Antiviral Activities of Green Tea Components against Grouper Iridovirus Infection In Vitro and In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish, Cells, Virus, and Reagents
2.2. Preparation of GTAE
2.3. Analysis of the Safe Working Concentrations of Green Tea Components with a Cell Viability Assay
2.4. Total RNA Extraction and Reverse Transcription (RT) Quantitative PCR (qPCR) Analysis
2.5. Monitoring SGIV Infection with an Aptamer (Q5c)-Based Fluorescent Molecular Probe (Q5c-AFMP)
2.6. Inhibitory Percentage of Green Tea Components against SGIV Infection
2.7. Antiviral Activity of Green Tea Components against SGIV Infection In Vitro
2.8. Antiviral Activity of Green Tea Components against SGIV Infection In Vivo
2.9. Statistical Analysis
3. Results
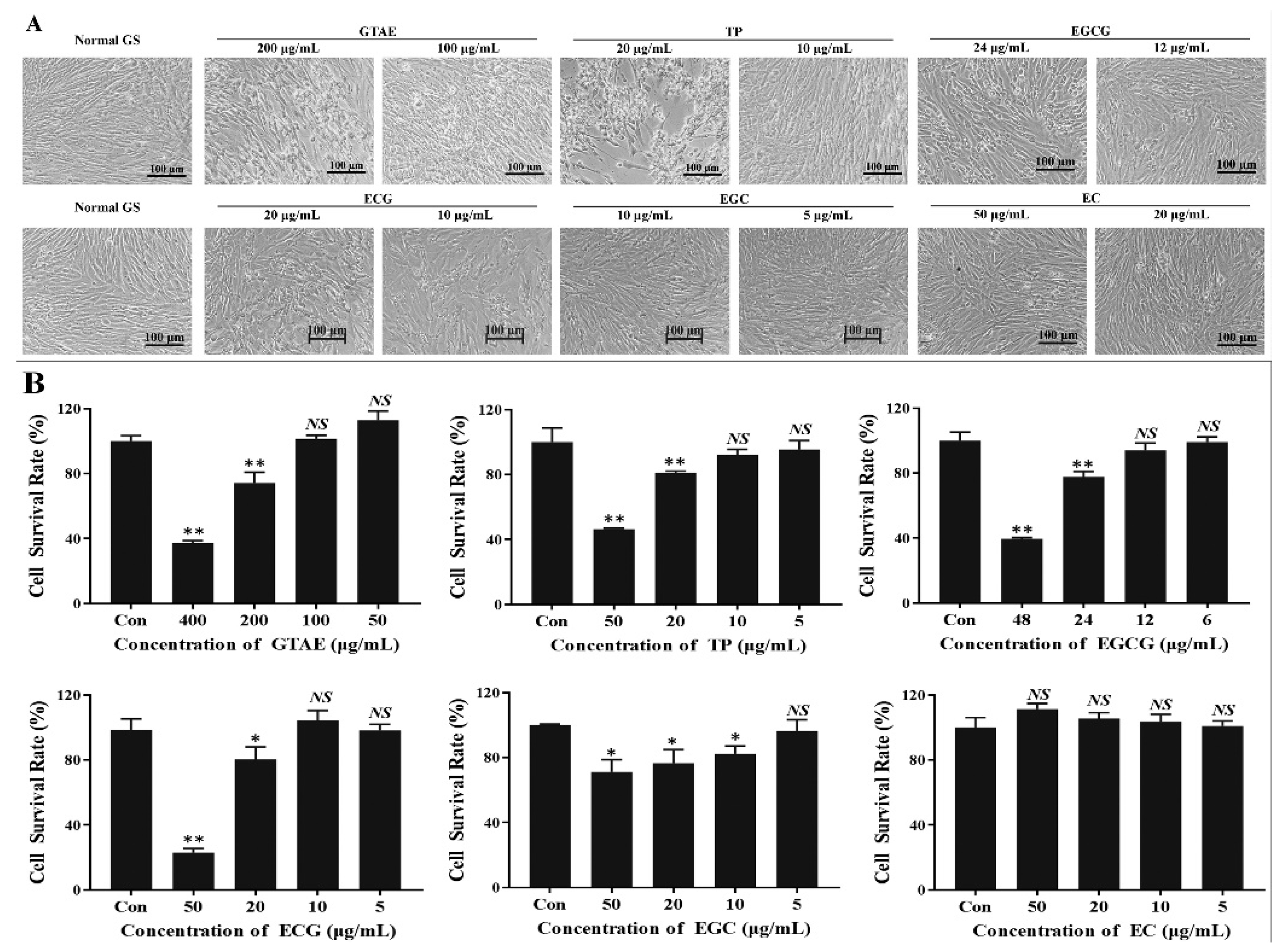
3.1. Safe Working Concentrations of Green Tea Components
3.2. Preliminary Screening of the Antiviral Effects of Green Tea Components In Vitro
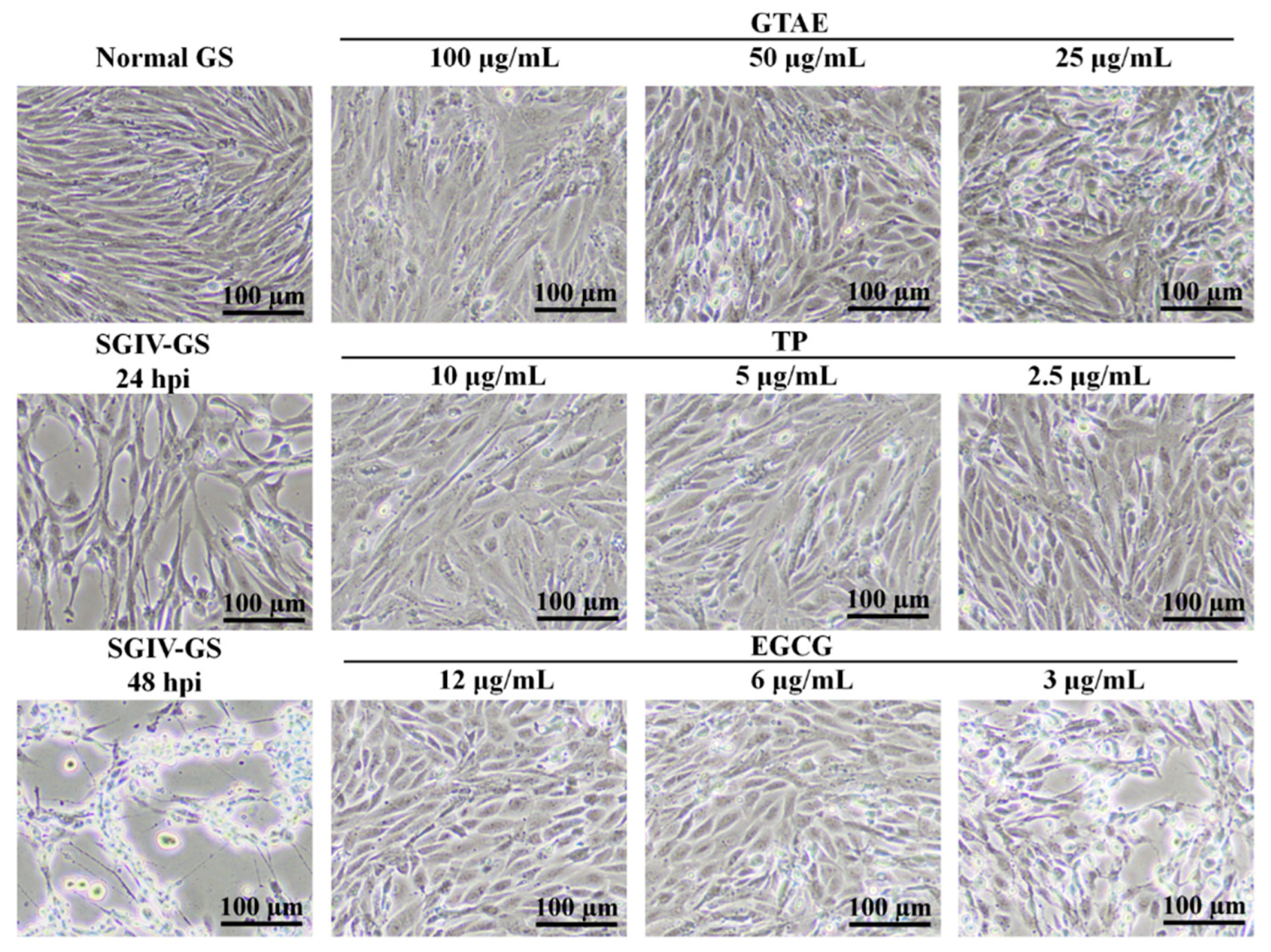
3.3. Antiviral Activity of Green Tea Components In Vitro
3.3.1. Antiviral Activity Analysis with Light Microscopy
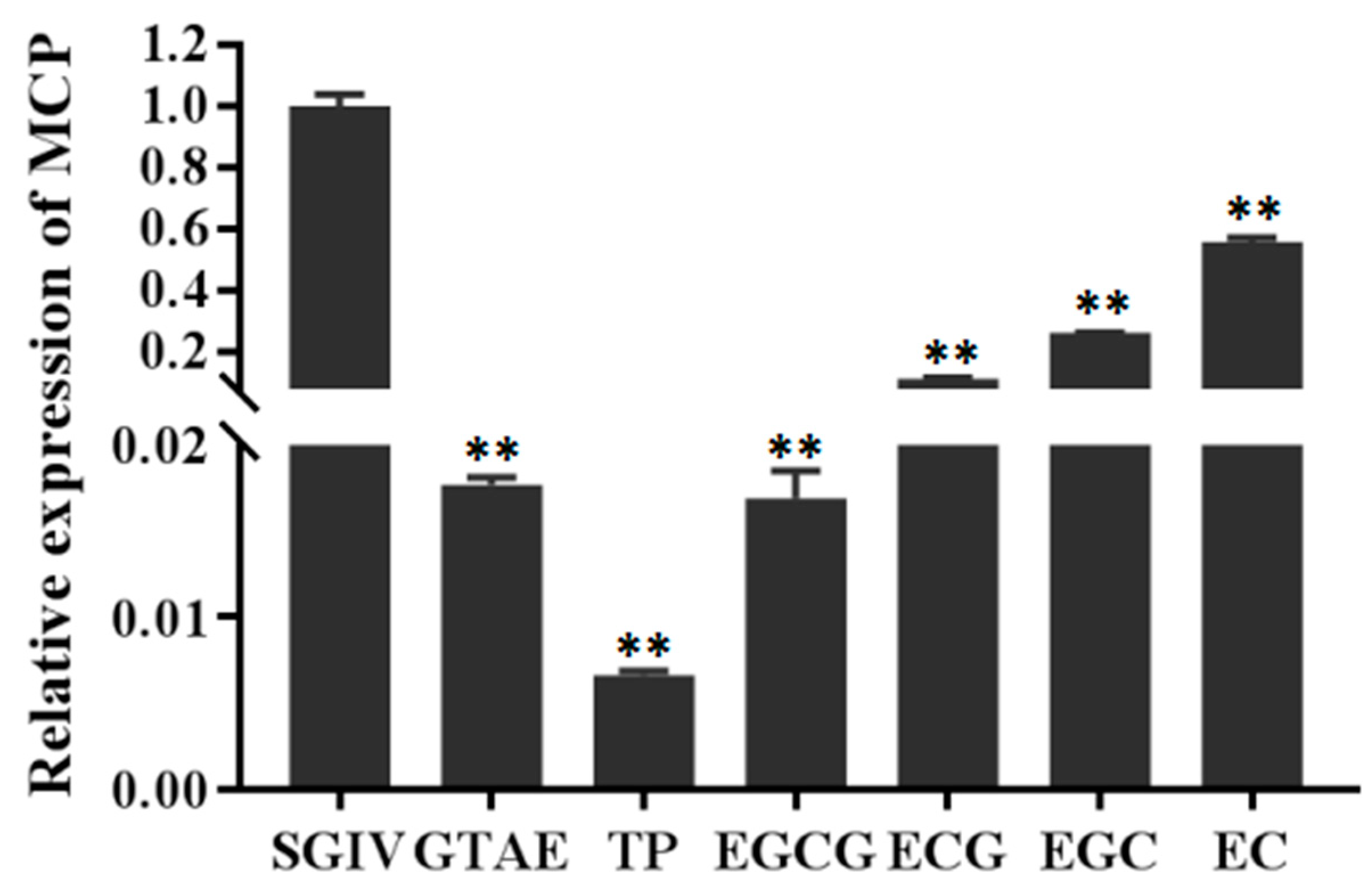
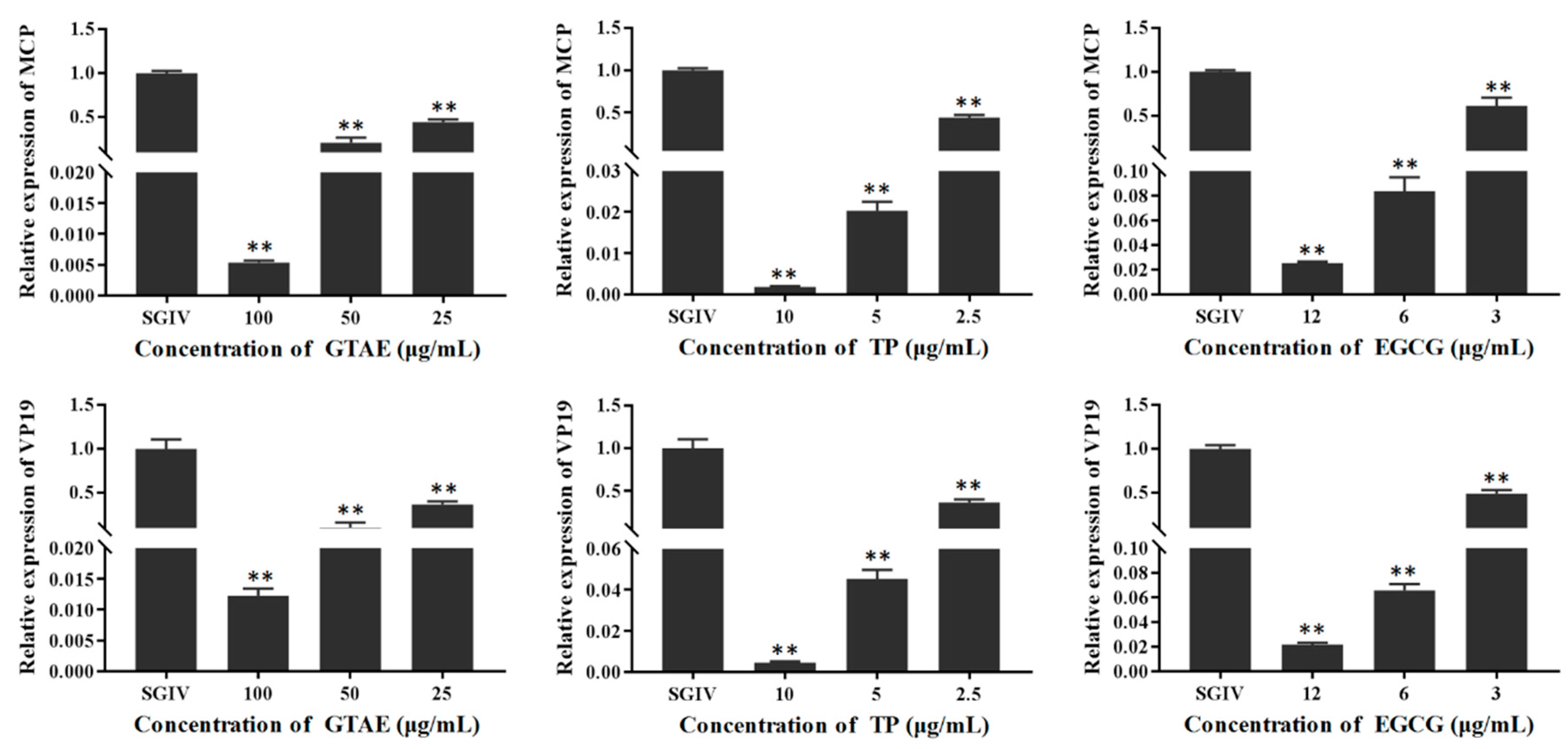
3.3.2. Antiviral Activity Analysis with qPCR
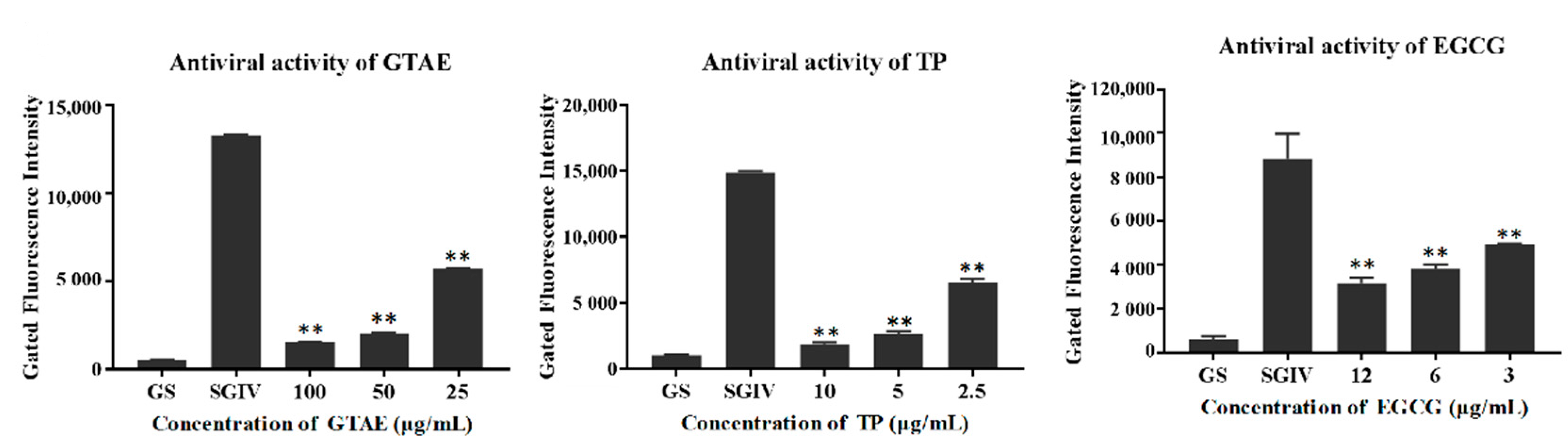
3.3.3. Antiviral Activity Analysis with Q5c-AFMP
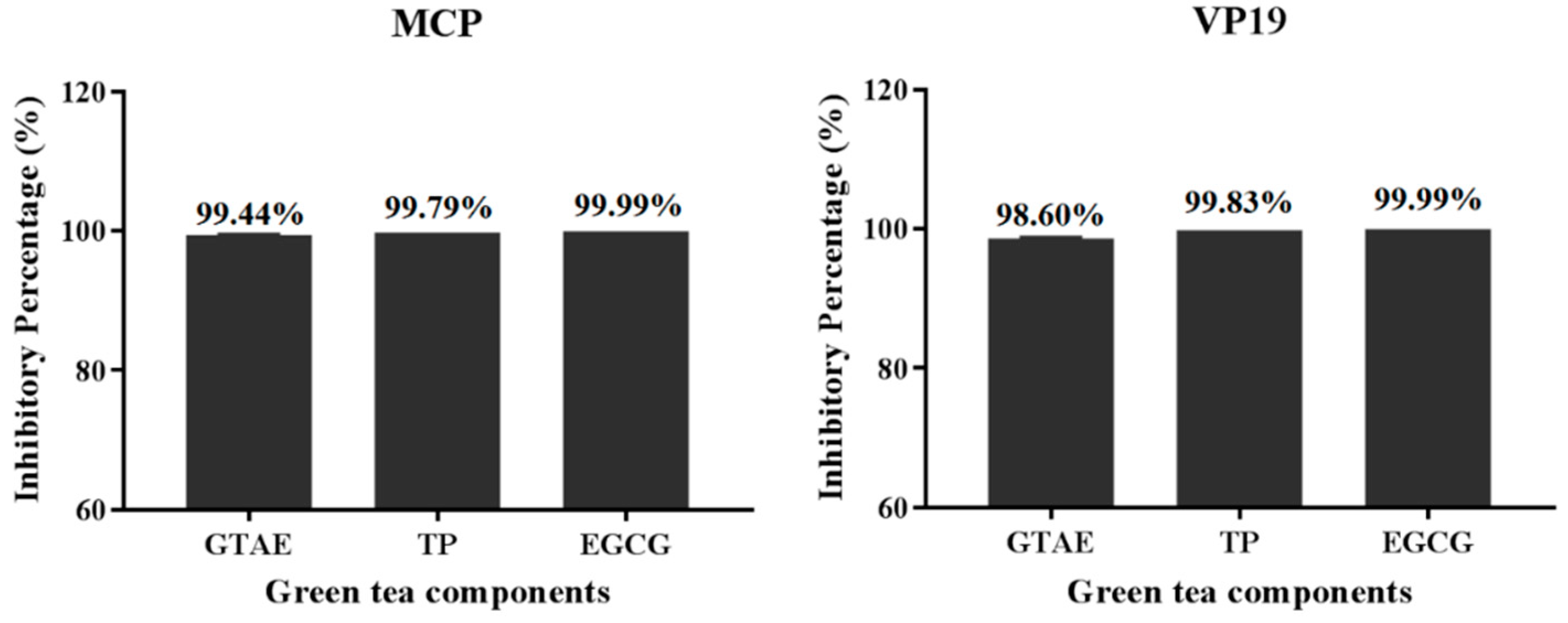
3.4. Percentage Inhibition of SGIV Infection by Each Green Tea Component
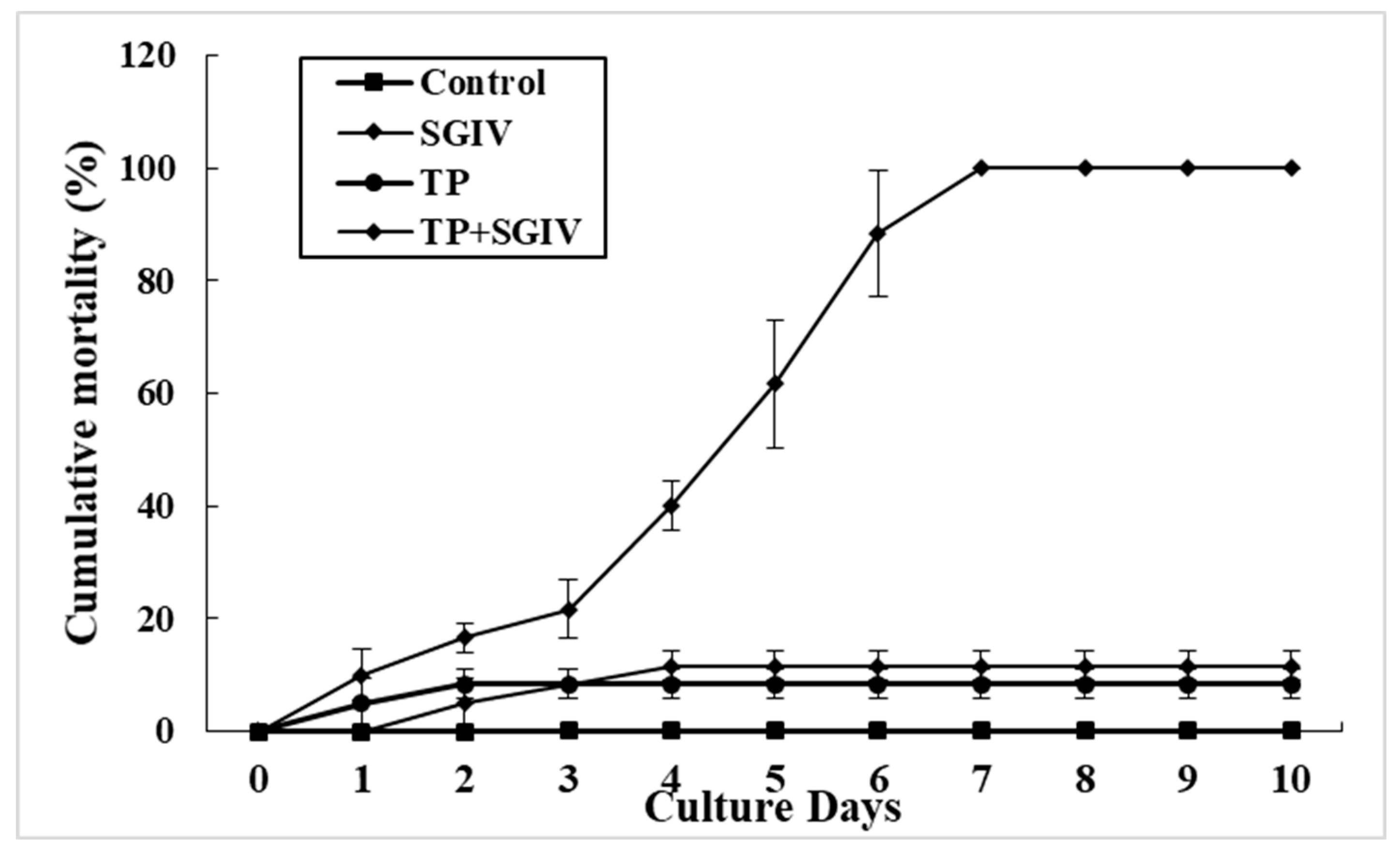
3.5. Antiviral Activity of TP against SGIV In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, Q.W.; Wu, T.H.; Jia, T.L.; Hegde, A.; Zhang, R.Q. Development and characterization of a new tropical marine fish cell line from grouper, Epinephelus coioides susceptible to iridovirus and nodavirus. J. Virol. Methods 2006, 131, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, S.; Zhou, L.; Yang, M.; Yu, Y.; Wei, J.; Jiang, G.; Qin, Q. Selection and characterization of novel DNA aptamers specifically recognized by Singapore grouper iridovirus-infected fish cells. J. Gen. Virol. 2015, 96, 3348–3359. [Google Scholar] [PubMed]
- Li, P.; Yu, Q.; Li, F.; Qin, X.; Dong, D.; Chen, B.; Qin, Q. First identification of the nervous necrosis virus isolated from cultured golden pompano (Trachinotus ovatus) in Guangxi, China. J. Fish Dis. 2018, 41, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Liu, M.Z.; Wei, S.N.; Xiao, H.H.; Wu, S.T.; Qin, X.L.; Shi, D.Q.; Li, S.Q.; Wang, T.X.; Li, P.F. Isolation of Nervous Necrosis Virus from Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂) Cultured in Guangxi, China. Fish Pathol. 2019, 54, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Zhou, S.Y.; Chen, C.; Weng, S.P.; Chan, S.M.; He, J.G. Complete genome sequence analysis of an iridovirus isolated from the orange-spotted grouper, Epinephelus coioides. Virology 2005, 339, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Huang, Y.; Xu, L.; Wei, S.; Ouyang, Z.; Feng, J.; Qin, Q. Identification and characterization of a novel lymphocystis disease virus isolate from cultured grouper in China. J. Fish Dis. 2015, 38, 379–387. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q.W.; Chang, S.F.; Ngoh-Lim, G.H.; Gibson-Kueh, S.; Shi, C.; Lam, T.J. Characterization of a novel ranavirus isolated from grouper Epinephelus tauvina. Dis. Aquat. Org. 2003, 53, 1–9. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, M.; Xiao, H.; Wu, S.; Qin, X.; Lu, Z.; Shi, D.; Li, S.; Mi, H.; Wang, Y.; et al. The inhibitory activities and antiviral mechanism of Viola philippica aqueous extracts against grouper iridovirus infection in vitro and in vivo. J. Fish Dis. 2019, 42, 859–868. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Khokhar, S.; Venema, D.; Hollman, P.; Dekker, M.; Jongen, W. A RP-HPLC method for the determination of tea catechins. Cancer Lett. 1997, 114, 171–172. [Google Scholar] [CrossRef]
- Cabrera, C.; Giménez, R.; López, M.C. Determination of Tea Components with Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 4427–4435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The green tea molecule EGCG inhibits Zika virus entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.; Li, S.; Zhan, J.; Ho, C. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Ru, G.; Xu, Y.; Lu, L. EGCG: Potential application as a protective agent against grass carp reovirus in aquaculture. J. Fish Dis. 2018, 41, 1259–1267. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, M.; Li, S.; Shi, D.; Zhu, D.; Ke, K.; Xu, Y.; Dong, D.; Zhu, L.; Yu, Q.; et al. Isolation and Characterization of a Ranavirus Associated with Disease Outbreaks in Cultured Hybrid Grouper (female symbol Tiger Grouper Epinephelus fuscoguttatus x male symbol Giant Grouper E. lanceolatus) in Guangxi, China. J. Aquat. Anim. Health 2019, 31, 364–370. [Google Scholar] [CrossRef]
- Liu, M.; Yu, Q.; Xiao, H.; Yi, Y.; Cheng, H.; Putra, D.F.; Huang, Y.; Zhang, Q.; Li, P. Antiviral activity of Illicium verum Hook. f. extracts against grouper iridovirus infection. J. Fish Dis. 2020, 43, 531–540. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Y.; Wei, S.; Li, P.; Zhou, L.; Ni, S.; Huang, X.; Qin, Q. A tumour necrosis factor receptor-like protein encoded by Singapore grouper iridovirus modulates cell proliferation, apoptosis and viral replication. J. Gen. Virol. 2016, 97, 756–766. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yu, Q.; Yi, Y.; Xiao, H.; Putra, D.F.; Ke, K.; Zhang, Q.; Li, P. Antiviral activities of Lonicera japonica Thunb. Components against grouper iridovirus in vitro and in vivo. Aquaculture 2020, 519, 734882. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Yu, F.; Li, F.; Li, W.; Yi, M.; Jia, K. Alpha-Lipoic Acid Exerts Its Antiviral Effect against Viral Hemorrhagic Septicemia Virus (VHSV) by Promoting Upregulation of Antiviral Genes and Suppressing VHSV-Induced Oxidative Stress. Virol. Sin. 2021, 36, 1520–1531. [Google Scholar] [CrossRef]
- Li, P.; Zhou, L.; Ni, S.; Xu, M.; Yu, Y.; Cai, J.; Wei, S.; Qin, Q. Establishment and characterization of a novel cell line from the brain of golden pompano (Trachinotus ovatus). Vitr. Cell. Dev. Biol. Anim. 2016, 52, 410–418. [Google Scholar] [CrossRef]
- Zhou, L.; Li, P.; Liu, J.; Ni, S.; Yu, Y.; Yang, M.; Wei, S.; Qin, Q. Establishment and characterization of a mid-kidney cell line derived from golden pompano Trachinotus ovatus, a new cell model for virus pathogenesis and toxicology studies. Vitr. Cell. Dev. Biol. Anim. 2017, 53, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Overview of antibacterial, antitoxin, antiviral, and antifungal activities of tea flavonoids and teas. Mol. Nutr. Food Res. 2007, 51, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antiviral Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, M.; Wu, S.; Wei, X.; Xiao, H.; Yi, Y.; Cheng, H.; Wang, S.; Zhang, Q.; Qin, Q.; et al. Specific Aptamer-Based Probe for Analyzing Biomarker MCP Entry Into Singapore Grouper Iridovirus-Infected Host Cells via Clathrin-Mediated Endocytosis. Front. Microbiol. 2020, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yu, Q.; Xiao, H.; Li, M.; Huang, Y.; Zhang, Q.; Li, P. The Inhibitory Activities and Antiviral Mechanism of Medicinal Plant Ingredient Quercetin Against Grouper Iridovirus Infection. Front. Microbiol. 2020, 11, 586331. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Suzuki, K.; Toda, M.; Okubo, S.; Hara, Y.; Shimamura, T. Inhibition of the infectivity of influenza virus by tea polyphenols. Antivir. Res. 1993, 21, 289–299. [Google Scholar] [CrossRef]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.W.; Hsu, F.L.; Lin, J.Y. Inhibitory Effects of Polyphenolic Catechins from Chinese Green Tea on HIV Reverse Transcriptase Activity. J. Biomed. Sci. 1994, 1, 163–166. [Google Scholar] [CrossRef]
- Hong, M.; Cheng, L.; Liu, Y.; Wu, Z.; Zhang, P.; Zhang, X. A Natural Plant Source-Tea Polyphenols, a Potential Drug for Improving Immunity and Combating Virus. Nutrients 2022, 14, 550. [Google Scholar] [CrossRef]
- Huang, C.F.; Lin, S.S.; Liao, P.H.; Young, S.C. Yang, C.C. The Immunopharmaceutical Effects and Mechanisms of Herb Medicine. Cell. Mol. Immunol. 2008, 5, 23–31. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, S.M.; Park, B.H.; Ji, S.C.; Lee, J.; Oh, B. Effect of dietary inclusion of various sources of green tea on growth, body composition and blood chemistry of the juvenile olive flounder, Paralichthys olivaceus. Fish Physiol. Biochem. 2007, 33, 49–57. [Google Scholar] [CrossRef]
- Nootash, S.; Sheikhzadeh, N.; Baradaran, B.; Oushani, A.K.; Maleki Moghadam, M.R.; Nofouzi, K.; Monfaredan, A.; Aghebati, L.; Zare, F.; Shabanzadeh, S. Green tea (Camellia sinensis) administration induces expression of immune relevant genes and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2013, 35, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Sheikhzadeh, N.; Nofouzi, K.; Delazar, A.; Oushani, A.K. Immunomodulatory effects of decaffeinated green tea (Camellia sinensis) on the immune system of rainbow trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2011, 31, 1268–1269. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Huang, S.; Xiao, S.; Xu, Y.; Wei, X.; Xiao, J.; Guo, Z.; Yu, Q.; Liu, M. Antiviral Activities of Green Tea Components against Grouper Iridovirus Infection In Vitro and In Vivo. Viruses 2022, 14, 1227. https://doi.org/10.3390/v14061227
Li P, Huang S, Xiao S, Xu Y, Wei X, Xiao J, Guo Z, Yu Q, Liu M. Antiviral Activities of Green Tea Components against Grouper Iridovirus Infection In Vitro and In Vivo. Viruses. 2022; 14(6):1227. https://doi.org/10.3390/v14061227
Chicago/Turabian StyleLi, Pengfei, Shuaishuai Huang, Shuangyan Xiao, Youhou Xu, Xinxian Wei, Jun Xiao, Zhongbao Guo, Qing Yu, and Mingzhu Liu. 2022. "Antiviral Activities of Green Tea Components against Grouper Iridovirus Infection In Vitro and In Vivo" Viruses 14, no. 6: 1227. https://doi.org/10.3390/v14061227





