Advances in Crosstalk between Porcine Circoviruses and Host
Abstract
1. Introduction
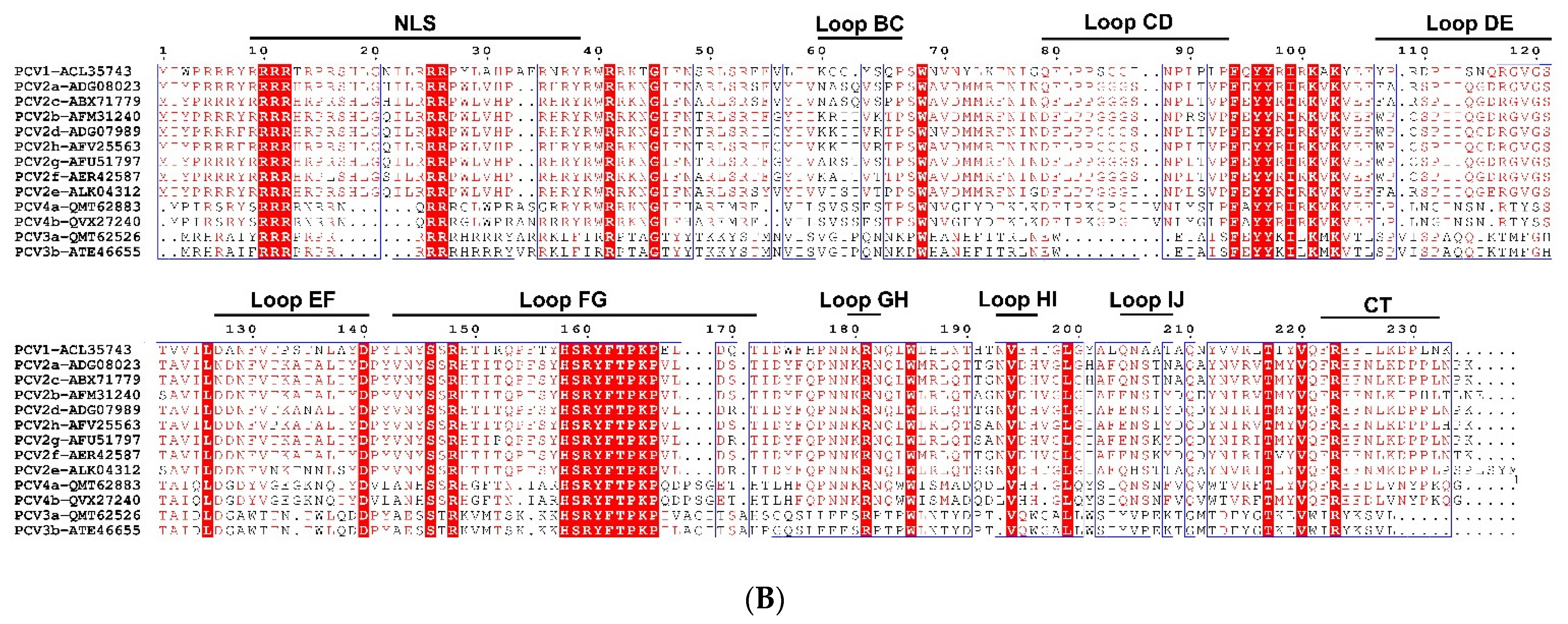
2. Genome, Protein, and Lifecycle of PCV
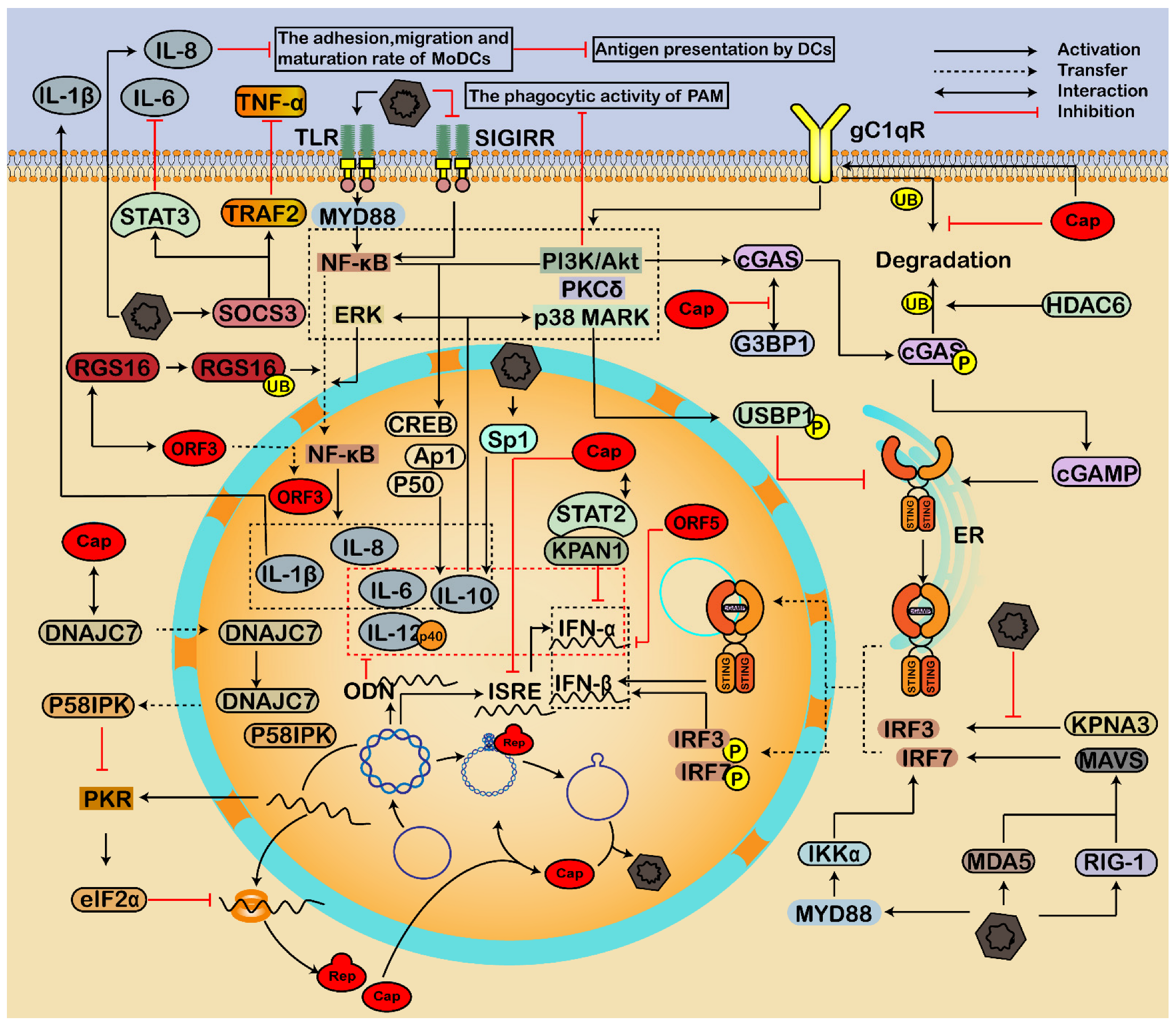
3. Crosstalk between PCV and Host
3.1. Essential Interaction for Viral Replication
3.2. Involving in Endoplasmic Reticulum Stress and Apoptosis
3.2.1. Endoplasmic Reticulum Pathway
3.2.2. Mitochondria-Mediated Pathway
3.2.3. Death Receptor Pathway
3.2.4. Other Pathways
3.3. Regulating Immune Response and Inflammatory Reactions
3.3.1. Regulation of Interferon
3.3.2. Modulation of Inflammatory Responses
3.4. Roles of Non-Coding RNAs on PCV Infection
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Chen, S.; Niu, G.; Zhang, X.; Ji, W.; Ren, Y.; Zhang, L.; Ren, L. Porcine Circovirus Type 4 Strains Circulating in China Are Relatively Stable and Have Higher Homology with Mink Circovirus than Other Porcine Circovirus Types. Int. J. Mol. Sci. 2022, 23, 3288. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Zhang, X.; Ji, W.; Chen, S.; Li, X.; Yang, L.; Zhang, L.; Ouyang, H.; Li, C.; Ren, L. Porcine circovirus 4 rescued from an infectious clone is replicable and pathogenic in vivo. Transbound. Emerg. Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Li, X.; Niu, G.; Ren, L. Recent Progress on Epidemiology and Pathobiology of Porcine Circovirus 3. Viruses 2021, 13, 1944. [Google Scholar] [CrossRef]
- Ren, L.; Chen, X.; Ouyang, H. Interactions of porcine circovirus 2 with its hosts. Virus Genes 2016, 52, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Tischer, I.; Rasch, R.; Tochtermann, G. Characterization of papovavirus-and picornavirus-like particles in permanent pig kidney cell lines. Zent. Bakteriol. Orig. A 1974, 226, 153–167. [Google Scholar]
- Meehan, B.M.; McNeilly, F.; Todd, D.; Kennedy, S.; Jewhurst, V.A.; Ellis, J.A.; Hassard, L.E.; Clark, E.G.; Haines, D.M.; Allan, G.M. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J. Gen. Virol. 1998, 79, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Giannitti, F.; Rossow, S.; Marthaler, D.; Knutson, T.P.; Li, L.; Deng, X.; Resende, T.; Vannucci, F.; Delwart, E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol. J. 2016, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, D.; Wang, J.; Zhu, S.; She, R.; Ren, X.; Tian, J.; Quan, R.; Hou, L.; Li, Z.; et al. Induction of Porcine Dermatitis and Nephropathy Syndrome in Piglets by Infection with Porcine Circovirus Type 3. J. Virol. 2019, 93, e02045-18. [Google Scholar] [CrossRef]
- Palinski, R.; Pineyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef]
- Zhang, H.H.; Hu, W.Q.; Li, J.Y.; Liu, T.N.; Zhou, J.Y.; Opriessnig, T.; Xiao, C.T. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound. Emerg. Dis. 2020, 67, 1057–1061. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, X.; Liu, X.; Ren, L. Co-Infection of Swine with Porcine Circovirus Type 2 and Other Swine Viruses. Viruses 2019, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Wei, Y.; Guo, L.; Wu, H.; Huang, L.; Liu, J.; Liu, C. Synergistic effects of sequential infection with highly pathogenic porcine reproductive and respiratory syndrome virus and porcine circovirus type 2. Virol. J. 2013, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Zhang, X.; Niu, G.; Yang, L.; Ji, W.; Zhang, L.; Ren, L. PCV2 and PRV Coinfection Induces Endoplasmic Reticulum Stress via PERK-eIF2alpha-ATF4-CHOP and IRE1-XBP1-EDEM Pathways. Int. J. Mol. Sci. 2022, 23, 4479. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zhang, L.; Niu, G.; Zhang, X.; Yang, L.; Ji, W.; Ren, L. Coinfection of Porcine Circovirus 2 and Pseudorabies Virus Enhances Immunosuppression and Inflammation through NF-kappaB, JAK/STAT, MAPK, and NLRP3 Pathways. Int. J. Mol. Sci. 2022, 23, 4469. [Google Scholar] [CrossRef]
- Ouyang, T.; Niu, G.; Liu, X.; Zhang, X.; Zhang, Y.; Ren, L. Recent progress on porcine circovirus type 3. Infect. Genet. Evol. 2019, 73, 227–233. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, T.; Ouyang, H.; Liu, X.; Niu, G.; Huo, W.; Yin, W.; Pang, D.; Ren, L. Human cells are permissive for the productive infection of porcine circovirus type 2 in vitro. Sci. Rep. 2019, 9, 5638. [Google Scholar] [CrossRef]
- Kim, D.Y.; Kim, H.R.; Park, J.H.; Kwon, N.Y.; Kim, J.M.; Kim, J.K.; Park, J.H.; Lee, K.K.; Kim, S.H.; Kim, W.I.; et al. Detection of a novel porcine circovirus 4 in Korean pig herds using a loop-mediated isothermal amplification assay. J. Virol. Methods 2022, 299, 114350. [Google Scholar] [CrossRef]
- Prinz, C.; Stillfried, M.; Neubert, L.K.; Denner, J. Detection of PCV3 in German wild boars. Virol. J. 2019, 16, 25. [Google Scholar] [CrossRef]
- Amoroso, M.G.; Serra, F.; Esposito, C.; D’Alessio, N.; Ferrara, G.; Cioffi, B.; Anzalone, A.; Pagnini, U.; De Carlo, E.; Fusco, G.; et al. Prevalence of Infection with Porcine Circovirus Types 2 and 3 in the Wild Boar Population in the Campania Region (Southern Italy). Animals 2021, 11, 3215. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Li, X.; Ouyang, T.; Niu, G.; Ouyang, H.; Ren, L. Genotyping based on complete coding sequences of porcine circovirus type 3 is stable and reliable. Infect. Genet. Evol. 2020, 78, 104116. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.Y.; Rho, S.B.; Kim, H.S.; Han, J.; Bae, J.; Lee, S.J.; Jung, W.W.; Chun, T. The ORF3 protein of porcine circovirus type 2 promotes secretion of IL-6 and IL-8 in porcine epithelial cells by facilitating proteasomal degradation of regulator of G protein signalling 16 through physical interaction. J. Gen. Virol. 2015, 96, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Cheng, A.; Wang, M.; Yin, Z.; Huang, J.; Jia, R. Apoptosis Triggered by ORF3 Proteins of the Circoviridae Family. Front. Cell. Infect. Microbiol. 2020, 10, 609071. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Guo, K.; Wang, T.; Zhang, C.; Zhang, Y. Porcine circovirus type 2 ORF4 protein binds heavy chain ferritin. J. Biosci. 2015, 40, 477–485. [Google Scholar] [CrossRef]
- Gao, Z.; Dong, Q.; Jiang, Y.; Opriessnig, T.; Wang, J.; Quan, Y.; Yang, Z. ORF4-protein deficient PCV2 mutants enhance virus-induced apoptosis and show differential expression of mRNAs in vitro. Virus Res. 2014, 183, 56–62. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cao, J.; Zhou, N.; Jin, Y.; Wu, J.; Zhou, J. Identification and functional analysis of the novel ORF4 protein encoded by porcine circovirus type 2. J. Virol. 2013, 87, 1420–1429. [Google Scholar] [CrossRef]
- Lv, J.; Jiang, Y.; Feng, Q.; Fan, Z.; Sun, Y.; Xu, P.; Hou, Y.; Zhang, X.; Fan, Y.; Xu, X.; et al. Porcine Circovirus Type 2 ORF5 Protein Induces Autophagy to Promote Viral Replication via the PERK-eIF2alpha-ATF4 and mTOR-ERK1/2-AMPK Signaling Pathways in PK-15 Cells. Front. Microbiol. 2020, 11, 320. [Google Scholar] [CrossRef]
- Ouyang, Y.; Xu, L.; Lv, J.; Hou, Y.; Fan, Z.; Xu, P.; Jiang, Y.; Wu, M.; Li, R.; Zhang, Y.; et al. Porcine circovirus type 2 ORF5 protein induces endoplasmic reticulum stress and unfolded protein response in porcine alveolar macrophages. Arch. Virol. 2019, 164, 1323–1334. [Google Scholar] [CrossRef]
- Choi, C.Y.; Choi, Y.C.; Park, I.B.; Lee, C.H.; Kang, S.J.; Chun, T. The ORF5 protein of porcine circovirus type 2 enhances viral replication by dampening type I interferon expression in porcine epithelial cells. Vet. Microbiol. 2018, 226, 50–58. [Google Scholar] [CrossRef]
- Kang, S.J.; Park, I.B.; Chun, T. Open reading frame 5 protein of porcine circovirus type 2 induces RNF128 (GRAIL) which inhibits mRNA transcription of interferon-beta in porcine epithelial cells. Res. Vet. Sci. 2021, 140, 79–82. [Google Scholar] [CrossRef]
- Wang, N.; Zhan, Y.; Wang, A.; Zhang, L.; Khayat, R.; Yang, Y. In silico analysis of surface structure variation of PCV2 capsid resulting from loop mutations of its capsid protein (Cap). J. Gen. Virol. 2016, 97, 3331–3344. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mai, J.; Lei, B.; Zhang, Y.; Yang, Y.; Wang, N. Structure, Antigenic Properties, and Highly Efficient Assembly of PCV4 Capsid Protein. Front. Vet. Sci. 2021, 8, 695466. [Google Scholar] [CrossRef] [PubMed]
- Bi, M.; Li, X.; Zhai, W.; Yin, B.; Tian, K.; Mo, X. Structural insight into the type-specific epitope of porcine circovirus type 3. Biosci. Rep. 2020, 40, BSR20201109. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Li, X.; Yin, B.; Deng, J.; Tian, K.; Yuan, A. Structural roles of PCV2 capsid protein N-terminus in PCV2 particle assembly and identification of PCV2 type-specific neutralizing epitope. PLoS Pathog. 2019, 15, e1007562. [Google Scholar] [CrossRef]
- Steiner, E.; Balmelli, C.; Herrmann, B.; Summerfield, A.; McCullough, K. Porcine circovirus type 2 displays pluripotency in cell targeting. Virology 2008, 378, 311–322. [Google Scholar] [CrossRef]
- Misinzo, G.; Delputte, P.L.; Lefebvre, D.J.; Nauwynck, H.J. Porcine circovirus 2 infection of epithelial cells is clathrin-, caveolae- and dynamin-independent, actin and Rho-GTPase-mediated, and enhanced by cholesterol depletion. Virus Res. 2009, 139, 1–9. [Google Scholar] [CrossRef]
- Meng, X.J. Porcine circovirus type 2 (PCV2): Pathogenesis and interaction with the immune system. Annu. Rev. Anim. Biosci. 2013, 1, 43–64. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, L.; Yang, Q. Infection of porcine circovirus 2 (PCV2) in intestinal porcine epithelial cell line (IPEC-J2) and interaction between PCV2 and IPEC-J2 microfilaments. Virol. J. 2014, 11, 193. [Google Scholar] [CrossRef]
- Wei, R.; Van Renne, N.; Nauwynck, H.J. Strain-Dependent Porcine Circovirus Type 2 (PCV2) Entry and Replication in T-Lymphoblasts. Viruses 2019, 11, 813. [Google Scholar] [CrossRef]
- Chen, G.H.; Mai, K.J.; Zhou, L.; Wu, R.T.; Tang, X.Y.; Wu, J.L.; He, L.L.; Lan, T.; Xie, Q.M.; Sun, Y.; et al. Detection and genome sequencing of porcine circovirus 3 in neonatal pigs with congenital tremors in South China. Transbound. Emerg. Dis. 2017, 64, 1650–1654. [Google Scholar] [CrossRef]
- Franzo, G.; Legnardi, M.; Hjulsager, C.K.; Klaumann, F.; Larsen, L.E.; Segales, J.; Drigo, M. Full-Genome sequencing of porcine circovirus 3 field strains from Denmark, Italy and Spain demonstrates a high within-Europe genetic heterogeneity. Transbound. Emerg. Dis. 2018, 65, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Yoo, S.J.; Park, C.K.; Lyoo, Y.S. Prevalence of novel porcine circovirus 3 in Korean pig populations. Vet. Microbiol. 2017, 207, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; McKillen, J.; Allan, G. Porcine circovirus type 3 in the UK. Vet. Rec. 2017, 181, 599. [Google Scholar] [CrossRef]
- Kedkovid, R.; Woonwong, Y.; Arunorat, J.; Sirisereewan, C.; Sangpratum, N.; Kesdangsakonwut, S.; Tummaruk, P.; Teankum, K.; Assavacheep, P.; Jittimanee, S.; et al. Porcine circovirus type 3 (PCV3) shedding in sow colostrum. Vet. Microbiol. 2018, 220, 12–17. [Google Scholar] [CrossRef]
- Misinzo, G.; Meerts, P.; Bublot, M.; Mast, J.; Weingartl, H.M.; Nauwynck, H.J. Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J. Gen. Virol. 2005, 86, 2057–2068. [Google Scholar] [CrossRef]
- Misinzo, G.; Delputte, P.L.; Meerts, P.; Lefebvre, D.J.; Nauwynck, H.J. Porcine circovirus 2 uses heparan sulfate and chondroitin sulfate B glycosaminoglycans as receptors for its attachment to host cells. J. Virol. 2006, 80, 3487–3494. [Google Scholar] [CrossRef]
- Dhindwal, S.; Avila, B.; Feng, S.; Khayat, R. Porcine Circovirus 2 Uses a Multitude of Weak Binding Sites to Interact with Heparan Sulfate, and the Interactions Do Not Follow the Symmetry of the Capsid. J. Virol. 2019, 93, e02222-18. [Google Scholar] [CrossRef] [PubMed]
- Vincent, I.E.; Carrasco, C.P.; Guzylack-Piriou, L.; Herrmann, B.; McNeilly, F.; Allan, G.M.; Summerfield, A.; McCullough, K.C. Subset-Dependent modulation of dendritic cell activity by circovirus type 2. Immunology 2005, 115, 388–398. [Google Scholar] [CrossRef]
- Nauwynck, H.J.; Sanchez, R.; Meerts, P.; Lefebvre, D.J.; Saha, D.; Huang, L.; Misinzo, G. Cell tropism and entry of porcine circovirus 2. Virus Res. 2012, 164, 43–45. [Google Scholar] [CrossRef]
- Cardin, A.D.; Weintraub, H.J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis 1989, 9, 21–32. [Google Scholar] [CrossRef]
- Shi, R.; Hou, L.; Wei, L.; Quan, R.; Zhou, B.; Jiang, H.; Wang, J.; Zhu, S.; Song, J.; Wang, D.; et al. Porcine Circovirus Type 3 Enters Into PK15 Cells Through Clathrin- and Dynamin-2-Mediated Endocytosis in a Rab5/Rab7 and pH-Dependent Fashion. Front. Microbiol. 2021, 12, 636307. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lin, C.; Wang, H.; Wang, L.; Zhou, N.; Jin, Y.; Liao, M.; Zhou, J. Circovirus transport proceeds via direct interaction of the cytoplasmic dynein IC1 subunit with the viral capsid protein. J. Virol. 2015, 89, 2777–2791. [Google Scholar] [CrossRef] [PubMed]
- Misinzo, G.; Delputte, P.L.; Nauwynck, H.J. Inhibition of endosome-lysosome system acidification enhances porcine circovirus 2 infection of porcine epithelial cells. J. Virol. 2008, 82, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zhang, M.; Li, W.; Wang, Y.; Liu, Y.; He, Q. Proteomic analysis of porcine alveolar macrophages infected with porcine circovirus type 2. J. Proteom. 2012, 75, 3258–3269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, J.; Wu, Y.; Zheng, X.; Ma, G.; Wang, Z.; Jin, Y.; He, J.; Yan, Y. Differential proteome analysis of host cells infected with porcine circovirus type 2. J. Proteome Res. 2009, 8, 5111–5119. [Google Scholar] [CrossRef]
- Wei, R.; Trus, I.; Yang, B.; Huang, L.; Nauwynck, H.J. Breed Differences in PCV2 Uptake and Disintegration in Porcine Monocytes. Viruses 2018, 10, 562. [Google Scholar] [CrossRef]
- Theerawatanasirikul, S.; Phecharat, N.; Prawettongsopon, C.; Chaicumpa, W.; Lekcharoensuk, P. Dynein light chain DYNLL1 subunit facilitates porcine circovirus type 2 intracellular transports along microtubules. Arch. Virol. 2017, 162, 677–686. [Google Scholar] [CrossRef]
- Chen, J.K.; Hsiao, C.; Lo, A.R.; Wang, C.Y. Characterization of the nuclear localization sequence of beak and feather disease virus capsid proteins and their assembly into virus-like particles. Virus Res. 2020, 289, 198144. [Google Scholar] [CrossRef]
- Song, J.; Hou, L.; Wang, D.; Wei, L.; Zhu, S.; Wang, J.; Quan, R.; Jiang, H.; Shi, R.; Liu, J. Nucleolar Phosphoprotein NPM1 Interacts with Porcine Circovirus Type 3 Cap Protein and Facilitates Viral Replication. Front. Microbiol. 2021, 12, 679341. [Google Scholar] [CrossRef]
- Zhou, J.; Qiu, Y.; Zhu, N.; Zhou, L.; Dai, B.; Feng, X.; Hou, L.; Liu, J. The Nucleolar Localization Signal of Porcine Circovirus Type 4 Capsid Protein Is Essential for Interaction with Serine-48 Residue of Nucleolar Phosphoprotein Nucleophosmin-1. Front. Microbiol. 2021, 12, 751382. [Google Scholar] [CrossRef]
- Cheung, A.K. Porcine circovirus: Transcription and DNA replication. Virus Res. 2012, 164, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Qiu, Y.; Wang, Y.; Yang, X.; Liu, C.; Shi, Y.; Feng, X.; Hou, L.; Liu, J. Contribution of DEAD-Box RNA Helicase 21 to the Nucleolar Localization of Porcine Circovirus Type 4 Capsid Protein. Front. Microbiol. 2022, 13, 802740. [Google Scholar] [CrossRef] [PubMed]
- Mankertz, A. Molecular interactions of porcine circoviruses type 1 and type 2 with its host. Virus Res. 2012, 164, 54–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lv, Q.Z.; Guo, K.K.; Zhang, Y.M. Current understanding of genomic DNA of porcine circovirus type 2. Virus Genes 2014, 49, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mankertz, A.; Hillenbrand, B. Replication of porcine circovirus type 1 requires two proteins encoded by the viral rep gene. Virology 2001, 279, 429–438. [Google Scholar] [CrossRef][Green Version]
- Sarker, S.; Terron, M.C.; Khandokar, Y.; Aragao, D.; Hardy, J.M.; Radjainia, M.; Jimenez-Zaragoza, M.; de Pablo, P.J.; Coulibaly, F.; Luque, D.; et al. Structural insights into the assembly and regulation of distinct viral capsid complexes. Nat. Commun. 2016, 7, 13014. [Google Scholar] [CrossRef]
- Hou, Q.; Hou, S.; Chen, Q.; Jia, H.; Xin, T.; Jiang, Y.; Guo, X.; Zhu, H. Nuclear localization signal regulates porcine circovirus type 2 capsid protein nuclear export through phosphorylation. Virus Res. 2018, 246, 12–22. [Google Scholar] [CrossRef]
- Rodriguez-Carino, C.; Sanchez-Chardi, A.; Segales, J. Subcellular immunolocalization of porcine circovirus type 2 (PCV2) in lymph nodes from pigs with post-weaning multisystemic wasting syndrome (PMWS). J. Comp. Pathol. 2010, 142, 291–299. [Google Scholar] [CrossRef]
- Yu, W.; Sun, Y.; He, Q.; Sun, C.; Dong, T.; Zhang, L.; Zhan, Y.; Wang, N.; Yang, Y.; Sun, Y. Mitochondrial Localization Signal of Porcine Circovirus Type 2 Capsid Protein Plays a Critical Role in Cap-Induced Apoptosis. Vet. Sci. 2021, 8, 272. [Google Scholar] [CrossRef]
- Finsterbusch, T.; Steinfeldt, T.; Doberstein, K.; Rodner, C.; Mankertz, A. Interaction of the replication proteins and the capsid protein of porcine circovirus type 1 and 2 with host proteins. Virology 2009, 386, 122–131. [Google Scholar] [CrossRef]
- Shuai, J.; Fu, L.; Zhang, X.; Zhu, B.; Li, X.; He, Y.; Fang, W. Functional exchangeability of the nuclear localization signal (NLS) of capsid protein between PCV1 and PCV2 in vitro: Implications for the role of NLS in viral replication. Virol. J. 2011, 8, 341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, J.; Li, H.; Zhang, Y.; Dong, W.; Jin, Y.; Yan, Y.; Gu, J.; Zhou, J. The serine-48 residue of nucleolar phosphoprotein nucleophosmin-1 plays critical role in subcellular localization and interaction with porcine circovirus type 3 capsid protein. Vet. Res. 2021, 52, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dai, Y.; Lin, C.; Zhang, Y.; Feng, Z.; Dong, W.; Jin, Y.; Yan, Y.; Zhou, J.; Gu, J. Nucleolar protein NPM1 is essential for circovirus replication by binding to viral capsid. Virulence 2020, 11, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Kouokam Fotso, G.B.; Bernard, C.; Bigault, L.; de Boisseson, C.; Mankertz, A.; Jestin, A.; Grasland, B. The expression level of gC1qR is down regulated at the early time of infection with porcine circovirus of type 2 (PCV-2) and gC1qR interacts differently with the Cap proteins of porcine circoviruses. Virus Res. 2016, 220, 21–32. [Google Scholar] [CrossRef]
- Wang, T.; Du, Q.; Niu, Y.; Zhang, X.; Wang, Z.; Wu, X.; Yang, X.; Zhao, X.; Liu, S.L.; Tong, D.; et al. Cellular p32 Is a Critical Regulator of Porcine Circovirus Type 2 Nuclear Egress. J. Virol. 2019, 93, e00979-19. [Google Scholar] [CrossRef]
- Ma, X.; Lv, C.; Wang, Q.; Li, C.; Wang, P.; Luo, C.; Wu, Y.; Wei, T.; Liu, S.; Adam, F.E.A.; et al. C1QBP inhibits proliferation of porcine circovirus type 2 by restricting nuclear import of the capsid protein. Arch. Virol. 2021, 166, 767–778. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, X.; Wang, D.; Jiang, Y.; Zhan, Y.; Li, M.; Zhou, Y.; Qin, Y.; Liu, J.; Wang, A.; et al. Inhibition of Abl or Src tyrosine kinase decreased porcine circovirus type 2 production in PK15 cells. Res. Vet. Sci. 2019, 124, 1–9. [Google Scholar] [CrossRef]
- Zhan, Y.; Yu, W.; Cai, X.; Lei, X.; Lei, H.; Wang, A.; Sun, Y.; Wang, N.; Deng, Z.; Yang, Y. The Carboxyl Terminus of the Porcine Circovirus Type 2 Capsid Protein Is Critical to Virus-Like Particle Assembly, Cell Entry, and Propagation. J. Virol. 2020, 94, e00042-20. [Google Scholar] [CrossRef]
- Wang, T.; Du, Q.; Wu, X.; Niu, Y.; Guan, L.; Wang, Z.; Zhao, X.; Liu, S.L.; Tong, D.; Huang, Y. Porcine MKRN1 Modulates the Replication and Pathogenesis of Porcine Circovirus Type 2 by Inducing Capsid Protein Ubiquitination and Degradation. J. Virol. 2018, 92, e00100-18. [Google Scholar] [CrossRef]
- Wei, L.; Liu, J. Porcine circovirus type 2 replication is impaired by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. Virology 2009, 386, 203–209. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiol. Rev. 2022, 102, 411–454. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, P.; Jia, R.; Wang, M.; Yin, Z.; Cheng, A. Regulation of Apoptosis during Porcine Circovirus Type 2 Infection. Front. Microbiol. 2018, 9, 2086. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Quarleri, J.; Cevallos, C.; Delpino, M.V. Apoptosis in infectious diseases as a mechanism of immune evasion and survival. Adv. Protein Chem. Struct. Biol. 2021, 125, 1–24. [Google Scholar]
- Darwich, L.; Segales, J.; Mateu, E. Pathogenesis of postweaning multisystemic wasting syndrome caused by Porcine circovirus 2: An immune riddle. Arch. Virol. 2004, 149, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Du, Q.; Han, C.; Wang, Z.; Zhang, X.; Wang, T.; Zhao, X.; Huang, Y.; Tong, D. p53 signaling modulation of cell cycle arrest and viral replication in porcine circovirus type 2 infection cells. Vet. Res. 2016, 47, 120. [Google Scholar] [CrossRef]
- Kohli, E.; Causse, S.; Baverel, V.; Dubrez, L.; Borges-Bonan, N.; Demidov, O.; Garrido, C. Endoplasmic Reticulum Chaperones in Viral Infection: Therapeutic Perspectives. Microbiol. Mol. Biol. Rev. 2021, 85, e0003521. [Google Scholar] [CrossRef]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef]
- Zhou, Y.; Qi, B.; Gu, Y.; Xu, F.; Du, H.; Li, X.; Fang, W. Porcine Circovirus 2 Deploys PERK Pathway and GRP78 for Its Enhanced Replication in PK-15 Cells. Viruses 2016, 8, 56. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Gu, Y.X.; Qi, B.Z.; Zhang, Y.K.; Li, X.L.; Fang, W.H. Porcine circovirus type 2 capsid protein induces unfolded protein response with subsequent activation of apoptosis. J. Zhejiang Univ. Sci. B 2017, 18, 316–323. [Google Scholar] [CrossRef]
- Sun, R.; Sun, S.; Zhang, Y.; Zhou, Y.; Shan, Y.; Li, X.; Fang, W. PCV2 Induces Reactive Oxygen Species to Promote Nucleocytoplasmic Translocation of the Viral DNA Binding Protein HMGB1 To Enhance Its Replication. J. Virol. 2020, 94, e00238-20. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Deng, Z.; Han, X.; Zhang, Y.; Zhou, Y.; Shan, Y.; Fang, W.; Li, X. Porcine Circovirus 2 Manipulates the PERK-ERO1alpha Axis of the Endoplasmic Reticulum to Favor Its Replication by Derepressing Viral DNA from HMGB1 Sequestration within Nuclei. J. Virol. 2021, 95, e0100921. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, A.K.; Kwang, J. ORF3 of porcine circovirus 2 enhances the in vitro and in vivo spread of the of the virus. Virology 2011, 410, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, L.; Jin, Y.; Lin, C.; Wang, H.; Zhou, N.; Xing, G.; Liao, M.; Zhou, J. Characterization of specific antigenic epitopes and the nuclear export signal of the Porcine circovirus 2 ORF3 protein. Vet. Microbiol. 2016, 184, 40–50. [Google Scholar] [CrossRef]
- Teras, M.; Viisileht, E.; Pahtma-Hall, M.; Rump, A.; Paalme, V.; Pata, P.; Pata, I.; Langevin, C.; Ruutel Boudinot, S. Porcine circovirus type 2 ORF3 protein induces apoptosis in melanoma cells. BMC Cancer 2018, 18, 1237. [Google Scholar] [CrossRef]
- Hung, L.C. The Monoclonal Antibody Recognized the Open Reading Frame Protein in Porcine Circovirus Type 2-Infected Peripheral Blood Mononuclear Cells. Viruses 2020, 12, 961. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Chen, I.; Lau, J.; He, F.; Lau, A.; Wang, Z.; Karuppannan, A.K.; Kwang, J. The ORF3 protein of porcine circovirus type 2 interacts with porcine ubiquitin E3 ligase Pirh2 and facilitates p53 expression in viral infection. J. Virol. 2007, 81, 9560–9567. [Google Scholar] [CrossRef]
- Karuppannan, A.K.; Liu, S.; Jia, Q.; Selvaraj, M.; Kwang, J. Porcine circovirus type 2 ORF3 protein competes with p53 in binding to Pirh2 and mediates the deregulation of p53 homeostasis. Virology 2010, 398, 1–11. [Google Scholar] [CrossRef]
- Lv, Q.; Guo, K.; Xu, H.; Wang, T.; Zhang, Y. Identification of Putative ORF5 Protein of Porcine Circovirus Type 2 and Functional Analysis of GFP-Fused ORF5 Protein. PLoS ONE 2015, 10, e0127859. [Google Scholar]
- Guo, K.; Xu, L.; Wu, M.; Hou, Y.; Jiang, Y.; Lv, J.; Xu, P.; Fan, Z.; Zhang, R.; Xing, F.; et al. A Host Factor GPNMB Restricts Porcine Circovirus Type 2 (PCV2) Replication and Interacts with PCV2 ORF5 Protein. Front. Microbiol. 2018, 9, 3295. [Google Scholar] [CrossRef]
- Guo, K.; Zhang, X.; Hou, Y.; Liu, J.; Feng, Q.; Wang, K.; Xu, L.; Zhang, Y. A novel PCV2 ORF5-interacting host factor YWHAB inhibits virus replication and alleviates PCV2-induced cellular response. Vet. Microbiol. 2020, 251, 108893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, R.; Geng, S.; Shan, Y.; Li, X.; Fang, W. Porcine Circovirus Type 2 Induces ORF3-Independent Mitochondrial Apoptosis via PERK Activation and Elevation of Cytosolic Calcium. J. Virol. 2019, 93, e01784-18. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Dai, L.; Han, H.; Zhang, S. PCV2 induces apoptosis and modulates calcium homeostasis in piglet lymphocytes in vitro. Res. Vet. Sci. 2012, 93, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, C.; Sun, P.; Shi, J.; Wu, X.; Liu, C.; Peng, Z.; Han, H.; Xu, S.; Yang, Y.; et al. PCV2 Triggers PK-15 Cell Apoptosis Through the PLC-IP3R-Ca(2+) Signaling Pathway. Front. Microbiol. 2021, 12, 674907. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, R.; Li, X.; Fang, W. Porcine Circovirus 2 Induction of ROS Is Responsible for Mitophagy in PK-15 Cells via Activation of Drp1 Phosphorylation. Viruses 2020, 12, 289. [Google Scholar] [CrossRef]
- Lv, Q.; Guo, K.; Zhang, G.; Zhang, Y. The ORF4 protein of porcine circovirus type 2 antagonizes apoptosis by stabilizing the concentration of ferritin heavy chain through physical interaction. J. Gen. Virol. 2016, 97, 1636–1646. [Google Scholar] [CrossRef][Green Version]
- Lin, C.; Gu, J.; Wang, H.; Zhou, J.; Li, J.; Wang, S.; Jin, Y.; Liu, C.; Liu, J.; Yang, H.; et al. Caspase-Dependent Apoptosis Induction via Viral Protein ORF4 of Porcine Circovirus 2 Binding to Mitochondrial Adenine Nucleotide Translocase 3. J. Virol. 2018, 92, e00238-18. [Google Scholar] [CrossRef]
- Chang, H.W.; Jeng, C.R.; Lin, C.M.; Liu, J.J.; Chang, C.C.; Tsai, Y.C.; Chia, M.Y.; Pang, V.F. The involvement of Fas/FasL interaction in porcine circovirus type 2 and porcine reproductive and respiratory syndrome virus co-inoculation-associated lymphocyte apoptosis in vitro. Vet. Microbiol. 2007, 122, 72–82. [Google Scholar] [CrossRef]
- Bian, L.; Meng, Y.; Zhang, M.; Li, D. MRE11-RAD50-NBS1 complex alterations and DNA damage response: Implications for cancer treatment. Mol. Cancer 2019, 18, 169. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, S.; Wang, J.; Quan, R.; Yan, X.; Li, Z.; Hou, L.; Wang, N.; Yang, Y.; Jiang, H.; et al. Induction of a Cellular DNA Damage Response by Porcine Circovirus Type 2 Facilitates Viral Replication and Mediates Apoptotic Responses. Sci. Rep. 2016, 6, 39444. [Google Scholar] [CrossRef]
- Wei, L.; Zhu, S.; Wang, J.; Zhang, C.; Quan, R.; Yan, X.; Liu, J. Regulatory role of ASK1 in porcine circovirus type 2-induced apoptosis. Virology 2013, 447, 285–291. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, L.; Zhu, Z.; Wang, J.; Liu, J. JNK and p38 mitogen-activated protein kinase pathways contribute to porcine circovirus type 2 infection. J. Virol. 2009, 83, 6039–6047. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, L.; Kwang, J.; Wang, J.; Shi, L.; Yang, B.; Li, Y.; Liu, J. Porcine circovirus type 2 induces the activation of nuclear factor kappa B by IkappaBalpha degradation. Virology 2008, 378, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhu, S.; Wang, J.; Liu, J. Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway during porcine circovirus type 2 infection facilitates cell survival and viral replication. J. Virol. 2012, 86, 13589–13597. [Google Scholar] [CrossRef]
- Ma, T.; Chen, X.; Ouyang, H.; Liu, X.; Ouyang, T.; Peng, Z.; Yang, X.; Chen, F.; Pang, D.; Bai, J.; et al. HMGCR inhibits the early stage of PCV2 infection, while PKC enhances the infection at the late stage. Virus Res. 2017, 229, 41–47. [Google Scholar] [CrossRef]
- Ouyang, T.; Niu, G.; Zhang, Y.; Liu, X.; Zhang, X.; Zhang, S.; Geng, Y.; Pang, D.; Ouyang, H.; Ren, L. Porcine HMGCR Inhibits Porcine Circovirus Type 2 Infection by Directly Interacting with the Viral Proteins. Viruses 2019, 11, 544. [Google Scholar] [CrossRef]
- Yang, X.; Ouyang, H.; Chen, F.; Pang, D.; Dong, M.; Yang, S.; Liu, X.; Peng, Z.; Wang, F.; Zhang, X.; et al. HMG-CoA reductase is negatively associated with PCV2 infection and PCV2-induced apoptotic cell death. J. Gen. Virol. 2014, 95, 1330–1337. [Google Scholar] [CrossRef][Green Version]
- Yang, L.; Liu, X.; Zhang, L.; Li, X.; Zhang, X.; Niu, G.; Ji, W.; Chen, S.; Ouyang, H.; Ren, L. Porcine TRIM21 Enhances Porcine Circovirus 2 Infection and Host Immune Responses, But Inhibits Apoptosis of PCV2-Infected Cells. Viruses 2022, 14, 156. [Google Scholar] [CrossRef]
- Han, C.; Du, Q.; Zhu, L.; Chen, N.; Luo, L.; Chen, Q.; Yin, J.; Wu, X.; Tong, D.; Huang, Y. Porcine DNAJB6 promotes PCV2 replication via enhancing the formation of autophagy in host cells. Vet. Res. 2020, 51, 61. [Google Scholar] [CrossRef]
- Rakibuzzaman, A.; Ramamoorthy, S. Comparative immunopathogenesis and biology of recently discovered porcine circoviruses. Transbound. Emerg. Dis. 2021, 68, 2957–2968. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, Y.; Lian, X.; Sun, H.; Wang, J.; Liu, W.; Meng, G.; Li, P.; Zhu, D.; Jin, Y.; et al. Functional analysis of the interferon-stimulated response element of porcine circovirus type 2 and its role during viral replication in vitro and in vivo. Virol. J. 2012, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Opriessnig, T.; Pal, N.; Huang, F.F.; Meng, X.J. Effect of an interferon-stimulated response element (ISRE) mutant of porcine circovirus type 2 (PCV2) on PCV2-induced pathological lesions in a porcine reproductive and respiratory syndrome virus (PRRSV) co-infection model. Vet. Microbiol. 2011, 147, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, S.; Huang, F.F.; Huang, Y.W.; Meng, X.J. Interferon-Mediated enhancement of in vitro replication of porcine circovirus type 2 is influenced by an interferon-stimulated response element in the PCV2 genome. Virus Res. 2009, 145, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Mutthi, P.; Theerawatanasirikul, S.; Roytrakul, S.; Paemanee, A.; Lekcharoensuk, C.; Hansoongnern, P.; Petcharat, N.; Thangthamniyom, N.; Lekcharoensuk, P. Interferon gamma induces cellular protein alteration and increases replication of porcine circovirus type 2 in PK-15 cells. Arch. Virol. 2018, 163, 2947–2957. [Google Scholar] [CrossRef]
- Shen, H.; Liu, X.; Zhang, P.; Wang, S.; Liu, Y.; Zhang, L.; Song, C. Porcine circovirus 3 Cap inhibits type I interferon signaling through interaction with STAT2. Virus Res. 2020, 275, 197804. [Google Scholar] [CrossRef]
- Wikstrom, F.H.; Fossum, C.; Fuxler, L.; Kruse, R.; Lovgren, T. Cytokine induction by immunostimulatory DNA in porcine PBMC is impaired by a hairpin forming sequence motif from the genome of Porcine Circovirus type 2 (PCV2). Vet. Immunol. Immunopathol. 2011, 139, 156–166. [Google Scholar] [CrossRef]
- Choi, C.Y.; Oh, H.N.; Jun Lee, S.; Chun, T. ORF2 protein of porcine circovirus type 2 promotes phagocytic activity of porcine macrophages by inhibiting proteasomal degradation of complement component 1, q subcomponent binding protein (C1QBP) through physical interaction. J. Gen. Virol. 2015, 96, 3294–3301. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Wu, X.; Ma, D.; Zhang, X.; Li, R.; Han, C.; Liu, H.; Yin, X.; Du, Q.; et al. PCV2 targets cGAS to inhibit type I interferon induction to promote other DNA virus infection. PLoS Pathog. 2021, 17, e1009940. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, L.; Lu, M.; Li, J.; Lv, Y. PCV2 infection activates the cGAS/STING signaling pathway to promote IFN-beta production and viral replication in PK-15 cells. Vet. Microbiol. 2018, 227, 34–40. [Google Scholar] [CrossRef]
- Huang, B.; Li, J.; Zhang, X.; Zhao, Q.; Lu, M.; Lv, Y. RIG-1 and MDA-5 signaling pathways contribute to IFN-beta production and viral replication in porcine circovirus virus type 2-infected PK-15 cells in vitro. Vet. Microbiol. 2017, 211, 36–42. [Google Scholar] [CrossRef]
- Dvorak, C.M.T.; Puvanendiran, S.; Murtaugh, M.P. Porcine circovirus 2 infection induces IFNbeta expression through increased expression of genes involved in RIG-I and IRF7 signaling pathways. Virus Res. 2018, 253, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Han, J.; Zhang, Y.; Duan, D.; Zhang, S. Porcine circovirus type 2 induces type I interferon production via MyD88-IKKalpha-IRFs signaling rather than NF-kappaB in porcine alveolar macrophages in vitro. Res. Vet. Sci. 2016, 104, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Shen, H.; Liu, X.; Wang, S.; Liu, Y.; Xu, Z.; Song, C. Porcine Circovirus Type 3 Cap Inhibits Type I Interferon Induction Through Interaction with G3BP1. Front. Vet. Sci. 2020, 7, 594438. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Z.; Qiao, D.; Yuan, Y.; Han, C.; Yang, N.; Li, R.; Du, Q.; Tong, D.; Huang, Y. Porcine circovirus type 2 infection attenuates the K63-linked ubiquitination of STING to inhibit IFN-beta induction via p38-MAPK pathway. Vet. Microbiol. 2021, 258, 109098. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, S.; Lu, M.; Huang, C.; Lv, Y. Nuclear transporter karyopherin subunit alpha 3 levels modulate Porcine circovirus type 2 replication in PK-15 cells. Virology 2020, 548, 31–38. [Google Scholar] [CrossRef]
- Li, J.; Lu, M.; Huang, B.; Lv, Y. Porcine circovirus type 2 inhibits inter-beta expression by targeting Karyopherin alpha-3 in PK-15 cells. Virology 2018, 520, 75–82. [Google Scholar] [CrossRef]
- Yang, S.; Liu, B.; Yin, S.; Shang, Y.; Zhang, X.; Khan, M.U.Z.; Liu, X.; Cai, J. Porcine Circovirus Type 2 Induces Single Immunoglobulin Interleukin-1 Related Receptor (SIGIRR) Downregulation to Promote Interleukin-1beta Upregulation in Porcine Alveolar Macrophage. Viruses 2019, 11, 1021. [Google Scholar] [CrossRef]
- Du, Q.; Huang, Y.; Wang, T.; Zhang, X.; Chen, Y.; Cui, B.; Li, D.; Zhao, X.; Zhang, W.; Chang, L.; et al. Porcine circovirus type 2 activates PI3K/Akt and p38 MAPK pathways to promote interleukin-10 production in macrophages via Cap interaction of gC1qR. Oncotarget 2016, 7, 17492–17507. [Google Scholar] [CrossRef]
- Han, J.; Zhang, S.; Zhang, Y.; Chen, M.; Lv, Y. Porcine circovirus type 2 increases interleukin-1beta and interleukin-10 production via the MyD88-NF-kappa B signaling pathway in porcine alveolar macrophages in vitro. J. Vet. Sci. 2017, 18, 183–191. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Qiao, J. TLR2/MyD88/NF-kappaB signalling pathway regulates IL-8 production in porcine alveolar macrophages infected with porcine circovirus 2. J. Gen. Virol. 2016, 97, 445–452. [Google Scholar] [CrossRef]
- Timmusk, S.; Merlot, E.; Lovgren, T.; Jarvekulg, L.; Berg, M.; Fossum, C. Regulator of G protein signalling 16 is a target for a porcine circovirus type 2 protein. J. Gen. Virol. 2009, 90, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bai, J.; Liu, P.; Wang, X.; Jiang, P. Suppressor of cytokine signaling 3 plays an important role in porcine circovirus type 2 subclinical infection by downregulating proinflammatory responses. Sci. Rep. 2016, 6, 32538. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Q.; Qiao, J.; Wang, J.; Cui, D.; Gu, K.; Zhou, S.; Li, H. Endothelial IL-8 induced by porcine circovirus type 2 affects dendritic cell maturation and antigen-presenting function. Virol. J. 2019, 16, 154. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Wang, T.; Liu, S.; Zhu, Y. Porcine circovirus type 2 exploits cap to inhibit PKR activation through interaction with Hsp40. Vet. Microbiol. 2021, 252, 108929. [Google Scholar] [CrossRef]
- Fang, M.; Yang, Y.; Wang, N.; Wang, A.; He, Y.; Wang, J.; Jiang, Y.; Deng, Z. Genome-Wide analysis of long non-coding RNA expression profile in porcine circovirus 2-infected intestinal porcine epithelial cell line by RNA sequencing. PeerJ 2019, 7, e6577. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Li, J.; Jiang, C.; Zeng, W.; Zhang, H.; Fan, S.; He, Q. PCV2 Regulates Cellular Inflammatory Responses through Dysregulating Cellular miRNA-mRNA Networks. Viruses 2019, 11, 1055. [Google Scholar] [CrossRef]
- Quan, R.; Wei, L.; Zhu, S.; Wang, J.; Cao, Y.; Xue, C.; Yan, X.; Liu, J. Involvement of miR-15a in G0/G1 Phase Cell Cycle Arrest Induced by Porcine Circovirus Type 2 Replication. Sci. Rep. 2016, 6, 27917. [Google Scholar] [CrossRef]
- Nunez-Hernandez, F.; Perez, L.J.; Munoz, M.; Vera, G.; Tomas, A.; Egea, R.; Cordoba, S.; Segales, J.; Sanchez, A.; Nunez, J.I. Identification of microRNAs in PCV2 subclinically infected pigs by high throughput sequencing. Vet. Res. 2015, 46, 18. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Wang, W.; Yu, Z.; Wen, L.; He, K.; Fan, H. MicroRNA-30a-5p promotes replication of porcine circovirus type 2 through enhancing autophagy by targeting 14-3-3. Arch. Virol. 2017, 162, 2643–2654. [Google Scholar] [CrossRef]
- Hong, J.S.; Kim, N.H.; Choi, C.Y.; Lee, J.S.; Na, D.; Chun, T.; Lee, Y.S. Changes in cellular microRNA expression induced by porcine circovirus type 2-encoded proteins. Vet. Res. 2015, 46, 39. [Google Scholar] [CrossRef][Green Version]
- Liu, G.; Wang, Y.; Jiang, S.; Sui, M.; Wang, C.; Kang, L.; Sun, Y.; Jiang, Y. Suppression of lymphocyte apoptosis in spleen by CXCL13 after porcine circovirus type 2 infection and regulatory mechanism of CXCL13 expression in pigs. Vet. Res. 2019, 50, 17. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Wu, X.; Wang, T.; Yang, X.; Wang, Z.; Niu, Y.; Zhao, X.; Liu, S.L.; Tong, D.; Huang, Y. Porcine Circovirus Type 2 Suppresses IL-12p40 Induction via Capsid/gC1qR-Mediated MicroRNAs and Signalings. J. Immunol. 2018, 201, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.; Li, Y.; Jiang, P.; Wang, Y.; Wang, P.; Kang, L.; Wang, Y.; Sun, Y.; Jiang, Y. Identification and characterization of microRNA in the lung tissue of pigs with different susceptibilities to PCV2 infection. Vet. Res. 2018, 49, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dong, L.; Duan, Z.; Guo, R.; Zhou, D.; Liu, Z.; Liang, W.; Liu, W.; Yuan, F.; Gao, T.; et al. Expression profile of long non-coding RNAs in porcine lymphnode response to porcine circovirus type 2 infection. Microb. Pathog. 2021, 158, 105118. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Jiang, C.; Cao, H.; Zeng, W.; Zhang, X.; Li, Z.; He, Q. Porcine circovirus type 2 infection activates NF-kappaB pathway and cellular inflammatory responses through circPDCD4/miR-21/PDCD4 axis in porcine kidney 15 cell. Virus Res. 2021, 298, 198385. [Google Scholar] [CrossRef]
- He, J.; Leng, C.; Pan, J.; Li, A.; Zhang, H.; Cong, F.; Wang, H. Identification of lncRNAs Involved in PCV2 Infection of PK-15 Cells. Pathogens 2020, 9, 479. [Google Scholar] [CrossRef]



| ncRNA | Function | Reference |
|---|---|---|
| miR-15a | Promotes PCV2-induced G0/G1 cell cycle arrest | [147] |
| miR-30a-5p | Promotes cell cycle arrest at the G2 phase to regulate PCV2 replication and autophagy by interacting directly with 14-3-3 | [149] |
| miR-129-5p | PCV2 ORF2 enhances ZNF265 by down-regulating miR-139-5p, affecting the transcription and splicing of host cells | [150] |
| Let-7e | Enhances RGS16 by downregulating let-7e, involving nuclear translocation of the PCV2 ORF3 protein | |
| miR-296-5p | Participates in regulating CXCL13 expression during the response to PCV2 infection | [151] |
| miR-23a/miR-29b | Suppresses IL-12p40 expression to lower host Th1 immunity to increase the risk of other pathogenic infection | [152] |
| miR-21 | PCV2 activated the NF-κB pathway and cellular inflammatory responses through regulating circPDCD4, miR-21, and PDCD4 in PK-15 cells | [146] |
| miR-122 | Indirectly suppresses PCV2 infection by targeting genes related to the host immune system | [153] |
| lncRNA | Participates in immunosuppressive pathogenesis induced by PCV2 infection through regulating cellular component biogenesis, immune responses, and protein binding | [154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, G.; Chen, S.; Li, X.; Zhang, L.; Ren, L. Advances in Crosstalk between Porcine Circoviruses and Host. Viruses 2022, 14, 1419. https://doi.org/10.3390/v14071419
Niu G, Chen S, Li X, Zhang L, Ren L. Advances in Crosstalk between Porcine Circoviruses and Host. Viruses. 2022; 14(7):1419. https://doi.org/10.3390/v14071419
Chicago/Turabian StyleNiu, Guyu, Si Chen, Xue Li, Liying Zhang, and Linzhu Ren. 2022. "Advances in Crosstalk between Porcine Circoviruses and Host" Viruses 14, no. 7: 1419. https://doi.org/10.3390/v14071419
APA StyleNiu, G., Chen, S., Li, X., Zhang, L., & Ren, L. (2022). Advances in Crosstalk between Porcine Circoviruses and Host. Viruses, 14(7), 1419. https://doi.org/10.3390/v14071419







