Differences in Humoral Immune Response against the Type 2 Porcine Reproductive and Respiratory Syndrome Virus via Different Immune Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Virus Strain
2.2. Animal Experiments
2.3. Sample Treatment
2.3.1. Serum Samples
2.3.2. Saliva Samples
2.3.3. Tissue Samples
2.3.4. PAMs Samples
2.4. PRRSV Viral Loads Detection
2.5. Anti-PRRSV Antibodies Detection
2.6. Anti-PRRSV NAs Detection
2.7. Statistical Analysis
3. Results
3.1. Viremia Detection in Serum Samples
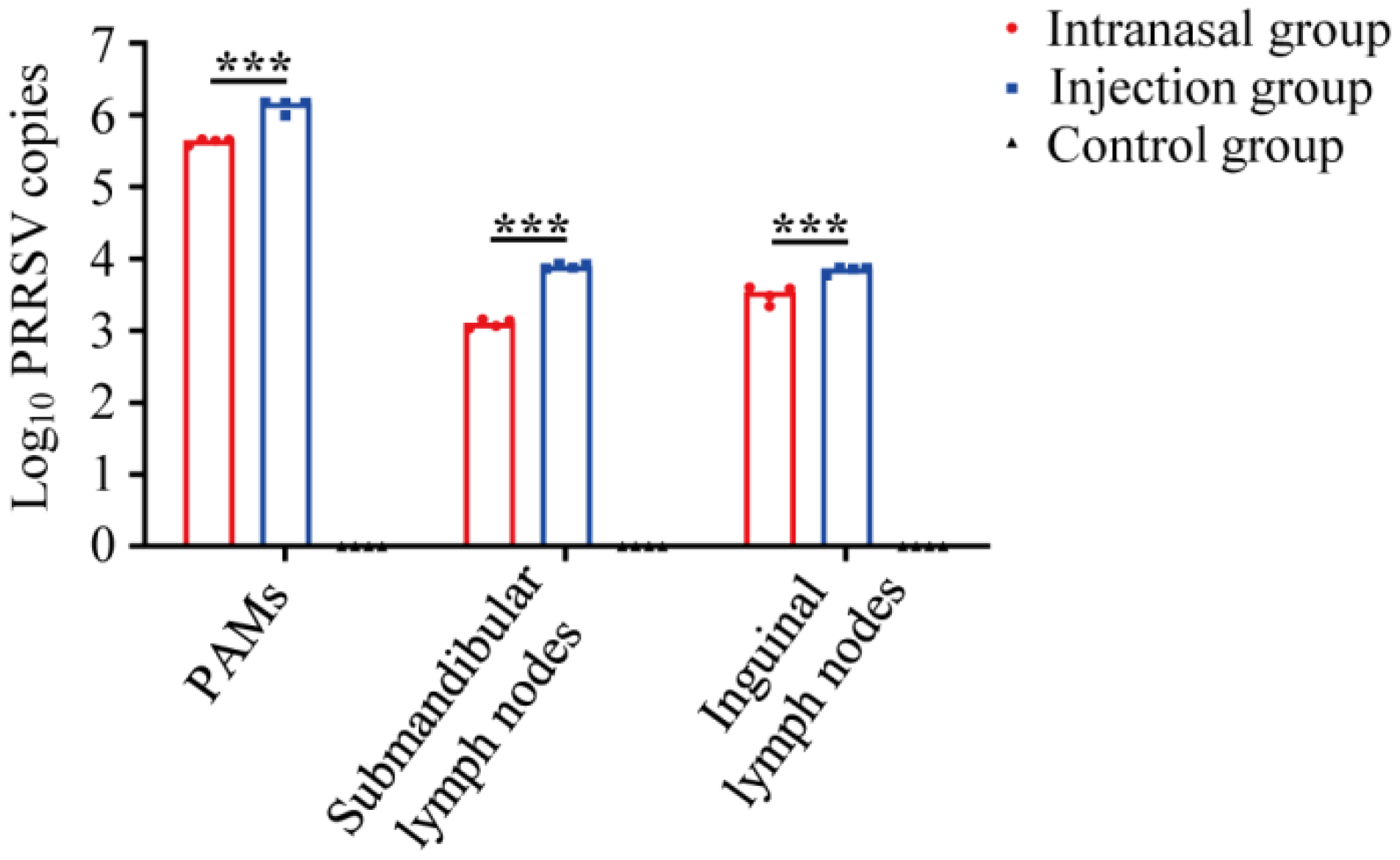
3.2. PRRSV Viral Loads Detection in Tissue Samples
3.3. Anti-PRRSV IgG Detection in Serum Samples
3.4. Anti-PRRSV SIgA Detection in Saliva Samples
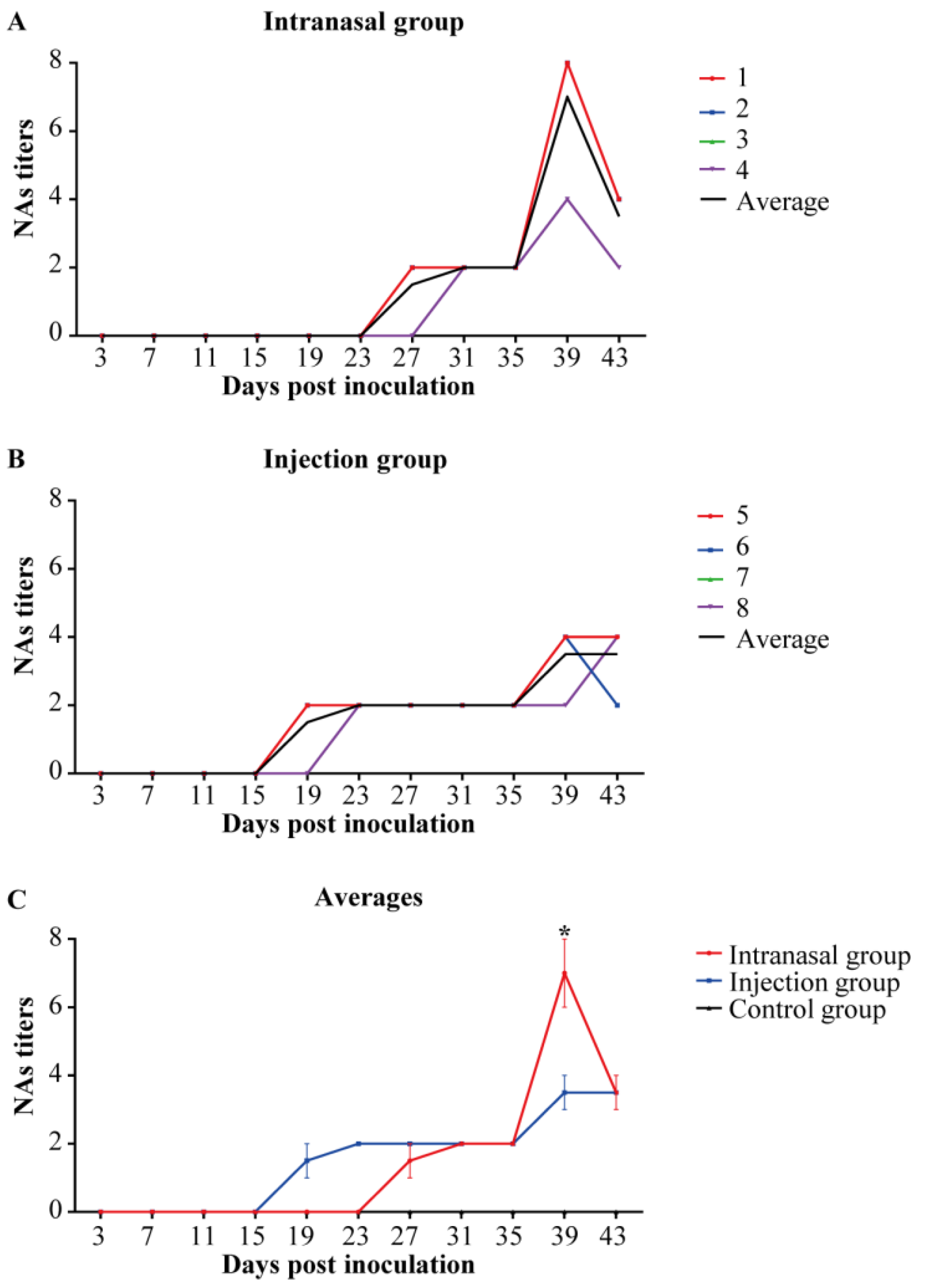
3.5. Anti-PRRSV NAs Detection in Serum Samples
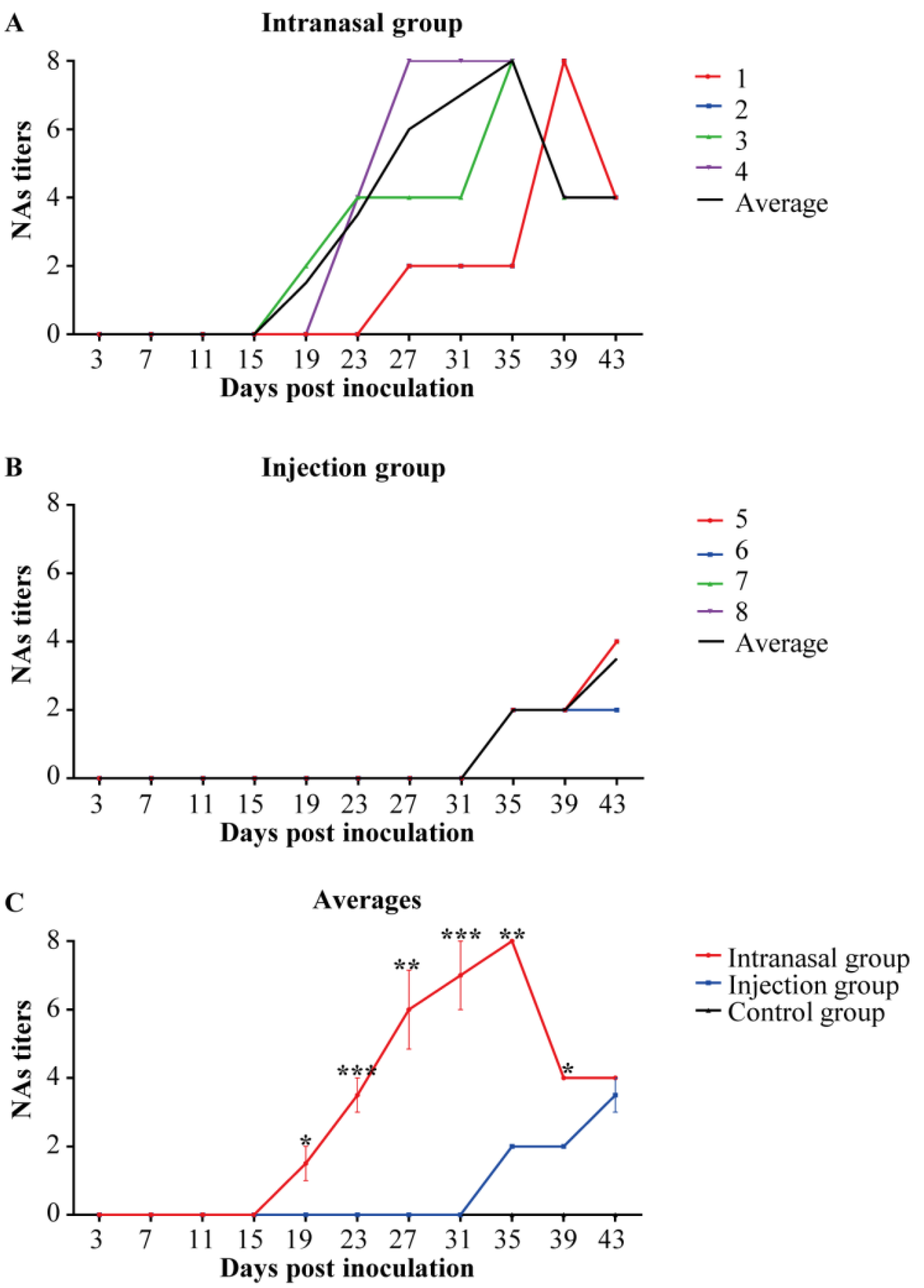
3.6. Anti-PRRSV NAs Detection in Saliva Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Davison, A.J.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; et al. Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses. Arch. Virol. 2021, 166, 2633–2648. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Park, C.; Choi, K.; Jeong, J.; Kang, I.; Park, S.J.; Chae, C. Comparison of Two Commercial Type 1 Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Modified Live Vaccines against Heterologous Type 1 and Type 2 Prrsv Challenge in Growing Pigs. Clin. Vaccine Immunol. 2015, 22, 631–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Choi, K.; Jeong, J.; Chae, C. Cross-Protection of a New Type 2 Porcine Reproductive and Respiratory Syndrome Virus (Prrsv) Modified Live Vaccine (Fostera PRRS) against Heterologous Type 1 Prrsv Challenge in Growing Pigs. Vet. Microbiol. 2015, 177, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Nan, Y.; Xiao, S.; Zhao, Q.; Zhou, E. Antiviral Strategies against PRRSV Infection. Trends Microbiol. 2017, 25, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Bello-Onaghise, G.; Wang, G.; Han, X.; Nsabimana, E.; Cui, W.; Yu, F.; Zhang, Y.; Wang, L.; Li, Z.; Cai, X.; et al. Antiviral Strategies of Chinese Herbal Medicine against Prrsv Infection. Front. Microbiol. 2020, 11, 1756. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. Commercial PRRS Modified-Live Virus Vaccines. Vaccines 2021, 9, 185. [Google Scholar] [CrossRef]
- Nan, Y.; Wu, C.; Gu, G.; Sun, W.; Zhang, Y.; Zhou, E. Improved Vaccine against PRRSV: Current Progress and Future Perspective. Front. Microbiol. 2017, 8, 1635. [Google Scholar] [CrossRef]
- Loving, C.L.; Osorio, F.A.; Murtaugh, M.P.; Zuckermann, F.A. Innate and Adaptive Immunity against Porcine Reproductive and Respiratory Syndrome Virus. Vet. Immunol. Immunopathol. 2015, 167, 1–14. [Google Scholar] [CrossRef]
- Terauchi, Y.; Sano, K.; Ainai, A.; Saito, S.; Taga, Y.; Ogawa-Goto, K.; Tamura, S.I.; Odagiri, T.; Tashiro, M.; Fujieda, M.; et al. IgA Polymerization Contributes to Efficient Virus Neutralization on Human Upper Respiratory Mucosa after Intranasal Inactivated Influenza Vaccine Administration. Hum. Vaccines Immunother. 2018, 14, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, A. Exploiting Mucosal Immunity for Antiviral Vaccines. Annu. Rev. Immunol. 2016, 34, 575–608. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Zhang, J.; Ly, H. Advances in Development and Application of Influenza Vaccines. Front. Immunol. 2021, 12, 711997. [Google Scholar] [CrossRef] [PubMed]
- Ku, M.; Bourgine, M.; Authie, P.; Lopez, J.; Nemirov, K.; Moncoq, F.; Noirat, A.; Vesin, B.; Nevo, F.; Blanc, C.; et al. Intranasal Vaccination with a Lentiviral Vector Protects against SARS-CoV-2 in Preclinical Animal Models. Cell Host Microbe 2021, 29, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Nan, Y.; Shen, M.; Ritthipichai, K.; Zhu, X.; Zhang, Y. Porcine Reproductive and Respiratory Syndrome Virus Inhibits Type I Interferon Signaling by Blocking Stat1/Stat2 Nuclear Translocation. J. Virol. 2010, 84, 11045–11055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Ma, G.; Liu, X.; Lu, Y.; Xi, S.; Ou, A.; Wei, J.; Li, B.; Shao, D.; Li, Y.; et al. Porcine Reproductive and Respiratory Syndrome Virus Counteracts Type I Interferon-Induced Early Antiviral State by Interfering Irf7 Activity. Vet. Microbiol. 2019, 229, 28–38. [Google Scholar] [CrossRef]
- Zhang, L.; Li, W.; Sun, Y.; Kong, L.; Xu, P.; Xia, P.; Zhang, G. Antibody-Mediated Porcine Reproductive and Respiratory Syndrome Virus Infection Downregulates the Production of Interferon-Alpha and Tumor Necrosis Factor-Alpha in Porcine Alveolar Macrophages Via Fc Gamma Receptor I and III. Viruses 2020, 12, 187. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Li, W.; Zhao, S.; Miao, X.; Kong, L.; Cui, Z.; Xu, P.; Chen, Y.; Zhang, G.; Chen, J.; et al. Comparison of Innate Immune Factors Induced by Prrsv Hen-3 Attenated Strain in Piglets Infected Via Intranasal Drip and Injection. Chin. J. Vet. Sci. 2021, 41, 1476–1481+1489. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Yang, Q.; Mu, C.; Duan, E.; Chen, J.; Yang, M.; Xia, P.; Cui, B. Ligation of Fc Gamma Receptor IIb Enhances Levels of Antiviral Cytokine in Response to PRRSV Infection in Vitro. Vet. Microbiol. 2012, 160, 473–480. [Google Scholar] [CrossRef]
- Duan, E.; Zhao, L.; Chen, J.; Zhang, Y.; Lu, X.; Zhang, Z.; Liu, X.; Xia, P.; Zhang, H.; Cui, B. Generation Rules of Anti-Main Structural Protein Antibodies Induced by Inactivated Prrsv Antigen. Immunol. J. 2011, 27, 234–238. [Google Scholar] [CrossRef]
- Wang, D. The Effect of Different Immunogens on PRRSV-Induced Neutralizing Antibody and Role of SHP1 in PRRSV Infection; Henan Agricultural University: Zhengzhou, China, 2015. [Google Scholar]
- Zhou, L.; Ge, X.; Yang, H. Porcine Reproductive and Respiratory Syndrome Modified Live Virus Vaccine: A “Leaky” Vaccine with Debatable Efficacy and Safety. Vaccines 2021, 9, 362. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I Interferons in Infectious Disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Madapong, A.; Temeeyasen, G.; Saeng-Chuto, K.; Tripipat, T.; Navasakuljinda, W.; Boonsoongnern, A.; Tantituvanont, A.; Nilubol, D. Humoral Immune Responses and Viral Shedding Following Vaccination with Modified Live Porcine Reproductive and Respiratory Syndrome Virus Vaccines. Arch. Virol. 2017, 162, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Goonewardene, K.B.; Chung, C.J.; Goolia, M.; Blakemore, L.; Fabian, A.; Mohamed, F.; Nfon, C.; Clavijo, A.; Dodd, K.A.; Ambagala, A. Evaluation of Oral Fluid as an Aggregate Sample for Early Detection of African Swine Fever Virus Using Four Independent Pen-Based Experimental Studies. Transbound. Emerg. Dis. 2021, 68, 2867–2877. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, M.L.; Gimenez-Lirola, L.; Ji, J.; Magtoto, R.; Henao-Diaz, Y.A.; Wang, C.; Baum, D.H.; Harmon, K.M.; Main, R.G.; Zimmerman, J.J. Detection of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV)-Specific IgM-IgA in Oral Fluid Samples Reveals PRRSV Infection in the Presence of Maternal Antibody. Vet. Microbiol. 2018, 214, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Kick, A.R.; Amaral, A.F.; Frias-De-Diego, A.; Cortes, L.M.; Fogle, J.E.; Crisci, E.; Almond, G.W.; Kaser, T. The Local and Systemic Humoral Immune Response against Homologous and Heterologous Strains of the Type 2 Porcine Reproductive and Respiratory Syndrome Virus. Front. Immunol. 2021, 12, 637613. [Google Scholar] [CrossRef]
- Wang, R.; Yang, L.; Zhang, Y.; Li, J.; Xu, L.; Xiao, Y.; Zhang, Q.; Bai, L.; Zhao, S.; Liu, E.; et al. Porcine Reproductive and Respiratory Syndrome Virus Induces Hmgb1 Secretion Via Activating Pkc-Delta to Trigger Inflammatory Response. Virology 2018, 518, 172–183. [Google Scholar] [CrossRef]
- Lemke, C.D.; Haynes, J.S.; Spaete, R.; Adolphson, D.; Vorwald, A.; Lager, K.; Butler, J.E. Lymphoid Hyperplasia Resulting in Immune Dysregulation Is Caused by Porcine Reproductive and Respiratory Syndrome Virus Infection in Neonatal Pigs. J. Immunol. 2004, 172, 1916–1925. [Google Scholar] [CrossRef]
- Nedumpun, T.; Sirisereewan, C.; Thanmuan, C.; Techapongtada, P.; Puntarotairung, R.; Naraprasertkul, S.; Thanawongnuwech, R.; Suradhat, S. Induction of Porcine Reproductive and Respiratory Syndrome Virus (Prrsv)-Specific Regulatory T Lymphocytes (Treg) in the Lungs and Tracheobronchial Lymph Nodes of Prrsv-Infected Pigs. Vet. Microbiol. 2018, 216, 13–19. [Google Scholar] [CrossRef]
- Renukaradhya, G.J.; Alekseev, K.; Jung, K.; Fang, Y.; Saif, L.J. Porcine Reproductive and Respiratory Syndrome Virus-Induced Immunosuppression Exacerbates the Inflammatory Response to Porcine Respiratory Coronavirus in Pigs. Viral Immunol. 2010, 23, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Wan, S.; Sun, N.; Sun, P.; Sun, Y.; Khan, A.; Guo, J.; Zheng, X.; Fan, K.; Yin, W.; et al. Damage to Intestinal Barrier Integrity in Piglets Caused by Porcine Reproductive and Respiratory Syndrome Virus Infection. Vet. Res. 2021, 52, 93. [Google Scholar] [CrossRef]
- Costers, S.; Lefebvre, D.J.; Delputte, P.L.; Nauwynck, H.J. Porcine Reproductive and Respiratory Syndrome Virus Modulates Apoptosis During Replication in Alveolar Macrophages. Arch. Virol. 2008, 153, 1453–1465. [Google Scholar] [CrossRef]
- Toman, M.; Celer, V.; Kavanova, L.; Leva, L.; Frolichova, J.; Ondrackova, P.; Kudlackova, H.; Nechvatalova, K.; Salat, J.; Faldyna, M. Dynamics and Differences in Systemic and Local Immune Responses after Vaccination with Inactivated and Live Commercial Vaccines and Subsequent Subclinical Infection with Prrs Virus. Front. Immunol. 2019, 10, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, K.; Binjawadagi, B.; Kittawornrat, A.; Olsen, C.; Hiremath, J.; Elkalifa, N.; Schleappi, R.; Wu, J.; Zimmerman, J.; Renukaradhya, G.J. Development and Validation of an Assay to Detect Porcine Reproductive and Respiratory Syndrome Virus-Specific Neutralizing Antibody Titers in Pig Oral Fluid Samples. Clin. Vaccine Immunol. 2013, 20, 1305–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, O.J.; Osorio, F.A. Role of Neutralizing Antibodies in Prrsv Protective Immunity. Vet. Immunol. Immunopathol. 2004, 102, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lobo, F.J.; Diez-Fuertes, F.; Simarro, I.; Castro, J.M.; Prieto, C. The Ability of Porcine Reproductive and Respiratory Syndrome Virus Isolates to Induce Broadly Reactive Neutralizing Antibodies Correlates with in Vivo Protection. Front. Immunol. 2021, 12, 691145. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wu, S.; Li, Y.; Sun, F.; Wang, Q.; Zhao, Q.; Yu, J.; Tian, F.; Wu, J.; Zhu, R.; et al. A Porcine Alveolar Macrophage Cell Line Stably Expressing CD163 Demonstrates Virus Replication and Cytokine Secretion Characteristics Similar to Primary Alveolar Macrophages Following PRRSV Infection. Vet. Microbiol. 2020, 244, 108690. [Google Scholar] [CrossRef]
- Shi, P.; Zhang, L.; Wang, J.; Lu, D.; Li, Y.; Ren, J.; Shen, M.; Zhang, L.; Huang, J. Porcine Fcepsilonri Mediates Porcine Reproductive and Respiratory Syndrome Virus Multiplication and Regulates the Inflammatory Reaction. Virol. Sin. 2018, 33, 249–260. [Google Scholar] [CrossRef]
- Su, J.; Zhou, L.; He, B.; Zhang, X.; Ge, X.; Han, J.; Guo, X.; Yang, H. Nsp2 and Gp5-M of Porcine Reproductive and Respiratory Syndrome Virus Contribute to Targets for Neutralizing Antibodies. Virol. Sin. 2019, 34, 631–640. [Google Scholar] [CrossRef]
- Tiboni, M.; Casettari, L.; Illum, L. Nasal Vaccination against SARS-CoV-2: Synergistic or Alternative to Intramuscular Vaccines? Int. J. Pharm. 2021, 603, 120686. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Sun, Y.; Zhao, S.; Cui, Z.; Chen, Y.; Xu, P.; Chen, J.; Zhang, Y.; Xia, P. Differences in Humoral Immune Response against the Type 2 Porcine Reproductive and Respiratory Syndrome Virus via Different Immune Pathways. Viruses 2022, 14, 1435. https://doi.org/10.3390/v14071435
Li W, Sun Y, Zhao S, Cui Z, Chen Y, Xu P, Chen J, Zhang Y, Xia P. Differences in Humoral Immune Response against the Type 2 Porcine Reproductive and Respiratory Syndrome Virus via Different Immune Pathways. Viruses. 2022; 14(7):1435. https://doi.org/10.3390/v14071435
Chicago/Turabian StyleLi, Wen, Yangyang Sun, Shijie Zhao, Zhiying Cui, Yu Chen, Pengli Xu, Jing Chen, Yina Zhang, and Pingan Xia. 2022. "Differences in Humoral Immune Response against the Type 2 Porcine Reproductive and Respiratory Syndrome Virus via Different Immune Pathways" Viruses 14, no. 7: 1435. https://doi.org/10.3390/v14071435





