusha, A.; Cunliffe, N.A.; French, N. The seroprevalence of SARS-CoV-2 during the first wave in Europe 2020: A systematic review. PLoS ONE 2021, 16, e0250541. [Google Scholar] [CrossRef]
- Jones, J.M.; Stone, M.; Sulaeman, H.; Fink, R.V.; Dave, H.; Levy, M.E.; Di Germanio, C.; Green, V.; Notari, E.; Saa, P.; et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020–May 2021. JAMA 2021, 326, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Raouf, M.; Rabeh, M.; Kaur, S.; Sharma, R.; Thottumkal, N.; Mohammed, R. Seroprevalence of IgG anti-SARS-CoV-2 among voluntary blood donors in Dubai: Demographic and risk factors. Dubai Med. J. 2021, 4, 204–211. [Google Scholar] [CrossRef]
- Shrotri, M.; Harris, R.J.; Rodger, A.; Planche, T.; Sanderson, F.; Mahungu, T.; McGregor, A.; Heath, P.T.; London, C.G.; Brown, C.S.; et al. Persistence of SARS-CoV-2 N-antibody response in healthcare workers, London, UK. Emerg. Infect. Dis. 2021, 27, 1155–1158. [Google Scholar] [CrossRef]
- Krutikov, M.; Palmer, T.; Tut, G.; Fuller, C.; Azmi, B.; Giddings, R.; Shrotri, M.; Kaur, N.; Sylla, P.; Lancaster, T.; et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in England. Lancet Healthy Longev. 2022, 3, e13–e21. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Yorsaeng, R.; Posuwan, N.; Puenpa, J.; Wanlapakorn, N.; Sudhinaraset, N.; Sripramote, M.; Chalongviriyalert, P.; Jirajariyavej, S.; Kiatpanabhikul, P.; et al. Long-term specific IgG response to SARS-CoV-2 nucleocapsid protein in recovered COVID-19 patients. Sci. Rep. 2021, 11, 23216. [Google Scholar] [CrossRef]
- Lai, C.C.; Wang, J.H.; Hsueh, P.R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. Int. J. Infect. Dis. 2020, 101, 314–322. [Google Scholar] [CrossRef]
- Xiang, T.; Liang, B.; Fang, Y.; Lu, S.; Li, S.; Wang, H.; Li, H.; Yang, X.; Shen, S.; Zhu, B.; et al. Declining levels of neutralizing antibodies against SARS-CoV-2 in convalescent COVID-19 patients one year post symptom onset. Front. Immunol. 2021, 12, 708523. [Google Scholar] [CrossRef]
- Zietz, M.; Zucker, J.; Tatonetti, N.P. Associations between blood type and COVID-19 infection, intubation, and death. Nat. Commun. 2020, 11, 5761. [Google Scholar] [CrossRef]
- Hoiland, R.L.; Fergusson, N.A.; Mitra, A.R.; Griesdale, D.E.G.; Devine, D.V.; Stukas, S.; Cooper, J.; Thiara, S.; Foster, D.; Chen, L.Y.C.; et al. The association of ABO blood group with indices of disease severity and multiorgan dysfunction in COVID-19. Blood Adv. 2020, 4, 4981–4989. [Google Scholar] [CrossRef]
- Latz, C.A.; Decarlo, C.; Boitano, L.; Png, C.Y.M.; Patell, R.; Conrad, M.F.; Eagleton, M.; Dua, A. Blood type and outcomes in patients with COVID-19. Ann. Hematol. 2020, 99, 2113–2118. [Google Scholar] [CrossRef]
- Leaf, R.K.; Al-Samkari, H.; Brenner, S.K.; Gupta, S.; Leaf, D. ABO phenotype and death in critically ill patients with COVID-19. Br. J. Haematol. 2020, 190, e204–e208. [Google Scholar] [CrossRef]
- Ray, J.G.; Schull, M.J.; Vermeulen, M.J.; Park, A.L. Association between ABO and Rh blood groups and SARS-CoV-2 infection or severe COVID-19 illness: A population-based cohort study. Ann. Intern. Med. 2020, 174, 308–315. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Huang, H.; Li, D.; Gu, D.; Lu, X.; Zheng, Z.; Liu, L.; Liu, T.; Liu, Y.; et al. Relationship between the ABO blood group and the COVID-19 susceptibility. Clin. Infect. Dis. 2021, 73, 328–331. [Google Scholar] [CrossRef]
- Shokri, P.; Golmohammadi, S.; Noori, M.; Nejadghaderi, S.A.; Carson-Chahhoud, K.; Safiri, S. The relationship between blood groups and risk of infection with SARS-CoV-2 or development of severe outcomes: A review. Rev. Med. Virol. 2022, 32, e2247. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Chen, J.; Cai, Y.; Deng, A.; Yang, M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br. J. Haematol. 2020, 190, 24–27. [Google Scholar] [CrossRef]
- Boukhari, R.; Breiman, A.; Jazat, J.; Ruvoen-Clouet, N.; Martinez, S.; Damais-Cepitelli, A.; Le Niger, C.; Devie-Hubert, I.; Penasse, F.; Mauriere, D.; et al. ABO blood group incompatibility protects against SARS-CoV-2 transmission. Front. Microbiol. 2021, 12, 799519. [Google Scholar] [CrossRef]
- Dzik, S.; Eliason, K.; Morris, E.B.; Kaufman, R.M.; North, C.M. COVID-19 and ABO blood groups. Transfusion 2020, 60, 1883–1884. [Google Scholar] [CrossRef]
- Boudin, L.; Janvier, F.; Bylicki, O.; Dutasta, F. ABO blood groups are not associated with risk of acquiring the SARS-CoV-2 infection in young adults. Haematologica 2020, 105, 2841–2843. [Google Scholar] [CrossRef]
- Kim, Y.; Latz, C.A.; DeCarlo, C.S.; Lee, S.; Png, C.Y.M.; Kibrik, P.; Sung, E.; Alabi, O.; Dua, A. Relationship between blood type and outcomes following COVID-19 infection. Semin. Vasc. Surg. 2021, 34, 125–131. [Google Scholar] [CrossRef]
- Singh, P.P.; Srivastava, A.K.; Upadhyay, S.K.; Singh, A.; Upadhyay, S.; Kumar, P.; Rai, V.; Shrivastava, P.; Chaubey, G.; Serosurveillance Consortium BHU. The association of ABO blood group with the asymptomatic COVID-19 cases in India. Transfus. Apher. Sci. 2021, 60, 103224. [Google Scholar] [CrossRef]
- Guillon, P.; Clement, M.; Sebille, V.; Rivain, J.G.; Chou, C.F.; Ruvoen-Clouet, N.; Le Pendu, J. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology 2008, 18, 1085–1093. [Google Scholar] [CrossRef] [Green Version]
- Barnkob, M.B.; Pottegard, A.; Stovring, H.; Haunstrup, T.M.; Homburg, K.; Larsen, R.; Hansen, M.B.; Titlestad, K.; Aagaard, B.; Moller, B.K.; et al. Reduced prevalence of SARS-CoV-2 infection in ABO blood group O. Blood Adv. 2020, 4, 4990–4993. [Google Scholar] [CrossRef]
- Deleers, M.; Breiman, A.; Daubie, V.; Maggetto, C.; Barreau, I.; Besse, T.; Clémenceau, B.; Ruvoën-Clouet, N.; Fils, J.-F.; Maillart, E.; et al. COVID-19 and blood groups: ABO antibody levels may also matter. Int. J. Infect. Dis. 2021, 104, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.A.; Quandelacy, T.M.; Kada, S.; Prasad, P.V.; Steele, M.; Brooks, J.T.; Slayton, R.B.; Biggerstaff, M.; Butler, J.C. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw. Open 2021, 4, e2035057. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.M.; Cabeen, R.P.; Toga, A.W.; Clark, K.A. Magnitude and timing of major white matter tract maturation from infancy through adolescence with NODDI. NeuroImage 2020, 212, 116672. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.F.; O’Connell, S.E.; Armbrust, T.; Mergaert, A.M.; Narpala, S.R.; Halfmann, P.J.; Bashar, S.J.; Glover, C.R.; Heffron, A.S.; Taylor, A.; et al. Specific COVID-19 symptoms correlate with high antibody levels against SARS-CoV-2. Immunohorizons 2021, 5, 466–476. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Mehta, O.P.; Bhandari, P.; Raut, A.; Kacimi, S.E.O.; Huy, N.T. Coronavirus disease (COVID-19): Comprehensive review of clinical presentation. Front. Public Health 2020, 8, 582932. [Google Scholar] [CrossRef]
- Tsai, P.-H.; Lai, W.-Y.; Lin, Y.-Y.; Luo, Y.-H.; Chen, H.-K.; Chen, Y.-M.; Lai, Y.-C.; Kuo, L.-C.; Chen, S.-D.; Chang, K.-J.; et al. Clinical manifestation and disease progression in COVID-19 infection. J. Chin. Med. Assoc. 2021, 84, 3–8. [Google Scholar] [CrossRef]
- Gold, J.; Okyay, R.; Licht, W.; Hurley, D. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]


| Time (Months After Donation) | SARS-CoV-2 Anti-N Total Antibody Assay ECLIA (COI) | Anti-SARS-CoV-2 IgG ELISA (Ratio Value) | Anti-SARS-CoV-2 Quantitative ECLIA (IU/mL) | Anti-SARS-CoV-2 sVNT (Inhibition in %) |
|---|---|---|---|---|
| Assay directed against viral | ||||
| N-protein | S-protein | S-protein | S-protein | |
| UNVACCINATED (n = 46) | ||||
| 3 months | 26.2 (9.5–104.0) | 18.3 (15.1–20.6) | 35.6 (15.2–103.0) | 61.9 (49.2–79.6) |
| 6 months | 14.8 (5.3–39.1) | 17.1 (16.6–18.7) | 42.1 (21.7–118.0) | 59.8 (45.8–79.9) |
| 9 months | 12.0 (3.7–47.0) | 18.4 (17.4–21.0) | 69.0 (28.4–209.0) | 63.4 (42.7–81.4) |
| VACCINATED (n = 74) | ||||
| 3 months | 38.8 (13.0–99.3) | 17.2 (15.7–19.8) | 107.0 (37.2–1674.0) | 79.2 (58.4–95.5) |
| 6 months | 20.7 (6.2–47.7) | 17.7 (16.9–18.9) | ≥2500.0 * (698.0–≥2500.0 *) | 95.6 (93.8–96.2) |
| 9 months | 10.3 (3.8–39.1) | 18.4 (18.3–21.0) | ≥2500.0 * (1324.0–≥2500.0 *) | 94.7 (94.1–95.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunhofer, V.; Weidner, L.; Hoeggerl, A.D.; Zimmermann, G.; Badstuber, N.; Grabmer, C.; Jungbauer, C.; Lindlbauer, N.; Held, N.; Pascariuc, M.; et al. Persistence of Naturally Acquired and Functional SARS-CoV-2 Antibodies in Blood Donors One Year after Infection. Viruses 2022, 14, 637. https://doi.org/10.3390/v14030637
Nunhofer V, Weidner L, Hoeggerl AD, Zimmermann G, Badstuber N, Grabmer C, Jungbauer C, Lindlbauer N, Held N, Pascariuc M, et al. Persistence of Naturally Acquired and Functional SARS-CoV-2 Antibodies in Blood Donors One Year after Infection. Viruses. 2022; 14(3):637. https://doi.org/10.3390/v14030637
Chicago/Turabian StyleNunhofer, Verena, Lisa Weidner, Alexandra Domnica Hoeggerl, Georg Zimmermann, Natalie Badstuber, Christoph Grabmer, Christof Jungbauer, Nadja Lindlbauer, Nina Held, Monica Pascariuc, and et al. 2022. "Persistence of Naturally Acquired and Functional SARS-CoV-2 Antibodies in Blood Donors One Year after Infection" Viruses 14, no. 3: 637. https://doi.org/10.3390/v14030637
APA StyleNunhofer, V., Weidner, L., Hoeggerl, A. D., Zimmermann, G., Badstuber, N., Grabmer, C., Jungbauer, C., Lindlbauer, N., Held, N., Pascariuc, M., Ortner, T., Rohde, E., & Laner-Plamberger, S. (2022). Persistence of Naturally Acquired and Functional SARS-CoV-2 Antibodies in Blood Donors One Year after Infection. Viruses, 14(3), 637. https://doi.org/10.3390/v14030637






