Use of a Novel Detection Tool to Survey Orthohantaviruses in Wild-Caught Rodent Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primer Design
2.2. Plasmid Construction and Cultured Viruses
2.3. Trapping and Sample Collection
2.4. RNA Extraction
2.5. Reverse Transcriptase-Quantitative Polymerase Chain Reaction (RT-qPCR) and Nested PCR
2.6. DNA gel Electrophoresis
2.7. Sanger Sequencing
2.8. Software Programs
3. Results
3.1. Primer Design for Pan-Orthohantaviruses Detection
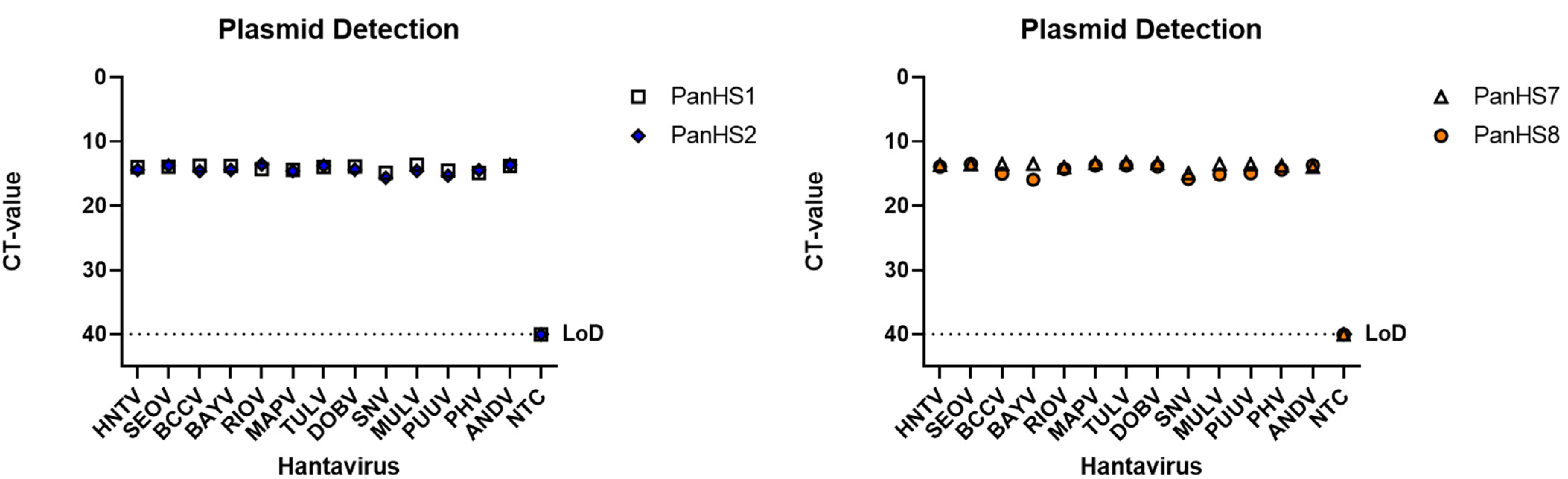
3.2. Validation of Pan-Orthohantavirus Primers for Detection of Both New and Old World Orthohantaviruses
3.3. Screening Wild-Caught Rodents Using Pan-Orthohantavirus Primers
3.4. Sequencing PCR Fragments of Positive Rodents to Confirm Orthohantavirus Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guo, W.-P.; Lin, X.-D.; Wang, W.; Tian, J.-H.; Cong, M.-L.; Zhang, H.-L.; Wang, M.-R.; Zhou, R.-H.; Wang, J.-B.; Li, M.-H.; et al. Phylogeny and Origins of Hantaviruses Harbored by Bats, Insectivores, and Rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, C.B.; Figueiredo, L.T.M.; Vapalahti, O. A Global Perspective on Hantavirus Ecology, Epidemiology, and Disease. Clin. Microbiol. Rev. 2010, 23, 412–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, E.; Dieterle, M.E.; Kleinfelter, L.M.; Slough, M.M.; Chandran, K.; Jangra, R.K. Hantavirus entry: Perspectives and recent advances. Adv. Appl. Microbiol. 2019, 104, 185–224. [Google Scholar] [CrossRef]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current Classification and Future Perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [Green Version]
- Arai, S.; Yanagihara, R. Genetic Diversity and Geographic Distribution of Bat-borne Hantaviruses. Curr. Issues Mol. Biol. 2020, 39, 1–28. [Google Scholar] [CrossRef]
- Yanagihara, R.; Gu, S.H.; Song, J.-W. Expanded Host Diversity and Global Distribution of Hantaviruses: Implications for Identifying and Investigating Previously Unrecognized Hantaviral Diseases. In Global Virology I-Identifying and Investigating Viral Diseases; Springer: New York, NY, USA, 2015; pp. 161–198. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the Etiologic Agent of Korean Hemorrhagic Fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef]
- Duchin, J.S.; Koster, F.T.; Peters, C.; Simpson, G.L.; Tempest, B.; Zaki, S.R.; Ksiazek, T.G.; Rollin, P.; Nichol, S.; Umland, E.T.; et al. Hantavirus Pulmonary Syndrome: A Clinical Description of 17 Patients with a Newly Recognized Disease. N. Engl. J. Med. 1994, 330, 949–955. [Google Scholar] [CrossRef]
- Nichol, S.T.; Spiropoulou, C.F.; Morzunov, S.; Rollin, P.E.; Ksiazek, T.G.; Feldmann, H.; Sanchez, A.; Childs, J.; Zaki, S.; Peters, C.J. Genetic Identification of a Hantavirus Associated with an Outbreak of Acute Respiratory Illness. Science 1993, 262, 914–917. [Google Scholar] [CrossRef]
- Noack, D.; Goeijenbier, M.; Reusken, C.B.E.M.; Koopmans, M.P.G.; Rockx, B.H.G. Orthohantavirus Pathogenesis and Cell Tropism. Front. Cell. Infect. Microbiol. 2020, 10, 399. [Google Scholar] [CrossRef]
- Lin, X.-D.; Guo, W.-P.; Wang, W.; Zou, Y.; Hao, Z.-Y.; Zhou, D.-J.; Dong, X.; Qu, Y.-G.; Li, M.-H.; Tian, H.-F.; et al. Migration of Norway Rats Resulted in the Worldwide Distribution of Seoul Hantavirus Today. J. Virol. 2012, 86, 972–981. [Google Scholar] [CrossRef] [Green Version]
- Knust, B.; Rollin, P. Twenty-Year Summary of Surveillance for Human Hantavirus Infections, United States. Emerg. Infect. Dis. 2013, 19, 1934–1937. [Google Scholar] [CrossRef] [Green Version]
- Yates, T.L.; Mills, J.N.; Parmenter, C.A.; Ksiazek, T.G.; Parmenter, R.; Castle, J.R.V.; Calisher, C.H.; Nichol, S.T.; Abbott, K.D.; Young, J.C.; et al. The Ecology and Evolutionary History of an Emergent Disease: Hantavirus Pulmonary Syndrome. Bioscience 2002, 52, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Childs, J.E.; Ksiazek, T.G.; Spiropoulou, C.F.; Krebs, J.W.; Morzunov, S.; Maupin, G.O.; Gage, K.L.; Rollin, P.E.; Sarisky, J.; Enscore, R.; et al. Serologic and Genetic Identification of Peromyscus maniculatus as the Primary Rodent Reservoir for a New Hantavirus in the Southwestern United States. J. Infect. Dis. 1994, 169, 1271–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, A.J.; Abbott, K.D.; Nichol, S.T. Genetic Identification and Characterization of Limestone Canyon Virus, a Unique Peromyscus-Borne Hantavirus. Virology 2001, 286, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Song, J.-W.; Baek, L.-J.; Gajdusek, D.; Yanagihara, R.; Gavrilovskaya, I.; Luft, B.; Mackow, E.; Hjelle, B. Isolation of pathogenic hantavirus from white-footed mouse (Peromyscus leucopus). Lancet 1994, 344, 1637. [Google Scholar] [CrossRef]
- Hjelle, B.; Torres-Pérez, F. Hantaviruses in the Americas and Their Role as Emerging Pathogens. Viruses 2010, 2, 2559–2586. [Google Scholar] [CrossRef] [Green Version]
- Hjelle, B.; Lee, S.W.; Song, W.; Torrez-Martinez, N.; Song, J.W.; Yanagihara, R.; Gavrilovskaya, I.; Mackow, E.R. Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: Genetic characterization of the M genome of New York virus. J. Virol. 1995, 69, 8137–8141. [Google Scholar] [CrossRef] [Green Version]
- Mills, J.N.; Amman, B.R.; Glass, G.E. Ecology of Hantaviruses and Their Hosts in North America. Vector-Borne Zoonotic Dis. 2010, 10, 563–574. [Google Scholar] [CrossRef]
- Jay, M. Seroepidemiologic Studies of Hantavirus Infection Among Wild Rodents in California. Emerg. Infect. Dis. 1997, 3, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Goodfellow, S.M.; Nofchissey, R.A.; Schwalm, K.C.; Cook, J.A.; Dunnum, J.L.; Guo, Y.; Ye, C.; Mertz, G.J.; Chandran, K.; Harkins, M.; et al. Tracing Transmission of Sin Nombre Virus and Discovery of Infection in Multiple Rodent Species. J. Virol. 2021, 95, e0153421. [Google Scholar] [CrossRef]
- Hjelle, B.; Krolikowski, J.; Torrez-Martinez, N.; Chavez-Giles, F.; Vanner, C.; Laposata, E. Phylogenetically distinct hantavirus implicated in a case of hantavirus pulmonary syndrome in the Northeastern United States. J. Med. Virol. 1995, 46, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.L.; Cajimat, M.N.; Romo, H.E.; Estrada-Franco, J.G.; Iñiguez-Dávalos, L.I.; Bradley, R.D.; Fulhorst, C.F. Geographic Distribution of Hantaviruses Associated with Neotomine and Sigmodontine Rodents, Mexico. Emerg. Infect. Dis. 2012, 18, 571–576. [Google Scholar] [CrossRef]
- Burns, J.E.; Metzger, M.E.; Messenger, S.; Fritz, C.L.; Vilcins, I.-M.E.; Enge, B.; Bronson, L.R.; Kramer, V.L.; Hu, R. Novel Focus of Sin Nombre Virus inPeromyscus eremicusMice, Death Valley National Park, California, USA. Emerg. Infect. Dis. 2018, 24, 1112–1115. [Google Scholar] [CrossRef] [Green Version]
- Padula, P.; Rossi, C.; Della Valle, M.; Martínez, P.; Colavecchia, S.; Edelstein, A.; Miguel, S.L.; Rabinovich, R.; Segura, E. De-velopment and evaluation of a solid-phase enzyme immunoassay based on Andes hantavirus recombinant nucleoprotein. J. Med. Microbiol. 2000, 49, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cautivo, K.; Schountz, T.; Acuña-Retamar, M.; Ferrés, M.; Torres-Pérez, F. Rapid Enzyme-Linked Immunosorbent Assay for the Detection of Hantavirus-Specific Antibodies in Divergent Small Mammals. Viruses 2014, 6, 2028–2037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjölander, K.B.; Elgh, F.; Kallio-Kokko, H.; Vapalahti, O.; Hägglund, M.; Palmcrantz, V.; Juto, P.; Vaheri, A.; Niklasson, B.; Lundkvist, A. Evaluation of serological methods for diagnosis of Puumala hantavirus infection (nephropathia epidemica). J. Clin. Microbiol. 1997, 35, 3264–3268. [Google Scholar] [CrossRef] [Green Version]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Denys, C.; Koivogui, L.; Ter Meulen, J.; Krüger, D.H. Hantavirus in African Wood Mouse, Guinea. Emerg. Infect. Dis. 2006, 12, 838–840. [Google Scholar] [CrossRef]
- Arai, S.; Aoki, K.; Sơn, N.T.; Tú, V.T.; Kikuchi, F.; Kinoshita, G.; Fukui, D.; Thành, H.T.; Gu, S.H.; Yoshikawa, Y.; et al. Đakrông virus, a novel mobatvirus (Hantaviridae) harbored by the Stoliczka’s Asian trident bat (Aselliscus stoliczkanus) in Vietnam. Sci. Rep. 2019, 9, 10239. [Google Scholar] [CrossRef] [Green Version]
- Nunes, B.T.D.; De Mendonça, M.H.R.; Simith, D.D.B.; Moraes, A.F.; Cardoso, C.C.; Prazeres, I.T.E.; De Aquino, A.A.; Santos, A.D.C.M.; Queiroz, A.L.N.; Rodrigues, D.S.G.; et al. Development of RT-qPCR and semi-nested RT-PCR assays for molecular diagnosis of hantavirus pulmonary syndrome. PLoS Negl. Trop. Dis. 2019, 13, e0007884. [Google Scholar] [CrossRef]
- Laenen, L.; Vergote, V.; Kafetzopoulou, L.E.; Wawina, T.B.; Vassou, D.; Cook, J.A.; Hugot, J.-P.; Deboutte, W.; Kang, H.J.; Witkowski, P.T.; et al. A Novel Hantavirus of the European Mole, Bruges Virus, Is Involved in Frequent Nova Virus Coinfec-tions. Genome Biol. Evol. 2018, 10, 45–55. [Google Scholar] [CrossRef]
- Moreli, M.L.; De Sousa, R.L.M.; Figueiredo, L.T.M. Detection of Brazilian hantavirus by reverse transcription polymerase chain reaction amplification of N gene in patients with hantavirus cardiopulmonary syndrome. Memórias Do Instituto Oswaldo Cruz 2004, 99, 633–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zana, B.; Kemenesi, G.; Buzás, D.; Csorba, G.; Görföl, T.; Khan, F.A.A.; Tahir, N.F.D.A.; Zeghbib, S.; Madai, M.; Papp, H.; et al. Molecular Identification of a Novel Hantavirus in Malaysian Bronze Tube-Nosed Bats (Murina aenea). Viruses 2019, 11, 887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikes, R.S.; Gannon, W.L. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. J. Mammal. 2011, 92, 235–253. [Google Scholar] [CrossRef]
- Dunnum, J.L.; Yanagihara, R.; Johnson, K.M.; Armien, B.; Batsaikhan, N.; Morgan, L.; Cook, J.A. Biospecimen Repositories and Integrated Databases as Critical Infrastructure for Pathogen Discovery and Pathobiology Research. PLoS Negl. Trop. Dis. 2017, 11, e0005133. [Google Scholar] [CrossRef] [Green Version]
- Galbreath, K.; Hoberg, E.P.; Cook, J.A.; Armién, B.; Bell, K.C.; Campbell, M.L.; Dunnum, J.L.; Dursahinhan, A.T.; Eckerlin, R.P.; Gardner, S.L.; et al. Building an integrated infrastructure for exploring biodiversity: Field collections and archives of mammals and parasites. J. Mammal. 2019, 100, 382–393. [Google Scholar] [CrossRef]
- Davenport, B.J.; Willis, D.G.; Prescott, J.; Farrell, R.M.; Coons, T.A.; Schountz, T. Generation of competent bone marrow-derived antigen presenting cells from the deer mouse (Peromyscus maniculatus). BMC Immunol. 2004, 5, 23. [Google Scholar] [CrossRef] [Green Version]
- Vapalahti, O.; Kallio-Kokko, H.; Närvänen, A.; Julkunen, I.; Lundkvist, Å.; Plyusnin, A.; Lehvaslaiho, H.; Brum-mer-Korvenkontio, M.; Vaheri, A.; Lankinen, H. Human B-cell epitopes of puumala virus nucleocapsid protein, the major antigen in early serological response. J. Med. Virol. 1995, 46, 293–303. [Google Scholar] [CrossRef]
- Rothenberger, S.; Torriani, G.; Johansson, M.U.; Kunz, S.; Engler, O. Conserved Endonuclease Function of Hantavirus L Polymerase. Viruses 2016, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Botten, J.; Mirowsky, K.; Kusewitt, D.; Bharadwaj, M.; Yee, J.; Ricci, R.; Feddersen, R.M.; Hjelle, B. Experimental infection model for Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus). Proc. Natl. Acad. Sci. USA 2000, 97, 10578–10583. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Seong, I.-W.; Baek, L.J.; Song, C.K.; Lee, P.W. Intraspecific Transmission of Hantaan Virus, Etiologic Agent of Korean Hemorrhagic Fever, in the Rodent Apodemus agrarius. Am. J. Trop. Med. Hyg. 1981, 30, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Raboni, S.M.; Levis, S.; Rosa, E.S.T.; Bisordi, I.; Delfraro, A.; Lemos, E.; Correia, D.C.; dos Santos, C.N.D. Hantavirus infection in Brazil: Development and evaluation of an enzyme immunoassay and immunoblotting based on N recombinant protein. Diagn. Microbiol. Infect. Dis. 2007, 58, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.R.; Greer, P.W.; Coffield, L.M.; Goldsmith, C.S.; Nolte, K.B.; Foucar, K.; Feddersen, R.M.; Zumwalt, R.E.; Miller, G.L.; Khan, A.S.; et al. Hantavirus pulmonary syndrome: Pathogenesis of an emerging infectious disease. Am. J. Pathol. 1995, 146, 552–579. [Google Scholar] [PubMed]
- Lederer, S.; Lattwein, E.; Hanke, M.; Sonnenberg, K.; Stoecker, W.; Lundkvist, Å.; Vaheri, A.; Vapalahti, O.; Chan, P.K.S.; Feldmann, H.; et al. Indirect Immunofluorescence Assay for the Simultaneous Detection of Antibodies against Clinically Im-portant Old and New World Hantaviruses. PLoS Negl. Trop. Dis. 2013, 7, e2157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terajima, M.; Hendershot, I.J.D.; Kariwa, H.; Koster, F.T.; Hjelle, B.; Goade, D.; DeFronzo, M.C.; Ennis, F.A. High Levels of Viremia in Patients with the Hantavirus Pulmonary Syndrome. J. Infect. Dis. 1999, 180, 2030–2034. [Google Scholar] [CrossRef]
- Mackay, I.M. Real-time PCR in virology. Nucleic Acids Res. 2002, 30, 1292–1305. [Google Scholar] [CrossRef] [Green Version]
- Aitichou, M.; Saleh, S.S.; McElroy, A.; Schmaljohn, C.; Ibrahim, M.S. Identification of Dobrava, Hantaan, Seoul, and Puumala viruses by one-step real-time RT-PCR. J. Virol. Methods 2005, 124, 21–26. [Google Scholar] [CrossRef]
- Garin, D.; Peyrefitte, C.; Crance, J.-M.; Le Faou, A.; Jouan, A.; Bouloy, M. Highly sensitive Taqman® PCR detection of Puumala hantavirus. Microbes Infect. 2001, 3, 739–745. [Google Scholar] [CrossRef]
- Kramski, M.; Meisel, H.; Klempa, B.; Krüger, D.H.; Pauli, G.; Nitsche, A. Detection and Typing of Human Pathogenic Han-taviruses by Real-Time Reverse Transcription-PCR and Pyrosequencing. Clin. Chem. 2007, 53, 1899–1905. [Google Scholar] [CrossRef] [Green Version]
- Näslund, J.; Kerner, A.; Drobni, P.; Bucht, G.; Evander, M.; Ahlm, C. Detection of Puumala and Rift Valley Fever virus by quantitative RT-PCR and virus viability tests in samples of blood dried and stored on filter paper. J. Virol. Methods 2011, 178, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Pang, Z.; Li, A.; Li, J.; Qu, J.; He, C.; Zhang, S.; Li, C.; Zhang, Q.; Liang, M.; Li, D. Comprehensive Multiplex One-Step Real-Time TaqMan qRT-PCR Assays for Detection and Quantification of Hemorrhagic Fever Viruses. PLoS ONE 2014, 9, e95635. [Google Scholar] [CrossRef]
- Wei, F.; Li, J.-L.; Ling, J.-X.; Chen, L.-J.; Li, N.; Liu, Y.-Y.; Luo, F.; Xiong, H.-R.; Hou, W.; Yang, Z.-Q. Establishment of SYBR green-based qPCR assay for rapid evaluation and quantification for anti-Hantaan virus compounds in vitro and in suckling mice. Virus Genes 2012, 46, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Vial, C.; Martinez-Valdebenito, C.; Rios, S.; Martinez, J.; Vial, P.A.; Ferres, M.; Rivera, J.C.; Perez, R.; Valdivieso, F. Molecular method for the detection of Andes hantavirus infection: Validation for clinical diagnostics. Diagn. Microbiol. Infect. Dis. 2016, 84, 36–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starr, E.P.; Nuccio, E.E.; Pett-Ridge, J.; Banfield, J.F.; Firestone, M.K. Metatranscriptomic reconstruction reveals RNA viruses with the potential to shape carbon cycling in soil. Proc. Natl. Acad. Sci. USA 2019, 116, 25900–25908. [Google Scholar] [CrossRef] [Green Version]
- Akopyants, N.S.; Lye, L.-F.; Dobson, D.E.; Lukeš, J.; Beverley, S.M. A Novel Bunyavirus-Like Virus of Trypanosomatid Protist Parasites. Genome Announc. 2016, 4, e00715–e00716. [Google Scholar] [CrossRef] [Green Version]
- Hierweger, M.M.; Koch, M.C.; Rupp, M.; Maes, P.; Di Paola, N.; Bruggmann, R.; Kuhn, J.H.; Schmidt-Posthaus, H.; Seuberlich, T. Novel Filoviruses, Hantavirus, and Rhabdovirus in Freshwater Fish, Switzerland, 2017. Emerg. Infect. Dis. 2021, 27, 3082–3091. [Google Scholar] [CrossRef]




| Primer | Sequence (5′-3′) | Target Segment (Gene) | Size (bp) | TM | |
|---|---|---|---|---|---|
| PanHS1 | Forward | GGRCARACHGCWGAYTGG | S (N) | 248 | 62 °C |
| Reverse | CCDGGHGTBADYTCHTCDGCYTTCAT | ||||
| PanHS2 | Forward | GAYATGMGDAAYACNATHATGGC | S (N) | 207 | 61 °C |
| Reverse | CWGGRTCCATRTCATCHCC | ||||
| PanHS7 | Forward | GGVCARACMGCWGAYTGG | S (N) | 248 | 57 °C |
| Reverse | CCWGGTGTNADYTCWTCDGC | ||||
| PanHS8 | Forward | CAGGAYATGVGRAAYACVATHATGGC | S (N) | 210 | 63 °C |
| Reverse | CTCWGGRTCCATRTCATCMCC |
| Genus Species | # Screened (%) | Sex M/F (% +) | Lung Tissue |
|---|---|---|---|
| PanHS8 + (%) | |||
| Peromyscus maniculatus | 28 (28%) | 16 (50%)/12 (42%) | 13 (46%) |
| Peromyscus leucopus | 14 (14%) | 8 (38%)/6 (67%) | 7 (50%) |
| Peromyscus truei | 29 (29%) | 13 (38%)/16 (63%) | 15 (52%) |
| Peromyscus boylii | 10 (10%) | 6 (33%)/4 (50%) | 4 (40%) |
| Peromyscus nasutus | 3 (3%) | 1 (0%)/2 (50%) | 1 (33%) |
| Mus musculus | 7 (7%) | 5 (40%)/2 (0%) | 2 (29%) |
| Thomomys bottae | 4 (4%) | 2 (100%)/2 (0%) | 2 (50%) |
| Neotomas albigula | 4 (4%) | 2 (100%)/2 (50%) | 3 (75%) |
| Reithrodontomys megalotis | 1 (1%) | 0 (0%)/1 (0%) | 0 (0%) |
| Total(s) | 100 (100%) | 53 (45%)/47 (49%) | 47 (47%) |
 | ||
| Repeated RT-qPCR Samples | Positive Percentage Accuracy | Negative Percentage Accuracy |
| Total (n = 73) | 82.6% | 90.0% |
| Rodent Samples Results | Number (%) of Hantavirus-Negative or Positive Rodents |
|---|---|
| Negatives | 53 (53%) |
| False positives | 2 (2%) |
| Positives | 45 (45%) |
| Total | 100 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodfellow, S.M.; Nofchissey, R.A.; Ye, C.; Dunnum, J.L.; Cook, J.A.; Bradfute, S.B. Use of a Novel Detection Tool to Survey Orthohantaviruses in Wild-Caught Rodent Populations. Viruses 2022, 14, 682. https://doi.org/10.3390/v14040682
Goodfellow SM, Nofchissey RA, Ye C, Dunnum JL, Cook JA, Bradfute SB. Use of a Novel Detection Tool to Survey Orthohantaviruses in Wild-Caught Rodent Populations. Viruses. 2022; 14(4):682. https://doi.org/10.3390/v14040682
Chicago/Turabian StyleGoodfellow, Samuel M., Robert A. Nofchissey, Chunyan Ye, Jonathan L. Dunnum, Joseph A. Cook, and Steven B. Bradfute. 2022. "Use of a Novel Detection Tool to Survey Orthohantaviruses in Wild-Caught Rodent Populations" Viruses 14, no. 4: 682. https://doi.org/10.3390/v14040682
APA StyleGoodfellow, S. M., Nofchissey, R. A., Ye, C., Dunnum, J. L., Cook, J. A., & Bradfute, S. B. (2022). Use of a Novel Detection Tool to Survey Orthohantaviruses in Wild-Caught Rodent Populations. Viruses, 14(4), 682. https://doi.org/10.3390/v14040682







