Calicivirus Infection in Cats
Abstract
:1. Introduction
2. Virus
3. Epidemiology
4. Pathogenesis
5. Immunity
5.1. Passive Immunity by Maternally Derived Antibodies
5.2. Active Immune Response
6. Clinical Signs
6.1. Acute Oral and Upper Respiratory Tract Disease
6.2. Feline Chronic Gingivostomatitis (FCGS)
6.3. Limping Syndrome
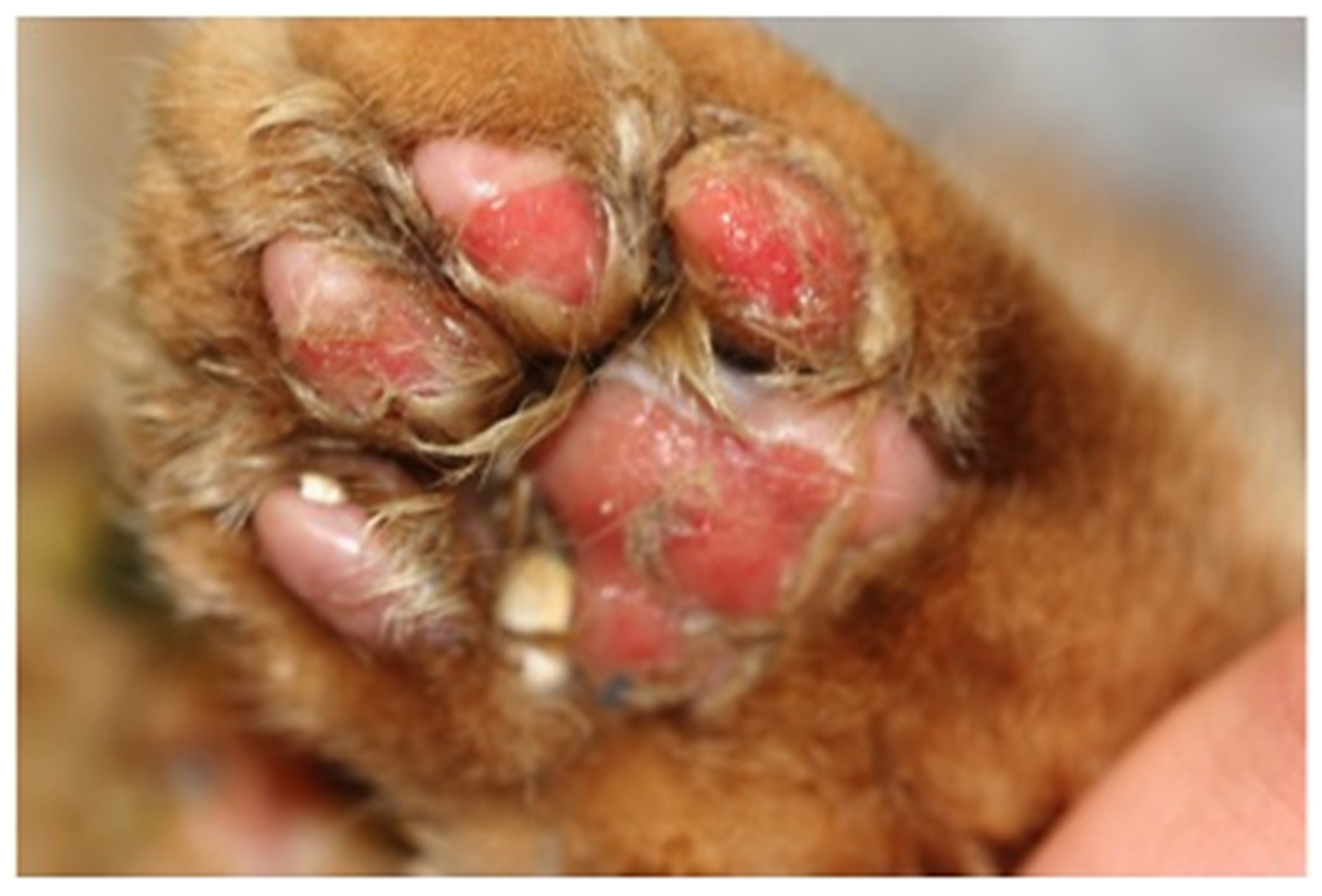
6.4. Paw and Mouth Disease
6.5. Virulent Systemic Feline Calicivirus Infection


| Not all cats with VS-FCV infection show all the typical clinical signs, which makes it difficult to recognize all affected cats. |


6.6. Other Clinical Presentations
7. Diagnosis
7.1. Detection of Nucleic Acids
| A negative RT-PCR result does not rule out FCV infection. However, in a cat that has typical clinical signs and a positive RT-PCR result, a causal relationship is likely. |
7.2. Virus Isolation
7.3. Antibody Detection
7.4. Diagnosis of VS-FCV Infections
| The diagnosis of VS-FCV infections relies on clinical signs typical for virulent systemic disease including systemic infection and organ involvement, often the occurrence in multicat environments, high contagiousness of the virus and epizootic spread of the infection, a high mortality rate of the disease, and the isolation of the same FCV strain from the blood, oropharyngeal swabs or cutaneous scrapings from ulcerated lesions of several diseased cats assessed by sequencing hypervariable regions of the capsid gene. |
8. Disease Management
8.1. Treatment of Cats with Acute Upper Respiratory Tract or Oral Disease
8.2. Antiviral Therapy of Acute Upper Respiratory Disease
8.3. Treatment of Cats with FCGS
8.4. Other Management Considerations for FCV Infection
- Avoid stress, consider environmental enrichment and management in multicat households. One study looked at the effect of a synthetic feline facial pheromone in two shelters in the USA [157] in reducing stress scores and/or the incidence of infections associated with FURTD, compared to placebo, but no evidence was found that the pheromone product had any effect on stress scores or incidence of infections associated with FURTD in the shelter-housed cats.
- Consider hygiene, partitions, grouping, order of cleaning (deal with ill cats last), etc. Effective barrier nursing is essential for hospitalised patients being treated with acute infections associated with FURTD. However, staff should be mindful that outwardly normal cats could be shedding FCV.
- Be careful with introduction of new cats into a household, particularly if you have a FCV-free household; quarantine for three weeks with the option to swab for FCV before introduction into the household.
- If there are recurrent problems with FCV in a multicat environment, reduce the number of cats within the individual group (see also “Managing FCV outbreaks in multicat communities” [158]).
8.5. Treatment of Cats with VS-FCV Infection
9. General Recommendations on Vaccine Type and Vaccination Protocol
9.1. Different FCV Strains
| Independent of the vaccine strains used, if FCV-associated disease is found to be occurring in fully vaccinated cats then changing to a different FCV vaccine strain should be considered. |
9.2. Vaccination and VS-FC Infection
9.3. Primary Vaccination Course
- Kittens: The ABCD recommends that all kittens should be vaccinated against FCV [159] (Table 1). MDA can interfere with the response to vaccination, and thus, the primary course of vaccination is usually started at approximately nine weeks of age, although some vaccines are licensed for use at an earlier age. Kittens should receive a second vaccination two to four weeks later, but not earlier than at 12 weeks of age. This protocol has been developed to ensure optimal protection. However, due to a longer persistence of MDA, some kittens might fail to respond to this protocol [77,185]. Therefore, in high-risk situations, particularly where FCV has been shown to cause disease in vaccinated kittens as well as if presence of high MDA is expected in the kittens, a third vaccination at 16 weeks should be considered. After the kitten primary vaccination course, all cats should receive an additional vaccine dose at 10 to 16 months of age: This will ensure adequate vaccine-induced immunity for cats that might not have adequately responded to the primary vaccination course. It is recommended that the same vaccine brand or at least the same vaccine strain(s) is used for the entire primary vaccination course.
- Older cats of uncertain FCV vaccination status should also receive two injections with an interval of two to four weeks, and a boost one year later, using vaccines containing the same virus strains (Table 1). This applies even if the vaccine contains modified live virus.
9.4. Revaccinations
10. Disease Control in Specific Situations
10.1. Shelters
10.2. Breeding Catteries
10.3. VS-FCV Outbreaks
11. Conclusions
Funding
Conflicts of Interest
References
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, A.; Willi, B.; Meli, M.L.; Boretti, F.S.; Hartnack, S.; Dreyfus, A.; Lutz, H.; Hofmann-Lehmann, R. Feline calicivirus and other respiratory pathogens in cats with Feline calicivirus-related symptoms and in clinically healthy cats in Switzerland. BMC Vet. Res. 2015, 11, 282. [Google Scholar] [CrossRef] [Green Version]
- Hurley, K.F.; Sykes, J.E. Update on feline calicivirus: New trends. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 759–772. [Google Scholar] [CrossRef]
- Slaviero, M.; Ehlers, L.P.; Argenta, F.F.; Savi, C.; Lopes, B.C.; Pavarini, S.P.; Driemeier, D.; Sonne, L. Causes and lesions of fatal pneumonia in domestic cats. J. Comp. Pathol. 2021, 189, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.; Bennett, D.; Carter, S.D.; Bennett, M.; Meanger, J.; Turner, P.C.; Carter, M.J.; Milton, I.; Gaskell, R.M. Acute arthritis of cats associated with feline calicivirus infection. Res. Vet. Sci. 1994, 56, 133–143. [Google Scholar] [CrossRef]
- Dawson, S.; McArdle, F.; Bennett, D.; Carter, S.D.; Bennett, M.; Ryvar, R.; Gaskell, R.M. Investigation of vaccine reactions and breakdowns after feline calicivirus vaccination. Vet. Rec. 1993, 132, 346–350. [Google Scholar] [CrossRef]
- Fried, W.A.; Soltero-Rivera, M.; Ramesh, A.; Lommer, M.J.; Arzi, B.; DeRisi, J.L.; Horst, J.A. Use of unbiased metagenomic and transcriptomic analyses to investigate the association between feline calicivirus and feline chronic gingivostomatitis in domestic cats. Am. J. Vet. Res. 2021, 82, 381–394. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Elliott, J.B.; Glasgow, A.; Poland, A.; Keel, K. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 2000, 73, 281–300. [Google Scholar] [CrossRef]
- Coyne, K.P.; Gaskell, R.M.; Dawson, S.; Porter, C.J.; Radford, A.D. Evolutionary mechanisms of persistence and diversification of a calicivirus within endemically infected natural host populations. J. Virol. 2007, 81, 1961–1971. [Google Scholar] [CrossRef] [Green Version]
- Geissler, K.; Schneider, K.; Platzer, G.; Truyen, B.; Kaaden, O.R.; Truyen, U. Genetic and antigenic heterogeneity among feline calicivirus isolates from distinct disease manifestations. Virus Res. 1997, 48, 193–206. [Google Scholar] [CrossRef]
- Bannasch, M.J.; Foley, J.E. Epidemiologic evaluation of multiple respiratory pathogens in cats in animal shelters. J. Feline Med. Surg. 2005, 7, 109–119. [Google Scholar] [CrossRef]
- Fastier, L.B. A new feline virus isolated in tissue culture. Am. J. Vet. Res. 1957, 18, 382–389. [Google Scholar]
- Green, K.Y.; Ando, T.; Balayan, M.S.; Berke, T.; Clarke, I.N.; Estes, M.K.; Matson, D.O.; Nakata, S.; Neill, J.D.; Studdert, M.J.; et al. Taxonomy of the caliciviruses. J. Infect. Dis. 2000, 181, S322–S330. [Google Scholar] [CrossRef] [Green Version]
- Sosnovtsev, S.V.; Garfield, M.; Green, K.Y. Processing map and essential cleavage sites of the nonstructural polyprotein encoded by ORF1 of the feline calicivirus genome. J. Virol. 2002, 76, 7060–7072. [Google Scholar] [CrossRef] [Green Version]
- Sosnovtsev, S.V.; Green, K.Y. Identification and genomic mapping of the ORF3 and VPg proteins in feline calicivirus virions. Virology 2000, 277, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Milton, I.D.; Turner, J.; Teelan, A.; Gaskell, R.; Turner, P.C.; Carter, M.J. Location of monoclonal antibody binding sites in the capsid protein of feline calicivirus. J. Gen. Virol. 1992, 73, 2435–2439. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Angulo, I.; Almanza, H.; Borrego, B.; Zamora-Ceballos, M.; Caston, J.R.; Mena, I.; Blanco, E.; Barcena, J. Precise location of linear epitopes on the capsid surface of feline calicivirus recognized by neutralizing and non-neutralizing monoclonal antibodies. Vet. Res. 2020, 51, 59. [Google Scholar] [CrossRef]
- Geissler, K.; Schneider, K.; Truyen, U. Mapping neutralizing and non-neutralizing epitopes on the capsid protein of feline calicivirus. J. Vet. Med. B Infect. Dis Vet. Public Health 2002, 49, 55–60. [Google Scholar] [CrossRef]
- Ohe, K.; Sakai, S.; Sunaga, F.; Murakami, M.; Kiuchi, A.; Fukuyama, M.; Furuhata, K.; Hara, M.; Soma, T.; Ishikawa, Y.; et al. Detection of feline calicivirus (FCV) from vaccinated cats and phylogenetic analysis of its capsid genes. Vet. Res. Commun. 2006, 30, 293–305. [Google Scholar] [CrossRef]
- Urban, C.; Luttermann, C. Major capsid protein synthesis from the genomic RNA of feline calicivirus. J. Virol. 2020, 94, e00280-20. [Google Scholar] [CrossRef]
- Campillay-Veliz, C.P.; Carvajal, J.J.; Avellaneda, A.M.; Escobar, D.; Covian, C.; Kalergis, A.M.; Lay, M.K. Human norovirus proteins: Implications in the replicative cycle, pathogenesis, and the host immune response. Front. Immunol. 2020, 11, 961. [Google Scholar] [CrossRef]
- Tohya, Y.; Yokoyama, N.; Maeda, K.; Kawaguchi, Y.; Mikami, T. Mapping of antigenic sites involved in neutralization on the capsid protein of feline calicivirus. J. Gen. Virol. 1997, 78, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Radford, A.D.; Bennett, M.; McArdle, F.; Dawson, S.; Turner, P.C.; Williams, R.A.; Glenn, M.A.; Gaskell, R.M. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and persistently infected cats. Vet. Microbiol. 1999, 69, 67–68. [Google Scholar] [CrossRef]
- Lu, Z.; Ledgerwood, E.D.; Hinchman, M.M.; Dick, R.; Parker, J.S.L. Conserved surface residues on the feline calicivirus capsid are essential for interaction with its receptor feline junctional adhesion molecule A (fJAM-A). J. Virol. 2018, 92, e00035-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conley, M.J.; McElwee, M.; Azmi, L.; Gabrielsen, M.; Byron, O.; Goodfellow, I.G.; Bhella, D. Calicivirus VP2 forms a portal-like assembly following receptor engagement. Nature 2019, 565, 377–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulet, H.; Brunet, S.; Soulier, M.; Leroy, V.; Goutebroze, S.; Chappuis, G. Comparison between acute oral/respiratory and chronic stomatitis/gingivitis isolates of feline calicivirus: Pathogenicity, antigenic profile and cross-neutralisation studies. Arch. Virol. 2000, 145, 243–261. [Google Scholar] [CrossRef] [PubMed]
- Glenn, M.; Radford, A.D.; Turner, P.C.; Carter, M.; Lowery, D.; DeSilver, D.A.; Meanger, J.; Baulch-Brown, C.; Bennett, M.; Gaskell, R.M. Nucleotide sequence of UK and Australian isolates of feline calicivirus (FCV) and phylogenetic analysis of FCVs. Vet. Microbiol. 1999, 67, 175–193. [Google Scholar] [CrossRef]
- Coyne, K.P.; Christley, R.M.; Pybus, O.G.; Dawson, S.; Gaskell, R.M.; Radford, A.D. Large-scale spatial and temporal genetic diversity of feline calicivirus. J. Virol. 2012, 86, 11356–11367. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Sehata, G.; Okada, N.; Iwamoto, K.; Masubuchi, K.; Kainuma, R.; Noda, T.; Igarashi, T.; Sawada, T.; Noro, T.; et al. Intranasal immunization with inactivated feline calicivirus particles confers robust protection against homologous virus and suppression against heterologous virus in cats. J. Gen. Virol. 2017, 98, 1730–1738. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, C.; Chung, H.C.; Park, Y.H.; Park, K.T. Full-length ORF2 sequence-based genetic and phylogenetic characterization of Korean feline caliciviruses. J. Vet. Sci. 2021, 22, e32. [Google Scholar] [CrossRef]
- Guo, J.; Ding, Y.; Sun, F.; Zhou, H.; He, P.; Chen, J.; Guo, J.; Zeng, H.; Long, J.; Wei, Z.; et al. Co-circulation and evolution of genogroups I and II of respiratory and enteric feline calicivirus isolates in cats. Transbound. Emerg. Dis. 2022. [Google Scholar] [CrossRef]
- Spiri, A.M.; Theze, J.; Meli, M.L.; Cattori, V.; Berger, A.; Steinrigl, A.; Pybus, O.G.; Hofmann-Lehmann, R.; Willi, B. Genetic diversity and phenotypic associations of feline caliciviruses from cats in Switzerland. J. Gen. Virol. 2016, 97, 3253–3266. [Google Scholar] [CrossRef]
- Pereira, J.J.; Baumworcel, N.; Fioretti, J.M.; Domingues, C.F.; Moraes, L.F.; Marinho, R.; Vieira, M.C.R.; Pinto, A.M.V.; de Castro, T.X. Molecular characterization of feline calicivirus variants from multicat household and public animal shelter in Rio de Janeiro, Brazil. Braz. J. Microbiol. 2018, 49, 777–784. [Google Scholar] [CrossRef]
- Spiri, A.M.; Meli, M.L.; Riond, B.; Herbert, I.; Hosie, M.J.; Hofmann-Lehmann, R. Environmental contamination and hygienic measures after feline calicivirus field strain infections of cats in a research facility. Viruses 2019, 11, 958. [Google Scholar] [CrossRef] [Green Version]
- Bordicchia, M.; Fumian, T.M.; Van Brussel, K.; Russo, A.G.; Carrai, M.; Le, S.J.; Pesavento, P.A.; Holmes, E.C.; Martella, V.; White, P.; et al. Feline calicivirus virulent systemic disease: Clinical epidemiology, analysis of viral isolates and in vitro efficacy of novel antivirals in Australian outbreaks. Viruses 2021, 13, 2040. [Google Scholar] [CrossRef]
- Hashimoto, M.; Roerink, F.; Tohya, Y.; Mochizuki, M. Genetic analysis of the RNA polymerase gene of caliciviruses from dogs and cats. J. Vet. Med. Sci. 1999, 61, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Roerink, F.; Hashimoto, M.; Tohya, Y.; Mochizuki, M. Genetic analysis of a canine calicivirus: Evidence for a new clade of animal caliciviruses. Vet. Microbiol. 1999, 69, 69–72. [Google Scholar] [CrossRef]
- Martella, V.; Pratelli, A.; Gentile, M.; Buonavoglia, D.; Decaro, N.; Fiorente, P.; Buonavoglia, C. Analysis of the capsid protein gene of a feline-like calicivirus isolated from a dog. Vet. Microbiol. 2002, 85, 315–322. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Rocco, C.; Ceci, C.; Marsilio, F. Characterization of a strain of feline calicivirus isolated from a dog faecal sample. Vet. Microbiol. 2009, 139, 52–57. [Google Scholar] [CrossRef]
- Binns, S.H.; Dawson, S.; Speakman, A.J.; Cuevas, L.E.; Hart, C.A.; Gaskell, C.J.; Morgan, K.L.; Gaskell, R.M. A study of feline upper respiratory tract disease with reference to prevalence and risk factors for infection with feline calicivirus and feline herpesvirus. J. Feline Med. Surg. 2000, 2, 123–133. [Google Scholar] [CrossRef]
- Helps, C.R.; Lait, P.; Damhuis, A.; Bjornehammar, U.; Bolta, D.; Brovida, C.; Chabanne, L.; Egberink, H.; Ferrand, G.; Fontbonne, A.; et al. Factors associated with upper respiratory tract disease caused by feline herpesvirus, feline calicivirus, Chlamydophila felis and Bordetella bronchiseptica in cats: Experience from 218 European catteries. Vet. Rec. 2005, 156, 669–673. [Google Scholar] [CrossRef] [Green Version]
- Wardley, R.C. Feline calicivirus carrier state. A study of the host/virus relationship. Arch. Virol. 1976, 52, 243–249. [Google Scholar] [CrossRef]
- Coyne, K.P.; Dawson, S.; Radford, A.D.; Cripps, P.J.; Porter, C.J.; McCracken, C.M.; Gaskell, R.M. Long-term analysis of feline calicivirus prevalence and viral shedding patterns in naturally infected colonies of domestic cats. Vet. Microbiol. 2006, 118, 12–25. [Google Scholar] [CrossRef]
- Kratzer, G.; Lewis, F.I.; Willi, B.; Meli, M.L.; Boretti, F.S.; Hofmann-Lehmann, R.; Torgerson, P.; Furrer, R.; Hartnack, S. Bayesian network modeling applied to feline calicivirus infection among cats in Switzerland. Front. Vet. Sci. 2020, 7, 73. [Google Scholar] [CrossRef] [Green Version]
- Radford, A.D.; Sommerville, L.; Ryvar, R.; Cox, M.B.; Johnson, D.R.; Dawson, S.; Gaskell, R.M. Endemic infection of a cat colony with a feline calicivirus closely related to an isolate used in live attenuated vaccines. Vaccine 2001, 19, 4358–4362. [Google Scholar] [CrossRef]
- Radford, A.D.; Dawson, S.; Ryvar, R.; Coyne, K.; Johnson, D.R.; Cox, M.B.; Acke, E.F.; Addie, D.D.; Gaskell, R.M. High genetic diversity of the immunodominant region of the feline calicivirus capsid gene in endemically infected cat colonies. Virus Genes 2003, 27, 145–155. [Google Scholar] [CrossRef]
- Dall’Ara, P.; Labriola, C.; Sala, E.; Spada, E.; Magistrelli, S.; Lauzi, S. Prevalence of serum antibody titres against feline panleukopenia, herpesvirus and calicivirus infections in stray cats of Milan, Italy. Prev. Vet. Med. 2019, 167, 32–38. [Google Scholar] [CrossRef]
- Tran, V.; Kelman, M.; Ward, M.; Westman, M. Risk of feline immunodeficiency virus (FIV) infection in pet cats in Australia is higher in areas of lower socioeconomic status. Animals 2019, 9, 592. [Google Scholar] [CrossRef] [Green Version]
- Radford, A.D.; Sommerville, L.M.; Dawson, S.; Kerins, A.M.; Ryvar, R.; Gaskell, R.M. Molecular analysis of isolates of feline calicivirus from a population of cats in a rescue shelter. Vet. Rec. 2001, 149, 477–481. [Google Scholar] [CrossRef]
- Radford, A.D.; Dawson, S.; Wharmby, C.; Ryvar, R.; Gaskell, R.M. Comparison of serological and sequence-based methods for typing feline calcivirus isolates from vaccine failures. Vet. Rec. 2000, 146, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.P.; Reed, F.C.; Porter, C.J.; Dawson, S.; Gaskell, R.M.; Radford, A.D. Recombination of feline calicivirus within an endemically infected cat colony. J. Gen. Virol. 2006, 87, 921–926. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.P.; Edwards, D.; Radford, A.D.; Cripps, P.; Jones, D.; Wood, J.L.; Gaskell, R.M.; Dawson, S. Longitudinal molecular epidemiological analysis of feline calicivirus infection in an animal shelter: A model for investigating calicivirus transmission within high-density, high-turnover populations. J. Clin. Microbiol. 2007, 45, 3239–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardley, R.C.; Povey, R.C. The clinical disease and patterns of excretion associated with three different strains of feline caliciviruses. Res. Vet. Sci. 1977, 23, 7–14. [Google Scholar] [CrossRef]
- Doultree, J.C.; Druce, J.D.; Birch, C.J.; Bowden, D.S.; Marshall, J.A. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 1999, 41, 51–57. [Google Scholar] [CrossRef]
- Duizer, E.; Bijkerk, P.; Rockx, B.; De Groot, A.; Twisk, F.; Koopmans, M. Inactivation of caliciviruses. Appl. Environ. Microbiol. 2004, 70, 4538–4543. [Google Scholar] [CrossRef] [Green Version]
- Clay, S.; Maherchandani, S.; Malik, Y.S.; Goyal, S.M. Survival on uncommon fomites of feline calicivirus, a surrogate of noroviruses. Am. J. Infect. Control 2006, 34, 41–43. [Google Scholar] [CrossRef]
- Schorr-Evans, E.M.; Poland, A.; Johnson, W.E.; Pedersen, N.C. An epizootic of highly virulent feline calicivirus disease in a hospital setting in New England. J. Feline Med. Surg. 2003, 5, 217–226. [Google Scholar] [CrossRef]
- Reynolds, B.S.; Poulet, H.; Pingret, J.L.; Jas, D.; Brunet, S.; Lemeter, C.; Etievant, M.; Boucraut-Baralon, C. A nosocomial outbreak of feline calicivirus associated virulent systemic disease in France. J. Feline Med. Surg. 2009, 11, 633–644. [Google Scholar] [CrossRef]
- Deschamps, J.Y.; Topie, E.; Roux, F. Nosocomial feline calicivirus-associated virulent systemic disease in a veterinary emergency and critical care unit in France. JFMS Open Rep. 2015, 1, 2055116915621581. [Google Scholar] [CrossRef] [Green Version]
- Mencke, N.; Vobis, M.; Mehlhorn, H.; D’haese, J.; Rehagen, M.; Mangold-Gehring, S.; Truyen, U. Transmission of feline calicivirus via the cat flea (Ctenocephalides felis). Parasitol. Res. 2009, 105, 185–189. [Google Scholar] [CrossRef]
- Gaskell, R.M.; Dawson, S.; Radford, A.D. Feline respiratory disease. In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; Saunders Elsevier: Philadelphia, PA, USA, 2006; pp. 145–154. [Google Scholar]
- Rodriguez, J.M.; Kohler, K.; Kipar, A. Calicivirus co-infections in herpesvirus pneumonia in kittens. Vet. J. 2018, 236, 1–3. [Google Scholar] [CrossRef]
- Bennett, D.; Gaskell, R.M.; Mills, A.; Knowles, J.; Carter, S.; McArdle, F. Detection of feline calicivirus antigens in the joints of infected cats. Vet. Rec. 1989, 124, 329–332. [Google Scholar] [CrossRef]
- Coyne, K.P.; Jones, B.R.; Kipar, A.; Chantrey, J.; Porter, C.J.; Barber, P.J.; Dawson, S.; Gaskell, R.M.; Radford, A.D. Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet. Rec. 2006, 158, 544–550. [Google Scholar] [CrossRef]
- Hurley, K.F. Virulent calicivirus infection in cats. In Proceedings of the American College of Veterinary Internal Medicine Congress, Amsterdam, The Netherlands, 14−16 September 2006. [Google Scholar]
- Ossiboff, R.J.; Sheh, A.; Shotton, J.; Pesavento, P.A.; Parker, J.S. Feline caliciviruses (FCVs) isolated from cats with virulent systemic disease possess in vitro phenotypes distinct from those of other FCV isolates. J. Gen. Virol. 2007, 88, 506–517. [Google Scholar] [CrossRef]
- Pesavento, P.A.; MacLachlan, N.J.; Dillard-Telm, L.; Grant, C.K.; Hurley, K.F. Pathologic, immunohistochemical, and electron microscopic findings in naturally occurring virulent systemic feline calicivirus infection in cats. Vet. Pathol. 2004, 41, 257–263. [Google Scholar] [CrossRef]
- Coutts, A.J.; Dawson, S.; Willoughby, K.; Gaskell, R.M. Isolation of feline respiratory viruses from clinically healthy cats at UK cat shows. Vet. Rec. 1994, 135, 555–556. [Google Scholar]
- Povey, R.C. Persistent viral infection. The carrier state. Vet. Clin. N. Am. Small Anim. Pract. 1986, 16, 1075–1095. [Google Scholar] [CrossRef]
- Johnson, R.P. Antigenic change in feline calicivirus during persistent infection. Can. J. Vet. Res. 1992, 56, 326–330. [Google Scholar]
- Kreutz, L.C.; Johnson, R.P.; Seal, B.S. Phenotypic and genotypic variation of feline calicivirus during persistent infection of cats. Vet. Microbiol. 1998, 59, 229–236. [Google Scholar] [CrossRef]
- Radford, A.D.; Turner, P.C.; Bennett, M.; McArdle, F.; Dawson, S.; Glenn, M.A.; Williams, R.A.; Gaskell, R.M. Quasispecies evolution of a hypervariable region of the feline calicivirus capsid gene in cell culture and in persistently infected cats. J. Gen. Virol. 1998, 79, 1–10. [Google Scholar] [CrossRef]
- Smith, S.L.; Afonso, M.M.; Pinchbeck, G.L.; Gaskell, R.M.; Dawson, S.; Radford, A.D. Temporally separated feline calicivirus isolates do not cluster phylogenetically and are similarly neutralised by high-titre vaccine strain FCV-F9 antisera in vitro. J. Feline Med. Surg. 2020, 22, 602–607. [Google Scholar] [CrossRef]
- Wu, H.; Huang, J.; Liu, Y.; Pan, Y.; Li, Y.; Miao, Q.; Qu, L.; Tian, J. Feline calicivirus proteinase-polymerase protein degrades mRNAs to inhibit host gene expression. J. Virol. 2021, 95, e0033621. [Google Scholar] [CrossRef]
- Tian, J.; Kang, H.; Huang, J.; Li, Z.; Pan, Y.; Li, Y.; Chen, S.; Zhang, J.; Yin, H.; Qu, L. Feline calicivirus strain 2280 p30 antagonizes type I interferon-mediated antiviral innate immunity through directly degrading IFNAR1 mRNA. PLoS Pathog. 2020, 16, e1008944. [Google Scholar] [CrossRef]
- Johnson, R.P.; Povey, R.C. Transfer and decline of maternal antibody to feline calicivirus. Can. Vet. J. 1983, 24, 6–9. [Google Scholar]
- Dawson, S.; Willoughby, K.; Gaskell, R.M.; Wood, G.; Chalmers, W.S. A field trial to assess the effect of vaccination against feline herpesvirus, feline calicivirus and feline panleucopenia virus in 6-week-old kittens. J. Feline Med. Surg. 2001, 3, 17–22. [Google Scholar] [CrossRef]
- Kahn, D.E.; Hoover, E.A.; Bittle, J.L. Induction of immunity to feline caliciviral disease. Infect. Immun. 1975, 11, 1003–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radford, A.D.; Willoughby, K.; Dawson, S.; McCracken, C.; Gaskell, R.M. The capsid gene of feline calicivirus contains linear B-cell epitopes in both variable and conserved regions. J. Virol. 1999, 73, 8496–8502. [Google Scholar] [CrossRef] [Green Version]
- Povey, C.; Ingersoll, J. Cross-protection among feline caliciviruses. Infect. Immun. 1975, 11, 877–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, J.O.; McArdle, F.; Dawson, S.; Carter, S.D.; Gaskell, C.J.; Gaskell, R.M. Studies on the role of feline calicivirus in chronic stomatitis in cats. Vet. Microbiol. 1991, 27, 205–219. [Google Scholar] [CrossRef]
- Spiri, A.M.; Novacco, M.; Meli, M.L.; Stirn, M.; Riond, B.; Fogle, J.E.; Boretti, F.S.; Herbert, I.; Hosie, M.J.; Hofmann-Lehmann, R. Modified-live feline calicivirus vaccination elicits cellular immunity against a current feline calicivirus field strain in an experimental feline challenge study. Viruses 2021, 13, 1736. [Google Scholar] [CrossRef] [PubMed]
- Poulet, H.; Brunet, S.; Leroy, V.; Chappuis, G. Immunisation with a combination of two complementary feline calicivirus strains induces a broad cross-protection against heterologous challenges. Vet. Microbiol. 2005, 106, 17–31. [Google Scholar] [CrossRef]
- Lesbros, C.; Martin, V.; Najbar, W.; Sanquer, A.; McGahie, D.; Eun, H.M.; Gueguen, S. Protective efficacy of the calicivirus valency of the leucofeligen vaccine against a virulent heterologous challenge in Kittens. Vet. Med. Int. 2013, 2013, 232397. [Google Scholar] [CrossRef]
- Tham, K.M.; Studdert, M.J. Antibody and cell-mediated immune responses to feline calicivirus following inactivated vaccine and challenge. J. Vet. Med. Ser. B Zent. Fur Vet. Reihe B Infect. Dis. Vet. Public Health 1987, 34, 640–654. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Z.; Fu, X.; Lv, Q.; Yang, Y.; Shi, F. Prevalence of feline calicivirus and the distribution of serum neutralizing antibody against isolate strains in cats of Hangzhou, China. J. Vet. Sci. 2021, 22, e73. [Google Scholar] [CrossRef]
- Ballin, A.C.; Schulz, B.; Helps, C.; Sauter-Louis, C.; Mueller, R.S.; Hartmann, K. Limited efficacy of topical recombinant feline interferon-omega for treatment of cats with acute upper respiratory viral disease. Vet. J. 2014, 202, 466–470. [Google Scholar] [CrossRef]
- Friedl, Y.; Schulz, B.; Knebl, A.; Helps, C.; Truyen, U.; Hartmann, K. Efficacy of passively transferred antibodies in cats with acute viral upper respiratory tract infection. Vet. J. 2014, 201, 316–321. [Google Scholar] [CrossRef]
- Gerriets, W.; Joy, N.; Huebner-Guthardt, J.; Eule, J.C. Feline calicivirus: A neglected cause of feline ocular surface infections? Vet. Ophthalmol. 2012, 15, 172–179. [Google Scholar] [CrossRef]
- Perry, R.; Tutt, C. Periodontal disease in cats back to basics—With an eye on the future. J. Feline Med. Surg. 2015, 17, 45–65. [Google Scholar] [CrossRef]
- Belgard, S.; Truyen, U.; Thibault, J.C.; Sauter-Louis, C.; Hartmann, K. Relevance of feline calicivirus, feline immunodeficiency virus, feline leukemia virus, feline herpesvirus and Bartonella henselae in cats with chronic gingivostomatitis. Berl. Munch. Tierarztl. Wochenschr. 2010, 123, 369–376. [Google Scholar]
- Dowers, K.L.; Hawley, J.R.; Brewer, M.M.; Morris, A.K.; Radecki, S.V.; Lappin, M.R. Association of Bartonella species, feline calicivirus, and feline herpesvirus 1 infection with gingivostomatitis in cats. J. Feline Med. Surg. 2010, 12, 314–321. [Google Scholar] [CrossRef]
- Fernandez, M.; Manzanilla, E.G.; Lloret, A.; Leon, M.; Thibault, J.C. Prevalence of feline herpesvirus-1, feline calicivirus, Chlamydophila felis and Mycoplasma felis DNA and associated risk factors in cats in Spain with upper respiratory tract disease, conjunctivitis and/or gingivostomatitis. J. Feline Med. Surg. 2017, 19, 461–469. [Google Scholar] [CrossRef]
- Nakanishi, H.; Furuya, M.; Soma, T.; Hayashiuchi, Y.; Yoshiuchi, R.; Matsubayashi, M.; Tani, H.; Sasai, K. Prevalence of microorganisms associated with feline gingivostomatitis. J. Feline Med. Surg. 2019, 21, 103–108. [Google Scholar] [CrossRef]
- Druet, I.; Hennet, P. Relationship between feline calicivirus load, oral lesions, and outcome in feline chronic gingivostomatitis (caudal stomatitis): Retrospective study in 104 cats. Front. Vet. Sci. 2017, 4, 209. [Google Scholar] [CrossRef] [Green Version]
- Rolim, V.M.; Pavarini, S.P.; Campos, F.S.; Pignone, V.; Faraco, C.; Muccillo, M.S.; Roehe, P.M.; da Costa, F.V.; Driemeier, D. Clinical, pathological, immunohistochemical and molecular characterization of feline chronic gingivostomatitis. J. Feline Med. Surg. 2017, 19, 403–409. [Google Scholar] [CrossRef]
- Thomas, S.; Lappin, D.F.; Spears, J.; Bennett, D.; Nile, C.; Riggio, M.P. Prevalence of feline calicivirus in cats with odontoclastic resorptive lesions and chronic gingivostomatitis. Res. Vet. Sci. 2017, 111, 124–126. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, N.C.; Laliberte, L.; Ekman, S. A transient febrile “limping” syndrome of kittens caused by two different strains of feline calicivirus. Feline Pract. 1983, 13, 26–35. [Google Scholar]
- TerWee, J.; Lauritzen, A.Y.; Sabara, M.; Dreier, K.J.; Kokjohn, K. Comparison of the primary signs induced by experimental exposure to either a pneumotrophic or a ‘limping’ strain of feline calicivirus. Vet. Microbiol. 1997, 56, 33–45. [Google Scholar] [CrossRef]
- Cooper, L.M.; Sabine, M. Paw and mouth disease in a cat. Aust. Vet. J. 1972, 48, 644. [Google Scholar] [CrossRef] [PubMed]
- Love, D.N.; Zuber, R.M. Feline calicivirus associated with pyrexia, profound anorexia and oral and perianal ulceration in a cat. Aust. Vet. Pract. 1987, 17, 136–137. [Google Scholar]
- Willi, B.; Spiri, A.M.; Meli, M.L.; Samman, A.; Hoffmann, K.; Sydler, T.; Cattori, V.; Graf, F.; Diserens, K.A.; Padrutt, I.; et al. Molecular characterization and virus neutralization patterns of severe, non-epizootic forms of feline calicivirus infections resembling virulent systemic disease in cats in Switzerland and in Liechtenstein. Vet. Microbiol. 2016, 182, 202–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.; Kershaw, O.; Klopfleisch, R. Feline calicivirus-associated virulent systemic disease: Not necessarily a local epizootic problem. Vet. Rec. 2011, 168, 589. [Google Scholar] [CrossRef]
- Battilani, M.; Vaccari, F.; Carelle, M.S.; Morandi, F.; Benazzi, C.; Kipar, A.; Dondi, F.; Scagliarini, A. Virulent feline calicivirus disease in a shelter in Italy: A case description. Res. Vet. Sci. 2013, 95, 283–290. [Google Scholar] [CrossRef]
- Hurley, K.E.; Pesavento, P.A.; Pedersen, N.C.; Poland, A.M.; Wilson, E.; Foley, J.E. An outbreak of virulent systemic feline calicivirus disease. J. Am. Vet. Med. Assoc. 2004, 224, 241–249. [Google Scholar] [CrossRef]
- Guo, H.; Miao, Q.; Zhu, J.; Yang, Z.; Liu, G. Isolation and molecular characterization of a virulent systemic feline calicivirus isolated in China. Infect. Genet. Evol. 2018, 65, 425–429. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, T.; Wei, J.; Jiang, Y.; Liu, X.; Song, W.; Guo, X.; Yuan, W.; Cui, Y.; Zhu, H.; et al. Isolation and phylogenetic analysis of strains of feline calicivirus in Beijing, China. Arch. Virol. 2021, 166, 2521–2527. [Google Scholar] [CrossRef]
- Schulz, B.S.; Hartmann, K.; Unterer, S.; Eichhorn, W.; Majzoub, M.; Homeier-Bachmann, T.; Truyen, U.; Ellenberger, C.; Huebner, J. Two outbreaks of virulent systemic feline calicivirus infection in cats in Germany. Berl. Munch. Tierarztl. Wochenschr. 2011, 124, 186–193. [Google Scholar]
- Caringella, F.; Elia, G.; Decaro, N.; Martella, V.; Lanave, G.; Varello, K.; Catella, C.; Diakoudi, G.; Carelli, G.; Colaianni, M.L.; et al. Feline calicivirus infection in cats with virulent systemic disease, Italy. Res. Vet. Sci. 2019, 124, 46–51. [Google Scholar] [CrossRef]
- Harrison, T.M.; Sikarskie, J.; Kruger, J.; Wise, A.; Mullaney, T.P.; Kiupel, M.; Maes, R.K. Systemic calicivirus epidemic in captive exotic felids. J. Zoo Wildl. Med. 2007, 38, 292–299. [Google Scholar] [CrossRef]
- Foley, J.; Hurley, K.; Pesavento, P.A.; Poland, A.; Pedersen, N.C. Virulent systemic feline calicivirus infection: Local cytokine modulation and contribution of viral mutants. J. Feline Med. Surg. 2006, 8, 55–61. [Google Scholar] [CrossRef]
- Klose, T.C.; MacPhail, C.M.; Schultheiss, P.C.; Rosychuk, R.A.; Hawley, J.R.; Lappin, M.R. Prevalence of select infectious agents in inflammatory aural and nasopharyngeal polyps from client-owned cats. J. Feline Med. Surg. 2010, 12, 769–774. [Google Scholar] [CrossRef]
- Larson, J.; Kruger, J.M.; Wise, A.G.; Kaneene, J.B.; Miller, R.; Fitzgerald, S.D.; Kiupel, M.; Maes, R.K. Nested case-control study of feline calicivirus viruria, oral carriage, and serum neutralizing antibodies in cats with idiopathic cystitis. J. Vet. Intern. Med. 2011, 25, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, B.; Lanave, G.; Di Profio, F.; Melegari, I.; Marsilio, F.; Camero, M.; Catella, C.; Capozza, P.; Banyai, K.; Barrs, V.R.; et al. Identification of feline calicivirus in cats with enteritis. Transbound. Emerg. Dis. 2020, 67, 2579–2588. [Google Scholar] [CrossRef]
- Mochizuki, M. Different stabilities to bile among feline calicivirus strains of respiratory and enteric origin. Vet. Microbiol. 1992, 31, 297–302. [Google Scholar] [CrossRef]
- Ruch-Gallie, R.A.; Veir, J.K.; Hawley, J.R.; Lappin, M.R. Results of molecular diagnostic assays targeting feline herpesvirus-1 and feline calicivirus in adult cats administered modified live vaccines. J. Feline Med. Surg. 2011, 13, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Sykes, J.E.; Studdert, V.P.; Browning, G.F. Detection and strain differentiation of feline calicivirus in conjunctival swabs by RT-PCR of the hypervariable region of the capsid protein gene. Arch. Virol. 1998, 143, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Helps, C.; Lait, P.; Tasker, S.; Harbour, D. Melting curve analysis of feline calicivirus isolates detected by real-time reverse transcription PCR. J. Virol. Methods 2002, 106, 241–244. [Google Scholar] [CrossRef]
- Scansen, B.A.; Wise, A.G.; Kruger, J.M.; Venta, P.J.; Maes, R.K. Evaluation of a p30 gene-based real-time reverse transcriptase polymerase chain reaction assay for detection of feline caliciviruses. J. Vet. Intern. Med. 2004, 18, 135–138. [Google Scholar] [CrossRef]
- Wilhelm, S.; Truyen, U. Real-time reverse transcription polymerase chain reaction assay to detect a broad range of feline calicivirus isolates. J. Virol. Methods 2006, 133, 105–108. [Google Scholar] [CrossRef]
- Meli, M.L.; Berger, A.; Willi, B.; Spiri, A.M.; Riond, B.; Hofmann-Lehmann, R. Molecular detection of feline calicivirus in clinical samples: A study comparing its detection by RT-qPCR directly from swabs and after virus isolation. J. Virol. Methods 2018, 251, 54–60. [Google Scholar] [CrossRef]
- Sykes, J.E.; Allen, J.L.; Studdert, V.P.; Browning, G.F. Detection of feline calicivirus, feline herpesvirus 1 and Chlamydia psittaci mucosal swabs by multiplex RT-PCR/PCR. Vet. Microbiol. 2001, 81, 95–108. [Google Scholar] [CrossRef]
- Schulz, C.; Hartmann, K.; Mueller, R.S.; Helps, C.; Schulz, B.S. Sampling sites for detection of feline herpesvirus-1, feline calicivirus and Chlamydia felis in cats with feline upper respiratory tract disease. J. Feline Med. Surg. 2015, 17, 1012–1019. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zheng, Y.; Yang, Y.; Xu, X.; Kang, H.; Jiang, Q.; Yang, M.; Qu, L.; Liu, J. Establishment and application of ERA-LFD method for rapid detection of feline calicivirus. Appl. Microbiol. Biotechnol. 2022, 106, 1651–1661. [Google Scholar] [CrossRef]
- Pedersen, N.C. Feline calicivirus. In Virus Infections of Carnivores; Appel, M.J., Ed.; Elsevier Science Publishers BV: New York, NY, USA, 1987; pp. 339–346. [Google Scholar]
- Gaskell, R.; Dawson, S. Feline respiratory disease. In Infectious Diseases of the Dog and Cat; Greene, C.E., Ed.; WB Saunders Company: Philadelphia, PA, USA, 1998; pp. 97–106. [Google Scholar]
- Marsilio, F.; Di Martino, B.; Decaro, N.; Buonavoglia, C. A novel nested PCR for the diagnosis of calicivirus infections in the cat. Vet. Microbiol. 2005, 105, 1–7. [Google Scholar] [CrossRef]
- Lappin, M.R.; Andrews, J.; Simpson, D.; Jensen, W.A. Use of serologic tests to predict resistance to feline herpesvirus 1, feline calicivirus, and feline parvovirus infection in cats. J. Am. Vet. Med. Assoc. 2002, 220, 38–42. [Google Scholar] [CrossRef]
- Bergmann, M.; Speck, S.; Rieger, A.; Truyen, U.; Hartmann, K. Antibody response to feline calicivirus vaccination in healthy adult cats. Viruses 2019, 11, 702. [Google Scholar] [CrossRef] [Green Version]
- Hellard, E.; Fouchet, D.; Santin-Janin, H.; Tarin, B.; Badol, V.; Coupier, C.; Leblanc, G.; Poulet, H.; Pontier, D. When cats’ ways of life interact with their viruses: A study in 15 natural populations of owned and unowned cats (Felis silvestris catus). Prev. Vet. Med. 2011, 101, 250–264. [Google Scholar] [CrossRef]
- Scott, F.W.; Geissinger, C.M. Long-term immunity in cats vaccinated with an inactivated trivalent vaccine. Am. J. Vet. Res. 1999, 60, 652–658. [Google Scholar]
- Gore, T.C.; Lakshmanan, N.; Williams, J.R.; Jirjis, F.F.; Chester, S.T.; Duncan, K.L.; Coyne, M.J.; Lum, M.A.; Sterner, F.J. Three-year duration of immunity in cats following vaccination against feline rhinotracheitis virus, feline calicivirus, and feline panleukopenia virus. Vet. Ther. 2006, 7, 213–222. [Google Scholar]
- Scott, F.W.; Geissinger, C.M. Duration of immunity in cats vaccinated with an inactivated feline panleukopenia, herpesvirus and calicivirus vaccine. Feline Pract. 1997, 25, 12–19. [Google Scholar]
- Abd-Eldaim, M.; Potgieter, L.; Kennedy, M. Genetic analysis of feline caliciviruses associated with a hemorrhagic-like disease. J. Vet. Diagn. Investig. 2005, 17, 420–429. [Google Scholar] [CrossRef] [Green Version]
- Brunet, S.; Sigoillot-Claude, C.; Pialot, D.; Poulet, H. Multiple correspondence analysis on amino acid properties within the variable region of the capsid protein shows differences between classical and virulent systemic feline calicivirus strains. Viruses 2019, 11, 1090. [Google Scholar] [CrossRef] [Green Version]
- Lappin, M.R.; Blondeau, J.; Boothe, D.; Breitschwerdt, E.B.; Guardabassi, L.; Lloyd, D.H.; Papich, M.G.; Rankin, S.C.; Sykes, J.E.; Turnidge, J.; et al. Antimicrobial use guidelines for treatment of respiratory tract disease in dogs and cats: Antimicrobial guidelines working group of the international society for companion animal infectious diseases. J. Vet. Intern. Med. 2017, 31, 279–294. [Google Scholar] [CrossRef]
- Li, D.; Cui, Z.; Li, G.; Zhang, L.; Zhang, Y.; Zhao, H.; Zhang, S.; Guo, Y.; Zhao, Y.; Men, F.; et al. Antiviral effect of copper chloride on feline calicivirus and synergy with ribavirin in vitro. BMC Vet. Res. 2020, 16, 231. [Google Scholar] [CrossRef]
- Povey, R.C. Effect of orally administered ribavirin on experimental feline calicivirus infection in cats. Am. J. Vet. Res. 1978, 39, 1337–1341. [Google Scholar] [PubMed]
- Synowiec, A.; Gryniuk, I.; Pachota, M.; Strzelec, L.; Roman, O.; Klysik-Trzcianska, K.; Zajac, M.; Drebot, I.; Gula, K.; Andruchowicz, A.; et al. Cat flu: Broad spectrum polymeric antivirals. Antivir. Res. 2019, 170, 104563. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, P.; Sheehy, P.A.; Fawcett, A.; Norris, J.M. Antiviral effect of mefloquine on feline calicivirus in vitro. Vet. Microbiol. 2015, 176, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Tuipulotu, D.E.; Fumian, T.M.; Netzler, N.E.; Mackenzie, J.M.; White, P.A. The adenosine analogue NITD008 has potent antiviral activity against human and animal caliciviruses. Viruses 2019, 11, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Z.; Wang, Q.; Li, D.; Zhao, S.; Zhang, Q.; Tan, Y.; Gong, Q.; Liu, T.; Shao, J.; Zhang, S.; et al. Icariin, formononetin and caffeic acid phenethyl ester inhibit feline calicivirus replication in vitro. Arch. Virol. 2021, 166, 2443–2450. [Google Scholar] [CrossRef]
- Cui, Z.; Li, D.; Yi, S.; Guo, Y.; Dong, G.; Niu, J.; Zhao, H.; Zhang, Y.; Zhang, S.; Cao, L.; et al. Equine immunoglobulin F(ab’)2 fragments protect cats against feline calicivirus infection. Int. Immunopharmacol. 2019, 75, 105714. [Google Scholar] [CrossRef]
- Fulton, R.W.; Burge, L.J. Susceptibility of feline herpesvirus 1 and a feline calicivirus to feline interferon and recombinant human leukocyte interferons. Antimicrob. Agents Chemother. 1985, 28, 698–699. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, M.; Nakatani, H.; Yoshida, M. Inhibitory effects of recombinant feline interferon on the replication of feline enteropathogenic viruses in vitro. Vet. Microbiol. 1994, 39, 145–152. [Google Scholar] [CrossRef]
- Taira, O.; Suzuki, M.; Takeuchi, Y.; Aramaki, Y.; Sakurai, I.; Watanabe, T.; Motokawa, K.; Arai, S.; Sato, H.; Maehara, N. Expression of feline interferon-alpha subtypes in Esherichia coli, and their antiviral activity and animal species specificity. J. Vet. Med. Sci. 2005, 67, 543–545. [Google Scholar] [CrossRef] [Green Version]
- Ohe, K.; Takahashi, T.; Hara, D.; Hara, M. Sensitivity of FCV to recombinant feline interferon (rFeIFN). Vet. Res. Commun. 2008, 32, 167–174. [Google Scholar] [CrossRef]
- Cui, Z.; Li, D.; Xie, Y.; Wang, K.; Zhang, Y.; Li, G.; Zhang, Q.; Chen, X.; Teng, Y.; Zhao, S.; et al. Nitazoxanide protects cats from feline calicivirus infection and acts synergistically with mizoribine in vitro. Antiviral Res. 2020, 182, 104827. [Google Scholar] [CrossRef]
- Vercelli, A.; Raviri, G.; Cornegliani, L. The use of oral cyclosporin to treat feline dermatoses: A retrospective analysis of 23 cases. Vet. Dermatol. 2006, 17, 201–206. [Google Scholar] [CrossRef]
- Hennet, P. Results of periodontal and extraction treatment in cats with gingivostomatitis. In Proceedings of the World Veterinary Dental Congress, Guarujá, SP, Brazil, 25−27 April 2007; p. 49. [Google Scholar]
- Arzi, B.; Clark, K.C.; Sundaram, A.; Spriet, M.; Verstraete, F.J.M.; Walker, N.J.; Loscar, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; et al. Therapeutic efficacy of fresh, allogeneic mesenchymal stem cells for severe refractory feline chronic gingivostomatitis. Stem Cells Transl. Med. 2017, 6, 1710–1722. [Google Scholar] [CrossRef]
- Arzi, B.; Mills-Ko, E.; Verstraete, F.J.; Kol, A.; Walker, N.J.; Badgley, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; Borjesson, D.L. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl. Med. 2016, 5, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Southerden, P.; Gorrel, C. Treatment of a case of refractory feline chronic gingivostomatitis with feline recombinant interferon omega. J. Small Anim. Pr. 2007, 48, 104–106. [Google Scholar] [CrossRef]
- Hennet, P.R.; Camy, G.A.; McGahie, D.M.; Albouy, M.V. Comparative efficacy of a recombinant feline interferon omega in refractory cases of calicivirus-positive cats with caudal stomatitis: A randomised, multi-centre, controlled, double-blind study in 39 cats. J. Feline Med. Surg. 2011, 13, 577–587. [Google Scholar] [CrossRef]
- Matsumoto, H.; Teshima, T.; Iizuka, Y.; Sakusabe, A.; Takahashi, D.; Amimoto, A.; Koyama, H. Evaluation of the efficacy of the subcutaneous low recombinant feline interferon-omega administration protocol for feline chronic gingivitis-stomatitis in feline calicivirus-positive cats. Res. Vet. Sci. 2018, 121, 53–58. [Google Scholar] [CrossRef]
- Leal, R.O.; Gil, S.; Brito, M.T.; McGahie, D.; Niza, M.M.; Tavares, L. The use of oral recombinant feline interferon omega in two cats with type II diabetes mellitus and concurrent feline chronic gingivostomatitis syndrome. Ir. Vet. J. 2013, 66, 19. [Google Scholar] [CrossRef] [Green Version]
- Chadwin, R.M.; Bain, M.J.; Kass, P.H. Effect of a synthetic feline facial pheromone product on stress scores and incidence of upper respiratory tract infection in shelter cats. J. Am. Vet. Med. Assoc. 2017, 251, 413–420. [Google Scholar] [CrossRef] [Green Version]
- ABCD. European Advisory Board for Cat Diseases (ABCD): Managing FCV Outbreaks in Multi-Cat Communities. Available online: http://www.abcdcatsvets.org/wp-content/uploads/2020/02/FCV-in-multi-cat-communities.pdf (accessed on 11 November 2020).
- ABCD. Vaccine Recommendations for Cats According to Their Lifestyle. Available online: http://www.abcdcatsvets.org/wp-content/uploads/2020/03/Tool_Vaccine-recommendations_Feb2020.pdf (accessed on 11 November 2020).
- Habacher, G.; Gruffydd-Jones, T.; Murray, J. Use of a web-based questionnaire to explore cat owners’ attitudes towards vaccination in cats. Vet. Rec. 2010, 167, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Gehrig, A.C.; Hartmann, K.; Gunther, F.; Klima, A.; Habacher, G.; Bergmann, M. A survey of vaccine history in German cats and owners’ attitudes to vaccination. J. Feline Med. Surg. 2018, 21, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.D.; Dawson, S.; Coyne, K.P.; Porter, C.J.; Gaskell, R.M. The challenge for the next generation of feline calicivirus vaccines. Vet. Microbiol. 2006, 117, 14–18. [Google Scholar] [CrossRef]
- Lappin, M.R.; Jensen, W.A.; Jensen, T.D.; Basaraba, R.J.; Brown, C.A.; Radecki, S.V.; Hawley, J.R. Investigation of the induction of antibodies against Crandell-Rees feline kidney cell lysates and feline renal cell lysates after parenteral administration of vaccines against feline viral rhinotracheitis, calicivirus, and panleukopenia in cats. Am. J. Vet. Res. 2005, 66, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Songaksorn, N.; Petsophonsakul, W.; Pringproa, K.; Lampang, K.N.; Sthitmatee, N.; Srifawattana, N.; Piyarungsri, K.; Thongkorn, K. Prevalence of autoantibodies that bind to kidney tissues in cats and association risk with antibodies to feline viral rhinotracheitis, calicivirus, and panleukopenia. J. Vet. Sci. 2021, 22, e38. [Google Scholar] [CrossRef]
- Jas, D.; Aeberle, C.; Lacombe, V.; Guiot, A.L.; Poulet, H. Onset of immunity in kittens after vaccination with a non-adjuvanted vaccine against feline panleucopenia, feline calicivirus and feline herpesvirus. Vet. J. 2009, 182, 86–93. [Google Scholar] [CrossRef]
- Jas, D.; Frances-Duvert, V.; Vernes, D.; Guigal, P.M.; Poulet, H. Three-year duration of immunity for feline herpesvirus and calicivirus evaluated in a controlled vaccination-challenge laboratory trial. Vet. Microbiol. 2015, 177, 123–131. [Google Scholar] [CrossRef]
- Almeras, T.; Schreiber, P.; Fournel, S.; Martin, V.; Nicolas, C.S.; Fontaine, C.; Lesbros, C.; Gueguen, S. Comparative efficacy of the Leucofeligen™ FeLV/RCP and Purevax™ RCP FeLV vaccines against infection with circulating feline Calicivirus. BMC Vet. Res. 2017, 13, 300. [Google Scholar] [CrossRef] [Green Version]
- Dawson, S.; Smyth, N.R.; Bennett, M.; Gaskell, R.M.; McCracken, C.M.; Brown, A.; Gaskell, C.J. Effect of primary-stage feline immunodeficiency virus infection on subsequent feline calicivirus vaccination and challenge in cats. Acquir. Immune Defic. Syndr. 1991, 5, 747–750. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Hawkins, K.F. Mechanisms for persistence of acute and chronic feline calicivirus infections in the face of vaccination. Vet. Microbiol. 1995, 47, 141–156. [Google Scholar] [CrossRef]
- Radford, A.D.; Bennett, M.; McArdle, F.; Dawson, S.; Turner, P.C.; Glenn, M.A.; Gaskell, R.M. The use of sequence analysis of a feline calicivirus (FCV) hypervariable region in the epidemiological investigation of FCV related disease and vaccine failures. Vaccine 1997, 15, 1451–1458. [Google Scholar] [CrossRef]
- Dawson, S.; McArdle, F.; Bennett, M.; Carter, M.; Milton, I.P.; Turner, P.; Meanger, J.; Gaskell, R.M. Typing of feline calicivirus isolates from different clinical groups by virus neutralisation tests. Vet. Rec. 1993, 133, 13–17. [Google Scholar] [CrossRef]
- Jas, D.; Frances-Duvert, V.; Brunet, S.; Oberli, F.; Guigal, P.M.; Poulet, H. Evaluation of safety and immunogenicity of feline vaccines with reduced volume. Vaccine 2021, 39, 1051–1057. [Google Scholar] [CrossRef]
- De Groot-Mijnes, J.D.; van der Most, R.G.; van Dun, J.M.; Lintelo, E.G.T.; Schuurman, N.M.; Egberink, H.F.; de Groot, R.J. Three-color flow cytometry detection of virus-specific CD4+ and CD8+ T cells in the cat. J. Immunol. Methods 2004, 285, 41–54. [Google Scholar] [CrossRef]
- Huang, C.; Hess, J.; Gill, M.; Hustead, D. A dual-strain feline calicivirus vaccine stimulates broader cross-neutralization antibodies than a single-strain vaccine and lessens clinical signs in vaccinated cats when challenged with a homologous feline calicivirus strain associated with virulent systemic disease. J. Feline Med. Surg. 2010, 12, 129–137. [Google Scholar] [CrossRef]
- Masubuchi, K.; Wakatsuki, A.; Iwamoto, K.; Takahashi, T.; Kokubu, T.; Shimizu, M. Immunological and genetic characterization of feline caliciviruses used in the development of a new trivalent inactivated vaccine in Japan. J. Vet. Med. Sci. 2010, 72, 1189–1194. [Google Scholar] [CrossRef] [Green Version]
- Lauritzen, A.; Jarrett, O.; Sabara, M. Serological analysis of feline calicivirus isolates from the United States and United Kingdom. Vet. Microbiol. 1997, 56, 55–63. [Google Scholar] [CrossRef]
- Afonso, M.M.; Pinchbeck, G.L.; Smith, S.L.; Daly, J.M.; Gaskell, R.M.; Dawson, S.; Radford, A.D. A multi-national European cross-sectional study of feline calicivirus epidemiology, diversity and vaccine cross-reactivity. Vaccine 2017, 35, 2753–2760. [Google Scholar] [CrossRef]
- Porter, C.J.; Radford, A.D.; Gaskell, R.M.; Ryvar, R.; Coyne, K.P.; Pinchbeck, G.L.; Dawson, S. Comparison of the ability of feline calicivirus (FCV) vaccines to neutralise a panel of current UK FCV isolates. J. Feline Med. Surg. 2008, 10, 32–40. [Google Scholar] [CrossRef]
- Wensman, J.J.; Samman, A.; Lindhe, A.; Thibault, J.C.; Berndtsson, L.T.; Hosie, M.J. Ability of vaccine strain induced antibodies to neutralize field isolates of caliciviruses from Swedish cats. Acta Vet. Scand. 2015, 57, 86. [Google Scholar] [CrossRef] [Green Version]
- Addie, D.; Poulet, H.; Golder, M.C.; Mcdonald, M.; Brunet, S.; Thibault, J.C.; Hosie, M.J. Ability of antibodies to two new caliciviral vaccine strains to neutralise feline calicivirus isolates from the UK. Vet. Rec. 2008, 163, 355–357. [Google Scholar] [CrossRef]
- Poulet, H.; Jas, D.; Lemeter, C.; Coupier, C.; Brunet, S. Efficacy of a bivalent inactivated non-adjuvanted feline calicivirus vaccine: Relation between in vitro cross-neutralization and heterologous protection in vivo. Vaccine 2008, 26, 3647–3654. [Google Scholar] [CrossRef]
- Hou, J.; Sanchez-Vizcaino, F.; McGahie, D.; Lesbros, C.; Almeras, T.; Howarth, D.; O’Hara, V.; Dawson, S.; Radford, A.D. European molecular epidemiology and strain diversity of feline calicivirus. Vet. Rec. 2016, 178, 114–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiri, A.M.; Riond, B.; Stirn, M.; Novacco, M.; Meli, M.L.; Boretti, F.S.; Herbert, I.; Hosie, M.J.; Hofmann-Lehmann, R. Modified-live feline calicivirus vaccination reduces viral RNA loads, duration of RNAemia, and the severity of clinical signs after heterologous feline calicivirus challenge. Viruses 2021, 13, 1505. [Google Scholar] [CrossRef] [PubMed]
- Brunet, S.; Jas, D.; David, F.; Bublot, M.; Poulet, H. Feline calicivirus: Vaccinations against virulent strains. In Proceedings of the Conference of the European Society of Veterinary Virology 2005: Comparative and Emerging Virus Infections of Dogs and Cats, Liverpool, UK, 2–4 May 2005. [Google Scholar]
- Poulet, H. Alternative early life vaccination programs for companion animals. J. Comp. Pathol. 2007, 137, S67–S71. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.; Skura, B.; Petric, M.; McIntyre, L.; Gamage, B.; Isaac-Renton, J. Efficacy of common disinfectant/cleaning agents in inactivating murine norovirus and feline calicivirus as surrogate viruses for human norovirus. Am. J. Infect. Control 2015, 43, 1208–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleraky, N.Z.; Potgieter, L.N.; Kennedy, M.A. Virucidal efficacy of four new disinfectants. J. Am. Anim. Hosp. Assoc. 2002, 38, 231–234. [Google Scholar] [CrossRef]
- ABCD. Disinfectant Choice in Feline Veterinary Hospitals, Shelters and Cat Households. Available online: http://www.abcdcatsvets.org/disinfectants/ (accessed on 19 April 2022).
- UCDavis. UCDavis Koret Shelter Medicine Program Resources. Available online: https://www.sheltermedicine.com/library/resources/?r=feline-calicivirus-virulent-systemic-feline-calicivirus-vs-fcv (accessed on 19 April 2022).
- Radford, A.D.; Addie, D.; Belak, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline calicivirus infection. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 556–564. [Google Scholar] [CrossRef]






| Primary Vaccination 1 | Primary Vaccination 2 | Primary Vaccination 3 | Final Primary Vaccination | Boosters | |
|---|---|---|---|---|---|
| Outdoor cats and indoor-only cats1 | |||||
| Kittens (age) | 8 to 9 weeks | 12 weeks | 16 weeks | 10 to 16 months | Annually (or up to every 3 years in low-risk situations) |
| Adult cat vaccinated <3 years ago | One immunisation | - | - | - | Annually (or up to every 3 years in low-risk situations) |
| Adult cat vaccinated ≥3 years ago | First immunisation | Second immunisation 2 to 4 weeks later | - | - | Annually (or up to every 3 years in low-risk situations) |
| Adult cat with no/unknown vaccination history | First immunisation | Second immunisation 2 to 4 weeks later | - | - | Annually (or up to every 3 years in low-risk situations) |
| Rescue shelter cats1,2 | |||||
| Kittens (age) | 6 weeks | 3 to 4 weeks later | 3 to 4 weeks later until 16 weeks | 10 to 16 months | Annually |
| Adult cat vaccinated <3 years ago | One immunisation | - | - | - | Annually |
| Adult cat vaccinated ≥3 years ago | First immunisation | Second immunisation 2 to 4 weeks later | - | - | Annually |
| Adult cat with no/unknown vaccination history | First immunisation | Second immunisation 2 to 4 weeks later | - | - | Annually |
| Breeding cats1 | |||||
| Kittens (age) 3 | 8 to 9 weeks | 12 weeks | 16 weeks | 10 to 16 months | Annually |
| Queens | - | - | - | - | Annually and before breeding if low MDA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann-Lehmann, R.; Hosie, M.J.; Hartmann, K.; Egberink, H.; Truyen, U.; Tasker, S.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; et al. Calicivirus Infection in Cats. Viruses 2022, 14, 937. https://doi.org/10.3390/v14050937
Hofmann-Lehmann R, Hosie MJ, Hartmann K, Egberink H, Truyen U, Tasker S, Belák S, Boucraut-Baralon C, Frymus T, Lloret A, et al. Calicivirus Infection in Cats. Viruses. 2022; 14(5):937. https://doi.org/10.3390/v14050937
Chicago/Turabian StyleHofmann-Lehmann, Regina, Margaret J. Hosie, Katrin Hartmann, Herman Egberink, Uwe Truyen, Séverine Tasker, Sándor Belák, Corine Boucraut-Baralon, Tadeusz Frymus, Albert Lloret, and et al. 2022. "Calicivirus Infection in Cats" Viruses 14, no. 5: 937. https://doi.org/10.3390/v14050937
APA StyleHofmann-Lehmann, R., Hosie, M. J., Hartmann, K., Egberink, H., Truyen, U., Tasker, S., Belák, S., Boucraut-Baralon, C., Frymus, T., Lloret, A., Marsilio, F., Pennisi, M. G., Addie, D. D., Lutz, H., Thiry, E., Radford, A. D., & Möstl, K. (2022). Calicivirus Infection in Cats. Viruses, 14(5), 937. https://doi.org/10.3390/v14050937











