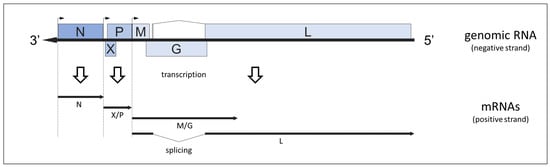
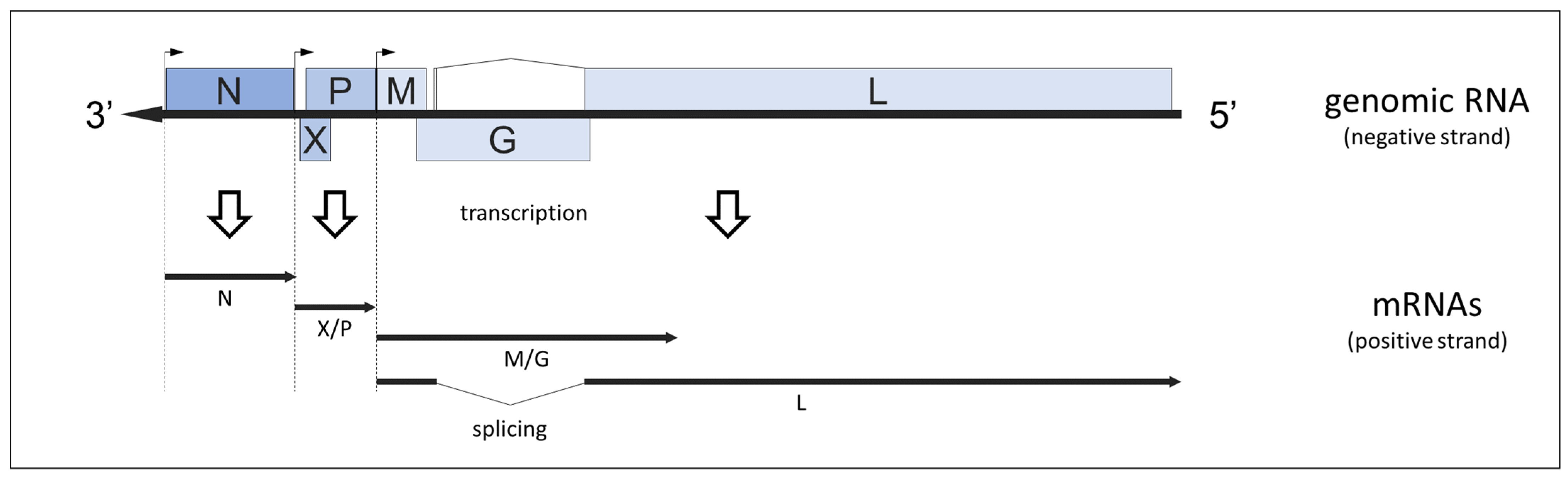
Abstract
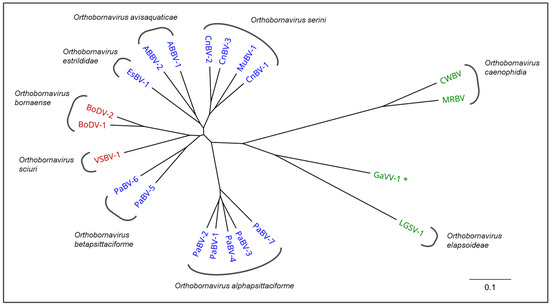
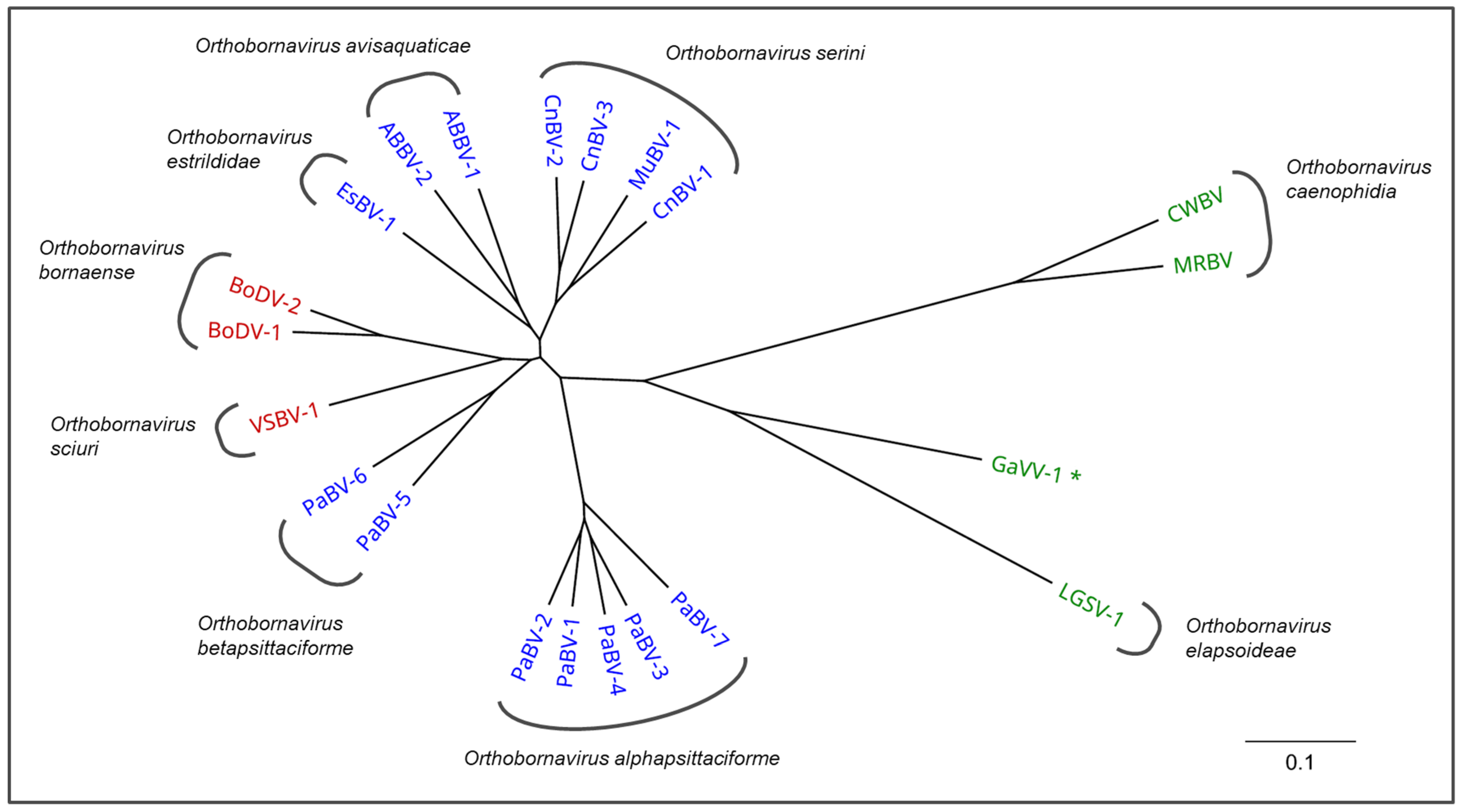
Avian bornaviruses constitute a genetically diverse group of at least 15 viruses belonging to the genus Orthobornavirus within the family Bornaviridae. After the discovery of the first avian bornaviruses in diseased psittacines in 2008, further viruses have been detected in passerines and aquatic birds. Parrot bornaviruses (PaBVs) possess the highest veterinary relevance amongst the avian bornaviruses as the causative agents of proventricular dilatation disease (PDD). PDD is a chronic and often fatal disease that may engulf a broad range of clinical presentations, typically including neurologic signs as well as impaired gastrointestinal motility, leading to proventricular dilatation. It occurs worldwide in captive psittacine populations and threatens private bird collections, zoological gardens and rehabilitation projects of endangered species. In contrast, only little is known about the pathogenic roles of passerine and waterbird bornaviruses. This comprehensive review summarizes the current knowledge on avian bornavirus infections, including their taxonomy, pathogenesis of associated diseases, epidemiology, diagnostic strategies and recent developments on prophylactic and therapeutic countermeasures.
1. Introduction
Avian bornaviruses were discovered in 2008 in parrots suffering from proventricular dilatation disease (PDD) [1,2]. PDD is a chronic neurologic and intestinal disorder of psittacine birds (order Psittaciformes) that was first described in the late 1970s in Europe and the USA. Since initially mainly macaws appeared to be affected, the disease was first described as ‘macaw wasting disease’. Additional synonyms included ‘neurotropic gastric dilatation’, ‘myenteric ganglioneuritis’ and ‘infiltrative splanchnic neuropathy’ [3,4,5,6]. However, PDD was soon found to occur in captive individuals of more than 70 psittacine species on several continents [3,4,5]. Diseases resembling PDD were sporadically detected also in non-psittacine species [5,7,8].
Although its etiology remained unknown for three decades, a transmissible nature of PDD was soon suspected based on field observations and experimental induction of the disease by transfer of tissue homogenate from diseased birds to healthy individuals [4,6]. The typical occurrence of non-suppurative encephalitis and ganglioneuritis with mononuclear infiltrates pointed towards a viral origin [3,5,9,10,11,12]. Several viruses had been discussed as possible causative agents of PDD, including paramyxoviruses, coronaviruses, alphaviruses and adenoviruses, but evidence was lacking [5,6,9]. Finally, in 2008, a group of avian bornaviruses was discovered in association with PDD [1,2], and Henle-Koch’s postulates were fulfilled by experimental reproduction of the disease [13,14,15,16,17].
Subsequently, additional genetically diverse avian bornaviruses were discovered not only in psittacines but also in birds of the orders Passeriformes, Anseriformes and Charadriiformes [18,19,20,21,22,23,24,25,26].
6. Clinical Signs, Gross Lesions and Histopathology
6.1. PaBV-Induced Disease in Psittacines
PDD and other bornavirus-induced diseases occur mainly in psittacines infected with parrot bornaviruses. The roles of PaBV-2 and PaBV-4 as causative agents of these disorders have been confirmed in numerous experimental infection studies in cockatiels [14,15,16,17,117,118,119,122,123], Patagonian conures [13] and African grey parrots [151]. Although final evidence is missing, it is widely assumed that further parrot bornaviruses, including the genetically more distantly related PaBV-5 and PaBV-6, are likewise pathogenic for psittacines.
Bornavirus-induced diseases in psittacines cover a considerable range of different clinical manifestations, with PDD being the most characteristic form. Typical PDD-like gastro-intestinal signs are proventricular dilatation (Figure 3D), delayed passage of ingesta, shedding of undigested seeds (Figure 3C) and diarrhea. As a result of the impaired digestion, birds often show body weight loss and emaciation [4,5,17,117,119,122,153]. In severe cases of proventricular dilatation, the proventriculus may rupture, resulting in peritonitis [4,5,153]. Neurological disorders represent an additional manifestation of avian bornavirus infections. Symptoms may include incoordination, seizures, tremors and lameness (Figure 3A,B) [4,15,17,151,154]. In addition, retinitis and blindness have been suggested as potential outcomes of avian bornavirus infections [155]. Behavioral disorders, such as feather plucking and auto-mutilation, have been described in association with avian bornavirus infections, but their causative relation requires further confirmation [84,151,154,156,157]. The course of disease is highly variable, ranging from peracute to chronic progression. Death without any prior clinical signs does occur in some birds [16,122]. However, the majority of birds die after chronic progression of the disease, whereas complete recovery is rarely reported [4,151,158]. The incubation period can be highly variable, ranging from three weeks to more than nine months in experimental studies, and a considerable proportion of infected birds may stay clinically healthy for several months or years or even become life-long healthy carriers [14,16,17,115,116,117,118,119,121,123,151].
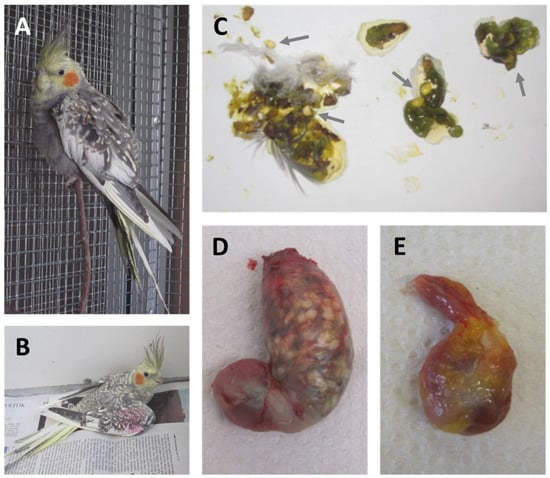
Figure 3.
Clinical signs and gross pathology in experimentally PaBV-infected cockatiels. (A) Apathy and (B) lameness of an acutely diseased PaBV-4-infected cockatiel. (C) Shedding of undigested seeds (black arrow) in the feces of a PaBV-4-infected bird. (D) Gizzard and severely dilated proventriculus of a PaBV-2-infected cockatiel. Seeds are visible through the stretched and translucid proventricular wall. (E) Gizzard and proventriculus of a healthy, non-infected control bird. Adapted with permission from Refs. [17,119]. Copyright 2014, 2016, Elsevier.
Typical gross lesions of PDD are a dilated proventriculus with a thin and often transparent wall, whereas prominent macroscopic lesions in other organs are rare [16,17,117,119,121,123,145]. Microscopic lesions in the central nervous system are characterized by non-suppurative encephalitis, including mononuclear perivascular cuffing and focal gliosis. Neuritis and ganglioneuritis with mononuclear infiltrations can be observed in peripheral nerves as well as in neuronal ganglia of a broad range of organs [13,25,118,119,121,122,145,146,148,150,159]. Inflammatory cells in bornavirus-associated lesions were identified as mainly CD3-positive T lymphocytes and Iba1-positive macrophages/microglia in perivascular cuffings in the brains of experimentally PaBV-2-infected cockatiels, whereas infiltrates in ganglia of proventriculus and intestine were composed of T lymphocytes, macrophages as well as PAX5-positive B lymphocytes [160].
6.2. Bornavirus-Induced Disease in Passerines and Aquatic Birds
The pathogenic potential of the non-psittacine avian bornaviruses known to date is a matter of controversial debate. PDD-like disease, neurological disorders and typical mononuclear infiltrations have been described for domestic canaries naturally infected with canary bornaviruses [8,19,26]. However, experimental infection of canaries with CnBV-1 and CnBV-2 did not result in clinical disease, and only minimal histopathologic alterations were observed [17,19,118]. Naturally ABBV-1-infected Canada geese as well as gulls infected with an ‘ABBV-1-like’ virus have been described to suffer from neurologic diseases and exhibit mononuclear infiltrations in the brain, but suitable bornavirus-negative controls to demonstrate an association of virus and disease were not included in these studies [23,97,101,103,161]. Experimental infections reproducing ABBV-1-induced disease have not been performed yet. Likewise, no information is available on the pathogenicity of EsBV-1 and MuBV-1 in estrildid finches or ABBV-2 in ducks [19,20,22].
6.3. Avian-Bornavirus-Induced Diseases in Supposed Non-Reservoir Hosts
Bornavirus-related diseases in avian hosts other than their presumed primary hosts have been described for natural PaBV-4 infection of a Himalayan monal [94] and a natural ABBV-1 infection of an emu [104]. In both cases, neurologic disease and non-purulent encephalitis have been described, resembling diseases caused by BoDV-1 and VSBV-1 in erroneous mammalian hosts. These two mammalian orthobornaviruses establish persistent infections without causing disease in their known reservoir hosts, namely bicolored white-toothed shrews or exotic squirrels, respectively [105,149]. However, following transmission to non-reservoir hosts, such as domestic mammals or humans, they induce usually fatal neurologic disorders due to non-purulent encephalitis [105,162,163]. Further research is required to better understand the virus–host interactions of avian bornaviruses under spill-over conditions.
11. Conclusions and Future Perspectives
Almost 15 years of active research have yielded ample knowledge on avian bornavirus infections and PDD. The considerable genetic and biological variability of this group of viruses is appreciated nowadays, and diagnostic tools are progressively implemented to cope with this fact. Important recent contributions have led to an incipient understanding of the pathogenesis of avian-bornavirus-induced diseases, revealing striking parallels to Borna disease in mammals. Experimental studies have provided a proof of concept for successful immunoprophylaxis against avian bornavirus infections using live viral vector vaccines.
However, many aspects of avian bornavirus infections and diseases remain elusive. The routes and mechanisms of natural bornavirus transmission are poorly understood, not only for avian bornaviruses but also for their mammalian relatives. Except for ABBV-1, the wild reservoirs of avian bornaviruses, their geographic origin and their way of introduction into captive bird populations are unknown. Likewise, the potential threat posed by their (re-)introduction into wild populations of endangered species remains largely speculative. Prophylactic measures are still restricted to quarantine, diagnostic monitoring and subsequent separation of infected birds since therapeutic approaches and vaccines are still in experimental phases and are unlikely to become available in practice soon. These and other issues provide manifold prospects for future avian bornavirus research. Finally, additional avian bornaviruses may be discovered, possibly belonging also to alternative bornavirus genera, such as Carbovirus or Cultervirus, potentially raising further open questions.
Funding
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) under grant RU-1923/2-1, donated to Dennis Rubbenstroth.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank Kore Schlottau for critically reviewing the manuscript.
Conflicts of Interest
The author declares no conflict of interest. The funders had no role in the writing of the manuscript, or in the decision to publish the review article.
References
- Honkavuori, K.S.; Shivaprasad, H.L.; Williams, B.L.; Quan, P.-L.; Hornig, M.; Street, C.; Palacios, G.; Hutchison, S.K.; Franca, M.; Egholm, M.; et al. Novel Borna Virus in Psittacine Birds with Proventricular Dilatation Disease. Emerg. Infect. Dis. 2008, 14, 1883–1886. [Google Scholar] [CrossRef] [PubMed]
- Kistler, A.L.; Gancz, A.; Clubb, S.; Skewes-Cox, P.; Fischer, K.; Sorber, K.; Chiu, C.Y.; Lublin, A.; Mechani, S.; Farnoushi, Y.; et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: Identification of a candidate etiologic agent. Virol. J. 2008, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.R.; Latimer, K.S.; Niagro, F.D.; Ritchie, B.W.; Campagnoli, R.P.; Norton, T.M.; McManamon, R.; Greenacre, C.B. A review of proventricular dilatation syndrome. J. Assoc. Avian Vet. 1994, 8, 69–75. [Google Scholar] [CrossRef]
- Lierz, M. Avian bornavirus and proventricular dilatation disease. In Current Therapy in Avian Medicine and Surgery; Speer, B., Ed.; Saunders: Philadelphia, PA, USA, 2015; pp. 28–46. [Google Scholar]
- Gancz, A.Y.; Clubb, S.; Shivaprasad, H.L. Advanced Diagnostic Approaches and Current Management of Proventricular Dilatation Disease. Vet. Clin. North Am. Exot. Anim. Pract. 2010, 13, 471–494. [Google Scholar] [CrossRef]
- Gregory, C.R.; Branson, R.W.; Latimer, K.S.; Steffens, W.L.; Campagnoli, R.P.; Pesti, D.; Lukert, P.D. Proventricular dilatation disease: A viral epornitic. In Proceedings of the Association of Avian Veterinarians; Association of Avian Veterinarians: Taeneck, NJ, USA, 1997; pp. 43–52. [Google Scholar]
- Daoust, P.-Y.; Julian, R.J.; Yason, C.V.; Artsob, H. Proventricular Impaction Associated with Nonsuppurative Encephalomyelitis and Ganglioneuritis in Two Canada Geese. J. Wildl. Dis. 1991, 27, 513–517. [Google Scholar] [CrossRef]
- Perpiñán, D.; Fernández-Bellon, H.; López, C.; Ramis, A. Lymphoplasmacytic Myenteric, Subepicardial, and Pulmonary Ganglioneuritis in Four Nonpsittacine Birds. J. Avian Med. Surg. 2007, 21, 210–214. [Google Scholar] [CrossRef]
- Mannl, A.; Gerlach, H.; Leipold, R. Neuropathic Gastric Dilatation in Psittaciformes. Avian Dis. 1987, 31, 214. [Google Scholar] [CrossRef]
- Berhane, Y.; Smith, D.A.; Newman, S.; Taylor, M.; Nagy, E.; Binnington, B.; Hunter, B. Peripheral neuritis in psittacine birds with proventricular dilatation disease. Avian Pathol. 2001, 30, 563–570. [Google Scholar] [CrossRef]
- Clark, F.D. Proventricular Dilatation Syndrome in Large Psittacine Birds. Avian Dis. 1984, 28, 813. [Google Scholar] [CrossRef]
- Gregory, C.R.; Latimer, K.S.; Campagnoli, R.P.; Ritchie, B.W. Histologic Evaluation of the Crop for Diagnosis of Proventricular Dilatation Syndrome in Psittacine Birds. J. Vet. Diagn. Investig. 1996, 8, 76–80. [Google Scholar] [CrossRef]
- Gray, P.; Hoppes, S.; Suchodolski, P.; Mirhosseini, N.; Payne, S.; Villanueva, I.; Shivaprasad, H.; Honkavuori, K.S.; Briese, T.; Reddy, S.M.; et al. Use of Avian Bornavirus Isolates to Induce Proventricular Dilatation Disease in Conures. Emerg. Infect. Dis. 2010, 16, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, N.; Gray, P.L.; Hoppes, S.; Tizard, I.; Shivaprasad, H.L.; Payne, S. Proventricular Dilatation Disease in Cockatiels (Nymphicus hollandicus) After Infection with a Genotype 2 Avian Bornavirus. J. Avian Med. Surg. 2011, 25, 199–204. [Google Scholar] [CrossRef]
- Payne, S.; Shivaprasad, H.L.; Mirhosseini, N.; Gray, P.; Hoppes, S.; Weissenböck, H.; Tizard, I. Unusual and severe lesions of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) acting as healthy carriers of avian bornavirus (ABV) and subsequently infected with a virulent strain of ABV. Avian Pathol. 2011, 40, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Piepenbring, A.K.; Enderlein, D.; Herzog, S.; Kaleta, E.F.; Heffels-Redmann, U.; Ressmeyer, S.; Herden, C.; Lierz, M. Pathogenesis of Avian Bornavirus in Experimentally Infected Cockatiels. Emerg. Infect. Dis. 2012, 18, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Brosinski, K.; Rinder, M.; Olbert, M.; Kaspers, B.; Korbel, R.; Staeheli, P. No contact transmission of avian bornavirus in experimentally infected cockatiels (Nymphicus hollandicus) and domestic canaries (Serinus canaria forma domestica). Vet. Microbiol. 2014, 172, 146–156. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Rinder, M.; Kaspers, B.; Staeheli, P. Efficient isolation of avian bornaviruses (ABV) from naturally infected psittacine birds and identification of a new ABV genotype from a salmon-crested cockatoo (Cacatua moluccensis). Vet. Microbiol. 2012, 161, 36–42. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Rinder, M.; Stein, M.; Höper, D.; Kaspers, B.; Brosinski, K.; Horie, M.; Schmidt, V.; Legler, M.; Korbel, R.; et al. Avian bornaviruses are widely distributed in canary birds (Serinus canaria f. domestica). Vet. Microbiol. 2013, 165, 287–295. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Schmidt, V.; Rinder, M.; Legler, M.; Corman, V.M.; Staeheli, P. Discovery of a new avian bornavirus genotype in estrildid finches (Estrildidae) in Germany. Vet. Microbiol. 2014, 168, 318–323. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Schmidt, V.; Rinder, M.; Legler, M.; Twietmeyer, S.; Schwemmer, P.; Corman, V.M. Phylogenetic Analysis Supports Horizontal Transmission as a Driving Force of the Spread of Avian Bornaviruses. PLoS ONE 2016, 11, e0160936. [Google Scholar] [CrossRef]
- Guo, J.; Shivaprasad, H.L.; Rech, R.R.; Heatley, J.J.; Tizard, I.; Payne, S. Characterization of a new genotype of avian bornavirus from wild ducks. Virol. J. 2014, 11, 197. [Google Scholar] [CrossRef]
- Payne, S.; Covaleda, L.; Jianhua, G.; Swafford, S.; Baroch, J.; Ferro, P.J.; Lupiani, B.; Heatley, J.; Tizard, I. Detection and Characterization of a Distinct Bornavirus Lineage from Healthy Canada Geese (Branta canadensis). J. Virol. 2011, 85, 12053–12056. [Google Scholar] [CrossRef]
- Philadelpho, N.A.; Rubbenstroth, D.; Guimarães, M.B.; Ferreira, A.J.P. Survey of bornaviruses in pet psittacines in Brazil reveals a novel parrot bornavirus. Vet. Microbiol. 2014, 174, 584–590. [Google Scholar] [CrossRef]
- Weissenböck, H.; Bakonyi, T.; Sekulin, K.; Ehrensperger, F.; Doneley, R.J.; Dürrwald, R.; Hoop, R.; Erdélyi, K.; Gál, J.; Kolodziejek, J.; et al. Avian Bornaviruses in Psittacine Birds from Europe and Australia with Proventricular Dilatation Disease. Emerg. Infect. Dis. 2009, 15, 1453–1459. [Google Scholar] [CrossRef]
- Weissenböck, H.; Sekulin, K.; Bakonyi, T.; Högler, S.; Nowotny, N. Novel Avian Bornavirus in a Nonpsittacine Species (Canary; Serinus canaria) with Enteric Ganglioneuritis and Encephalitis. J. Virol. 2009, 83, 11367–11371. [Google Scholar] [CrossRef]
- Kuhn, J.H.; Dürrwald, R.; Bào, Y.; Briese, T.; Carbone, K.; Clawson, A.N.; DeRisi, J.L.; Garten, W.; Jahrling, P.B.; Kolodziejek, J.; et al. Taxonomic reorganization of the family Bornaviridae. Arch. Virol. 2015, 160, 621–632. [Google Scholar] [CrossRef]
- Rubbenstroth, D.; Briese, T.; Durrwald, R.; Horie, M.; Hyndman, T.H.; Kuhn, J.H.; Nowotny, N.; Payne, S.; Stenglein, M.D.; Tomonaga, K.; et al. ICTV Virus Taxonomy Profile: Bornaviridae. J. Gen. Virol. 2021, 102, 001613. [Google Scholar] [CrossRef]
- Afonso, C.L.; Amarasinghe, G.K.; Bányai, K.; Bào, Y.; Basler, C.F.; Bavari, S.; Bejerman, N.; Blasdell, K.; Briand, F.-X.; Briese, T.; et al. Taxonomy of the order Mononegavirales: Update 2016. Arch. Virol. 2016, 161, 2351–2360. [Google Scholar] [CrossRef]
- Amarasinghe, G.K.; Bào, Y.; Basler, C.F.; Bavari, S.; Beer, M.; Bejerman, N.; Blasdell, K.R.; Bochnowski, A.; Briese, T.; Bukreyev, A.; et al. Taxonomy of the order Mononegavirales: Update 2017. Arch. Virol. 2017, 162, 2493–2504. [Google Scholar] [CrossRef]
- Amarasinghe, G.K.; Ceballos, N.G.A.; Banyard, A.C.; Basler, C.F.; Bavari, S.; Bennett, A.J.; Blasdell, K.R.; Briese, T.; Bukreyev, A.; Caì, Y.; et al. Taxonomy of the order Mononegavirales: Update 2018. Arch. Virol. 2018, 163, 2283–2294. [Google Scholar] [CrossRef]
- Amarasinghe, G.K.; Ayllón, M.A.; Bào, Y.; Basler, C.F.; Bavari, S.; Blasdell, K.R.; Briese, T.; Brown, P.A.; Bukreyev, A.; Balkema-Buschmann, A.; et al. Taxonomy of the order Mononegavirales: Update 2019. Arch. Virol. 2019, 164, 1967–1980. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Davison, A.J.; Dempsey, D.M.; Dutilh, B.E.; García, M.L.; et al. Changes to virus taxonomy and to the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2021). Arch. Virol. 2021, 166, 2633–2648. [Google Scholar] [CrossRef] [PubMed]
- Richt, J.A.; Rott, R. Borna Disease Virus: A Mystery as an Emerging Zoonotic Pathogen. Vet. J. 2001, 161, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Rubbenstroth, D.; Schlottau, K.; Schwemmle, M.; Rissland, J.; Beer, M. Human bornavirus research: Back on track! PLoS Pathog. 2019, 15, e1007873. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Tappe, D.; Höper, D.; Herden, C.; Boldt, A.; Mawrin, C.; Niederstraßer, O.; Müller, T.; Jenckel, M.; van der Grinten, E.; et al. A Variegated Squirrel Bornavirus Associated with Fatal Human Encephalitis. N. Engl. J. Med. 2015, 373, 154–162. [Google Scholar] [CrossRef]
- Tappe, D.; Frank, C.; Homeier-Bachmann, T.; Wilking, H.; Allendorf, V.; Schlottau, K.; Muñoz-Fontela, C.; Rottstegge, M.; Port, J.R.; Rissland, J.; et al. Analysis of exotic squirrel trade and detection of human infections with variegated squirrel bornavirus 1, Germany, 2005 to 2018. Eurosurveillance 2019, 24, 1800483. [Google Scholar] [CrossRef] [PubMed]
- Tappe, D.; Schlottau, K.; Cadar, D.; Hoffmann, B.; Al, D.T.E.; Bewig, B.; Hoffmann, D.; Eisermann, P.; Fickenscher, H.; Krumbholz, A.; et al. Occupation-Associated Fatal Limbic Encephalitis Caused by Variegated Squirrel Bornavirus 1, Germany, 2013. Emerg. Infect. Dis. 2018, 24, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Stenglein, M.D.; Leavitt, E.B.; Abramovitch, M.A.; McGuire, J.A.; DeRisi, J.L. Genome Sequence of a Bornavirus Recovered from an African Garter Snake (Elapsoidea loveridgei). Genome Announc. 2014, 2, e00779-14. [Google Scholar] [CrossRef]
- Fujino, K.; Horie, M.; Honda, T.; Nakamura, S.; Matsumoto, Y.; Francischetti, I.M.B.; Tomonaga, K. Evolutionarily Conserved Interaction between the Phosphoproteins and X Proteins of Bornaviruses from Different Vertebrate Species. PLoS ONE 2012, 7, e51161. [Google Scholar] [CrossRef]
- Pfaff, F.; Rubbenstroth, D. Two novel bornaviruses identified in colubrid and viperid snakes. Arch. Virol. 2021, 166, 2611–2614. [Google Scholar] [CrossRef]
- Hyndman, T.H.; Shilton, C.M.; Stenglein, M.D.; Wellehan, J.F.X., Jr. Divergent bornaviruses from Australian carpet pythons with neurological disease date the origin of extant Bornaviridae prior to the end-Cretaceous extinction. PLoS Pathog. 2018, 14, e1006881. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Chen, X.; Tian, J.-H.; Chen, L.-J.; Li, K.; Wang, W.; Eden, J.-S.; Shen, J.-J.; Liu, L.; et al. The evolutionary history of vertebrate RNA viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Briese, T.; Schneemann, A.; Lewis, A.J.; Park, Y.S.; Kim, S.; Ludwig, H.; Lipkin, W.I. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. USA 1994, 91, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, K.; Kobayashi, T.; Ikuta, K. Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 2002, 4, 491–500. [Google Scholar] [CrossRef]
- Briese, T.; de la Torre, J.C.; Lewis, A.; Ludwig, H.; Lipkin, W.I. Borna disease virus, a negative-strand RNA virus, transcribes in the nucleus of infected cells. Proc. Natl. Acad. Sci. USA 1992, 89, 11486–11489. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.A.; Schneemann, A.; Lipkin, W.I. RNA splicing in Borna disease virus, a nonsegmented, negative-strand RNA virus. J. Virol. 1994, 68, 5007–5012. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ohtaki, N.; Hayashi, Y.; Ikuta, K.; Tomonaga, K. Autogenous Translational Regulation of the Borna Disease Virus Negative Control Factor X from Polycistronic mRNA Using Host RNA Helicases. PLoS Pathog. 2009, 5, e1000654. [Google Scholar] [CrossRef]
- Poenisch, M.; Staeheli, P.; Schneider, U. Viral accessory protein X stimulates the assembly of functional Borna disease virus polymerase complexes. J. Gen. Virol. 2008, 89 Pt 6, 1442–1445. [Google Scholar] [CrossRef]
- Luo, M.; Green, T.J.; Zhang, X.; Tsao, J.; Qiu, S. Structural comparisons of the nucleoprotein from three negative strand RNA virus families. Virol. J. 2007, 4, 72. [Google Scholar] [CrossRef][Green Version]
- Rudolph, M.G.; Kraus, I.; Dickmanns, A.; Eickmann, M.; Garten, W.; Ficner, R. Crystal Structure of the Borna Disease Virus Nucleoprotein. Structure 2003, 11, 1219–1226. [Google Scholar] [CrossRef]
- Hock, M.; Kraus, I.; Schoehn, G.; Jamin, M.; Andrei-Selmer, C.; Garten, W.; Weissenhorn, W. RNA induced polymerization of the Borna disease virus nucleoprotein. Virology 2010, 397, 64–72. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shoya, Y.; Koda, T.; Takashima, I.; Lai, P.K.; Ikuta, K.; Kakinuma, M.; Kishi, M. Nuclear Targeting Activity Associated with the Amino Terminal Region of the Borna Disease Virus Nucleoprotein. Virology 1998, 243, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kamitani, W.; Zhang, G.; Watanabe, M.; Tomonaga, K.; Ikuta, K. Borna Disease Virus Nucleoprotein Requires both Nuclear Localization and Export Activities for Viral Nucleocytoplasmic Shuttling. J. Virol. 2001, 75, 3404–3412. [Google Scholar] [CrossRef] [PubMed]
- Schwemmle, M.; Salvatore, M.; Shi, L.; Richt, J.; Lee, C.H.; Lipkin, W.I. Interactions of the Borna Disease Virus P, N, and X Proteins and Their Functional Implications. J. Biol. Chem. 1998, 273, 9007–9012. [Google Scholar] [CrossRef] [PubMed]
- Schwemmle, M.; De, B.; Shi, L.; Banerjee, A.; Lipkin, W.I. Borna disease virus P-protein is phosphorylated by protein kinase Cepsilon and casein kinase II. J. Biol. Chem. 1997, 272, 21818–21823. [Google Scholar] [CrossRef] [PubMed]
- Schwemmle, M.; Jehle, C.; Shoemaker, T.; Lipkin, W.I. Characterization of the major nuclear localization signal of the Borna disease virus phosphoprotein. J. Gen. Virol. 1999, 80 Pt 1, 97–100. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Horie, M.; Tomonaga, K.; Suzuki, Y. No Evidence for Natural Selection on Endogenous Borna-Like Nucleoprotein Elements after the Divergence of Old World and New World Monkeys. PLoS ONE 2011, 6, e24403. [Google Scholar] [CrossRef]
- Schneider, U.; Naegele, M.; Staeheli, P.; Schwemmle, M. Active Borna Disease Virus Polymerase Complex Requires a Distinct Nucleoprotein-to-Phosphoprotein Ratio but No Viral X Protein. J. Virol. 2003, 77, 11781–11789. [Google Scholar] [CrossRef]
- Schwardt, M.; Mayer, D.; Frank, R.; Schneider, U.; Eickmann, M.; Planz, O.; Wolff, T.; Schwemmle, M. The negative regulator of Borna disease virus polymerase is a non-structural protein. J. Gen. Virol. 2005, 86 Pt 11, 3163–3169. [Google Scholar] [CrossRef]
- Yanai, H.; Hayashi, Y.; Watanabe, Y.; Ohtaki, N.; Kobayashi, T.; Nozaki, Y.; Ikuta, K.; Tomonaga, K. Development of a novel Borna disease virus reverse genetics system using RNA polymerase II promoter and SV40 nuclear import signal. Microbes Infect. 2006, 8, 1522–1529. [Google Scholar] [CrossRef]
- Poenisch, M.; Unterstab, G.; Wolff, T.; Staeheli, P.; Schneider, U. The X protein of Borna disease virus regulates viral polymerase activity through interaction with the P protein. J. Gen. Virol. 2004, 85 Pt 7, 1895–1898. [Google Scholar] [CrossRef]
- Kraus, I.; Eickmann, M.; Kiermayer, S.; Scheffczik, H.; Fluess, M.; Richt, J.A.; Garten, W. Open Reading Frame III of Borna Disease Virus Encodes a Nonglycosylated Matrix Protein. J. Virol. 2001, 75, 12098–12104. [Google Scholar] [CrossRef] [PubMed]
- Chase, G.; Mayer, D.; Hildebrand, A.; Frank, R.; Hayashi, Y.; Tomonaga, K.; Schwemmle, M. Borna Disease Virus Matrix Protein Is an Integral Component of the Viral Ribonucleoprotein Complex That Does Not Interfere with Polymerase Activity. J. Virol. 2007, 81, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Hatalski, C.G.; Kliche, S.; Stitz, L.; Lipkin, W.I. Neutralizing antibodies in Borna disease virus-infected rats. J. Virol. 1995, 69, 741–747. [Google Scholar] [CrossRef]
- Stoyloff, R.; Bode, L.; Borchers, K.; Ludwig, H. Neutralization of borna disease virus depends upon terminal carbohydrate residues (alpha-D-man, beta-D-GlcNAc) of glycoproteins gp17 and gp94. Intervirology 1998, 41, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Dunia, D.; Cubitt, B.; de la Torre, J.C. Mechanism of Borna Disease Virus Entry into Cells. J. Virol. 1998, 72, 783–788. [Google Scholar] [CrossRef]
- Schneider, P.A.; Hatalski, C.G.; Lewis, A.J.; Lipkin, W.I. Biochemical and functional analysis of the Borna disease virus G protein. J. Virol. 1997, 71, 331–336. [Google Scholar] [CrossRef]
- Perez, M.; Watanabe, M.; Whitt, M.A.; de la Torre, J.C. N-Terminal Domain of Borna Disease Virus G (p56) Protein Is Sufficient for Virus Receptor Recognition and Cell Entry. J. Virol. 2001, 75, 7078–7085. [Google Scholar] [CrossRef]
- Gonzalez-Dunia, D.; Cubitt, B.; Grasser, F.A.; de la Torre, J.C. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J. Virol. 1997, 71, 3208–3218. [Google Scholar] [CrossRef]
- Richt, J.A.; Furbringer, T.; Koch, A.; Pfeuffer, I.; Herden, C.; Bause-Niedrig, I.; Garten, W. Processing of the Borna Disease Virus Glycoprotein gp94 by the Subtilisin-Like Endoprotease Furin. J. Virol. 1998, 72, 4528–4533. [Google Scholar] [CrossRef]
- Bajramovic, J.J.; Munter, S.; Syan, S.; Nehrbass, U.; Brahic, M.; Gonzalez-Dunia, D. Borna Disease Virus Glycoprotein Is Required for Viral Dissemination in Neurons. J. Virol. 2003, 77, 12222–12231. [Google Scholar] [CrossRef]
- Eickmann, M.; Kiermayer, S.; Kraus, I.; Gössl, M.; Richt, J.A.; Garten, W. Maturation of Borna disease virus glycoprotein. FEBS Lett. 2005, 579, 4751–4756. [Google Scholar] [CrossRef] [PubMed]
- Daito, T.; Fujino, K.; Watanabe, Y.; Ikuta, K.; Tomonaga, K. Analysis of Intracellular Distribution of Borna Disease Virus Glycoprotein Fused with Fluorescent Markers in Living Cells. J. Vet. Med. Sci. 2011, 73, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, V.; Rinder, M.; Kaspers, B.; Staeheli, P.; Rubbenstroth, D. Impact of antigenic diversity on laboratory diagnosis of Avian bornavirus infections in birds. J. Vet. Diagn. Investig. 2014, 26, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Priestnall, S.L.; Schöniger, S.; Ivens, P.A.; Eickmann, M.; Brachthäuser, L.; Kehr, K.; Tupper, C.; Piercy, R.J.; Menzies-Gow, N.J.; Herden, C. Borna disease virus infection of a horse in Great Britain. Vet. Rec. 2011, 168, 380. [Google Scholar] [CrossRef] [PubMed]
- Werner-Keišs, N.; Garten, W.; Richt, J.A.; Porombka, D.; Algermissen, D.; Herzog, S.; Baumgärtner, W.; Herden, C. Restricted expression of Borna disease virus glycoprotein in brains of experimentally infected Lewis rats. Neuropathol. Appl. Neurobiol. 2008, 34, 590–602. [Google Scholar] [CrossRef]
- Walker, M.P.; Jordan, I.; Briese, T.; Fischer, N.; Lipkin, W.I. Expression and Characterization of the Borna Disease Virus Polymerase. J. Virol. 2000, 74, 4425–4428. [Google Scholar] [CrossRef]
- Poenisch, M.; Burger, N.; Staeheli, P.; Bauer, G.; Schneider, U. Protein X of Borna Disease Virus Inhibits Apoptosis and Promotes Viral Persistence in the Central Nervous Systems of Newborn-Infected Rats. J. Virol. 2009, 83, 4297–4307. [Google Scholar] [CrossRef][Green Version]
- Szelechowski, M.; Bétourné, A.; Monnet, Y.; Ferré, C.A.; Thouard, A.; Foret, C.; Peyrin, J.-M.; Hunot, S.; Gonzalez-Dunia, D. A viral peptide that targets mitochondria protects against neuronal degeneration in models of Parkinson’s disease. Nat. Commun. 2014, 5, 5181. [Google Scholar] [CrossRef]
- Kohno, T.; Goto, T.; Takasaki, T.; Morita, C.; Nakaya, T.; Ikuta, K.; Kurane, I.; Sano, K.; Nakai, M. Fine Structure and Morphogenesis of Borna Disease Virus. J. Virol. 1999, 73, 760–766. [Google Scholar] [CrossRef]
- Duchala, C.S.; Carbone, K.M.; Narayan, O. Preliminary Studies on the Biology of Borna Disease Virus. J. Gen. Virol. 1989, 70 Pt 12, 3507–3511. [Google Scholar] [CrossRef]
- Heffels-Redmann, U.; Enderlein, D.; Herzog, S.; Herden, C.; Piepenbring, A.; Neumann, D.; Muller, H.; Capelli, S.; Müller, H.; Oberhäuser, K.; et al. Occurrence of avian bornavirus infection in captive psittacines in various European countries and its association with proventricular dilatation disease. Avian Pathol. 2011, 40, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Ueda, K.; Ueda, A.; Honda, T.; Tomonaga, K. Detection of Avian bornavirus 5 RNA in Eclectus roratus with feather picking disorder. Microbiol. Immunol. 2012, 56, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Last, R.D.; Weissenböck, H.; Nedorost, N.; Shivaprasad, H. Avian bornavirus genotype 4 recovered from naturally infected psittacine birds with proventricular dilatation disease in South Africa. J. South Afr. Vet. Assoc. 2012, 83, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rinder, M.; Ackermann, A.; Kempf, H.; Kaspers, B.; Korbel, R.; Staeheli, P. Broad Tissue and Cell Tropism of Avian Bornavirus in Parrots with Proventricular Dilatation Disease. J. Virol. 2009, 83, 5401–5407. [Google Scholar] [CrossRef] [PubMed]
- Donatti, R.V.; Resende, M.; Junior, F.F.; Marques, M.V.R.; Ecco, R.; Shivaprasad, H.L.; De Resende, J.S.; Martins, N.R.D.S. Fatal Proventricular Dilatation Disease in Captive Native Psittacines in Brazil. Avian Dis. 2014, 58, 187–193. [Google Scholar] [CrossRef]
- Nedorost, N.; Maderner, A.; Kolodziejek, J.; Lussy, H.; Nowotny, N.; Weissenböck, H. Identification of mixed infections with different genotypes of Avian Bornavirus in psittacine birds with proventricular dilatation disease. Avian Dis. 2012, 56, 414–417. [Google Scholar] [CrossRef]
- Weissenböck, H.; Fragner, K.; Nedorost, N.; Mostegl, M.M.; Sekulin, K.; Maderner, A.; Bakonyi, T.; Nowotny, N. Localization of avian bornavirus RNA by in situ hybridization in tissues of psittacine birds with proventricular dilatation disease. Vet. Microbiol. 2010, 145, 9–16. [Google Scholar] [CrossRef]
- Kessler, S.; Heenemann, K.; Krause, T.; Twietmeyer, S.; Fuchs, J.; Lierz, M.; Corman, V.M.; Vahlenkamp, T.M.; Rubbenstroth, D. Monitoring of free-ranging and captive Psittacula populations in Western Europe for avian bornaviruses, circoviruses and polyomaviruses. Avian Pathol. 2019. epub ahead of print. [Google Scholar] [CrossRef]
- Villanueva, I.; Gray, P.; Mirhosseini, N.; Payne, S.; Hoppes, S.; Honkavuori, K.S.; Briese, T.; Turner, D.; Tizard, I. The diagnosis of proventricular dilatation disease: Use of a Western blot assay to detect antibodies against avian Borna virus. Vet. Microbiol. 2009, 143, 196–201. [Google Scholar] [CrossRef]
- Encinas-Nagel, N.; Enderlein, D.; Piepenbring, A.; Herden, C.; Heffels-Redmann, U.; Felippe, P.A.; Arns, C.; Hafez, H.M.; Lierz, M. Avian Bornavirus in Free-Ranging Psittacine Birds, Brazil. Emerg. Infect. Dis. 2014, 20, 2103–2106. [Google Scholar] [CrossRef]
- Sutherland, M.; Phalen, D.N.; Herzog, S.; Maier-Sam, K.; Lierz, M. Detection of Immunoreactivity to Psittaciform Bornavirus in the Serum of a Wild Cacatuid in Victoria, Australia. J. Wildl. Dis. 2021, 57, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Bourque, L.; Laniesse, D.; Beaufrère, H.; Pastor, A.; Ojkic, D.; Smith, D.A. Identification of avian bornavirus in a Himalayan monal (Lophophorus impejanus) with neurological disease. Avian Pathol. 2015, 44, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Sassa, Y.; Bui, V.N.; Saitoh, K.; Watanabe, Y.; Koyama, S.; Endoh, D.; Horie, M.; Tomonaga, K.; Furuya, T.; Nagai, M.; et al. Parrot bornavirus-2 and -4 RNA detected in wild bird samples in Japan are phylogenetically adjacent to those found in pet birds in Japan. Virus Genes 2015, 51, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Okanoya, K. Molecular characterization of the song control nucleus HVC in Bengalese finch brain. Brain Res. 2010, 1360, 56–76. [Google Scholar] [CrossRef] [PubMed]
- Delnatte, P.; Ojkic, D.; DeLay, J.; Campbell, D.; Crawshaw, G.; Smith, D.A. Pathology and diagnosis of avian bornavirus infection in wild Canada geese (Branta canadensis), trumpeter swans (Cygnus buccinator) and mute swans (Cygnus olor) in Canada: A retrospective study. Avian Pathol. 2013, 42, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Delnatte, P.; Nagy, E.; Ojkic, D.; Leishman, D.; Crawshaw, G.; Elias, K.; Smith, D.A. Avian bornavirus in free-ranging waterfowl: Prevalence of antibodies and cloacal shedding of viral RNA. J. Wildl. Dis. 2014, 50, 512–523. [Google Scholar] [CrossRef]
- Guo, J.; Covaleda, L.; Heatley, J.; Baroch, J.; Tizard, I.; Payne, S. Widespread avian bornavirus infection in mute swans in the Northeast United States. Vet. Med. Res. Rep. 2012, 3, 49–52. [Google Scholar]
- Thomsen, A.F.; Nielsen, J.B.; Hjulsager, C.K.; Chriél, M.; Smith, D.A.; Bertelsen, M.F. Aquatic Bird Bornavirus 1 in Wild Geese, Denmark. Emerg. Infect. Dis. 2015, 21, 2201–2203. [Google Scholar] [CrossRef]
- Murray, M.; Guo, J.; Tizard, I.; Jennings, S.; Shivaprasad, H.L.; Payne, S.; Ellis, J.C.; Van Wettere, A.J.; O’Brien, K.M. Aquatic Bird Bornavirus-Associated Disease in Free-Living Canada Geese (Branta canadensis) in the Northeastern USA. J. Wildl. Dis. 2017, 53, 607–611. [Google Scholar] [CrossRef]
- Swieton, E.; Dziadek, K.; Śmietanka, K. Avian Bornaviruses in Wild Aquatic Birds of the Anseriformes Order in Poland. Pathogens 2022, 11, 98. [Google Scholar] [CrossRef]
- Guo, J.; Tizard, I.; Baroch, J.; Shivaprasad, H.L.; Payne, S.L. Avian Bornaviruses in North American Gulls. J. Wildl. Dis. 2015, 51, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.M.W.; Ojkic, D.; Dutton, C.J.; Smith, D.A. Aquatic bird bornavirus 1 infection in a captive Emu (Dromaius novaehollandiae): Presumed natural transmission from free-ranging wild waterfowl. Avian Pathol. 2018, 47, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Dürrwald, R.; Nowotny, N.; Beer, M.; Kuhn, J.H. Infections caused by Bornaviruses. In Clinical Virology, 4th ed.; Richman, D.D., Whitley, R.J., Hayden, F.G., Eds.; American Society for Microbiology: Washington, DC, USA, 2016; pp. 1395–1407. [Google Scholar]
- Ludwig, H. Essentials in Bornavirus Virology—An Epilogue. APMIS 2008, 116, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Matthias, D. Weitere Untersuchungen zur Bornaschen Krankheit der Pferde und Schafe. Arch. Exp. Veterinärmed 1958, 12, 920–947. [Google Scholar]
- Berg, M.; Johansson, M.; Montell, H.; Berg, A.-L. Wild birds as a possible natural reservoir of Borna disease virus. Epidemiol. Infect. 2001, 127, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Dürrwald, R.; Kolodziejek, J.; Muluneh, A.; Herzog, S.; Nowotny, N. Epidemiological pattern of classical Borna disease and regional genetic clustering of Borna disease viruses point towards the existence of to-date unknown endemic reservoir host populations. Microbes Infect. 2006, 8, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Ashash, E.; Malkinson, M.; Meir, R.; Perl, S.; Weisman, Y. Causes of Losses Including a Borna Disease Paralytic Syndrome Affecting Young Ostriches of One Breeding Organization over a Five-Year Period (1989–1993). Avian Dis. 1996, 40, 240. [Google Scholar] [CrossRef] [PubMed]
- Malkinson, M.; Weisman, Y.; Ashash, E.; Bode, L.; Ludwig, H. Borna disease in ostriches. Vet. Rec. 1993, 133, 304. [Google Scholar] [CrossRef] [PubMed]
- Ashash, E.; Malkinson, M.; Meir, R.; Weisman, Y. Serum therapy of Borna disease in ostriches. Vet. Rec. 1994, 135, 608. [Google Scholar]
- Weisman, Y.; Malkinson, M.; Perl, S.; Machany, S.; Lublin, A.; Ashash, E. Paresis in young ostriches. Vet. Rec. 1993, 132, 284. [Google Scholar] [CrossRef] [PubMed]
- Weisman, Y.; Malkinson, M.; Ashash, E.; Nir, A. Serum therapy of a paretic syndrome of ostriches. Vet. Rec. 1993, 133, 172. [Google Scholar] [CrossRef] [PubMed]
- Heffels-Redmann, U.; Enderlein, D.; Herzog, S.; Piepenbring, A.; Bürkle, M.; Neumann, D.; Herden, C.; Lierz, M. Follow-Up Investigations on Different Courses of Natural Avian Bornavirus Infections in Psittacines. Avian Dis. 2012, 56, 153–159. [Google Scholar] [CrossRef] [PubMed]
- De Kloet, S.R.; Dorrestein, G.M. Presence of Avian Bornavirus RNA and Anti-Avian Bornavirus Antibodies in Apparently Healthy Macaws. Avian Dis. 2009, 53, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Piepenbring, A.K.; Enderlein, D.; Herzog, S.; Al-Ibadi, B.; Heffels-Redmann, U.; Heckmann, J.; Lange-Herbst, H.; Herden, C.; Lierz, M. Parrot Bornavirus (PaBV)-2 isolate causes different disease patterns in cockatiels than PaBV-4. Avian Pathol. 2016, 45, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Olbert, M.; Römer-Oberdörfer, A.; Herden, C.; Malberg, S.; Runge, S.; Staeheli, P.; Rubbenstroth, D. Viral vector vaccines expressing nucleoprotein and phosphoprotein genes of avian bornaviruses ameliorate homologous challenge infections in cockatiels and common canaries. Sci. Rep. 2016, 6, 36840. [Google Scholar] [CrossRef]
- Runge, S.; Olbert, M.; Herden, C.; Malberg, S.; Römer-Oberdörfer, A.; Staeheli, P.; Rubbenstroth, D. Viral vector vaccines protect cockatiels from inflammatory lesions after heterologous parrot bornavirus 2 challenge infection. Vaccine 2017, 35, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Hoppes, S.; Heatley, J.J.; Guo, J.; Turner, D.; Shivaprasad, H.L.; Tizard, I. Meloxicam treatmen in cockatiels (Nymphicus hollandicus) infected with Avian Bornavirus. J. Exp. Pet. Med. 2013, 22, 275–279. [Google Scholar] [CrossRef]
- Gancz, A.Y.; Kistler, A.L.; Greninger, A.L.; Farnoushi, Y.; Mechani, S.; Perl, S.; Berkowitz, A.; Perez, N.; Clubb, S.; DeRisi, J.L.; et al. Experimental induction of proventricular dilatation disease in cockatiels (Nymphicus hollandicus) inoculated with brain homogenates containing avian bornavirus 4. Virol. J. 2009, 6, 100–111. [Google Scholar] [CrossRef]
- De Araújo, J.L.; Rech, R.R.; Heatley, J.J.; Guo, J.; Giaretta, P.R.; Tizard, I.; Rodrigues-Hoffmann, A. From nerves to brain to gastrointestinal tract: A time-based study of parrot bornavirus 2 (PaBV-2) pathogenesis in cockatiels (Nymphicus hollandicus). PLoS ONE 2017, 12, e0187797. [Google Scholar] [CrossRef]
- Hameed, S.S.; Guo, J.; Tizard, I.; Shivaprasad, H.L.; Payne, S. Studies on immunity and immunopathogenesis of parrot bornaviral disease in cockatiels. Virology 2018, 515, 81–91. [Google Scholar] [CrossRef]
- Gartner, A.M.; Link, J.; Bücking, B.; Enderlein, D.; Herzog, S.; Petzold, J.; Malberg, S.; Herden, C.; Lierz, M. Age-dependent development and clinical characteristics of an experimental parrot bornavirus-4 (PaBV-4) infection in cockatiels (Nymphicus hollandicus). Avian Pathol. 2021, 50, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, J.; Enderlein, D.; Gartner, A.M.; Bücking, B.; Herzog, S.; Heffels-Redmann, U.; Malberg, S.; Herden, C.; Lierz, M. Wounds as the Portal of Entrance for Parrot Bornavirus 4 (PaBV-4) and Retrograde Axonal Transport in Experimentally Infected Cockatiels (Nymphicus hollandicus). Avian Dis. 2020, 64, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, J.; Enderlein, D.; Piepenbring, A.K.; Herzog, S.; Heffels-Redmann, U.; Malberg, S.; Herden, C.; Lierz, M. Investigation of Different Infection Routes of Parrot Bornavirus in Cockatiels. Avian Dis. 2017, 61, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lierz, M.; Piepenbring, A.; Herden, C.; Oberhäuser, K.; Heffels-Redmann, U.; Enderlein, D. Vertical Transmission of Avian Bornavirus in Psittacines. Emerg. Infect. Dis. 2011, 17, 2390–2391. [Google Scholar] [CrossRef]
- Kerski, A.; de Kloet, A.H.; de Kloet, S.R. Vertical transmission of avian bornavirus in Psittaciformes: Avian bornavirus RNA and anti-avian bornavirus antibodies in eggs, embryos, and hatchlings obtained from infected sun conures (Aratinga solstitialis). Avian Dis. 2012, 56, 471–478. [Google Scholar] [CrossRef]
- Monaco, E.; Hoppes, S.; Guo, J.; Tizard, I. The Detection of Avian Bornavirus within Psittacine Eggs. J. Avian Med. Surg. 2012, 26, 144–148. [Google Scholar] [CrossRef]
- Delnatte, P.; Nagy, E.; Ojkic, D.; Crawshaw, G.; Smith, D.A. Investigation into the possibility of vertical transmission of avian bornavirus in free-ranging Canada geese (Branta canadensis). Avian Pathol. 2014, 43, 301–304. [Google Scholar] [CrossRef]
- Bilzer, T.; Planz, O.; Lipkin, W.I.; Stitz, L. Presence of CD4+ and CD8+ T Cells and Expression of MHC Class I and MHC Class II Antigen in Horses with Borna Disease Virus-Induced Encephalitis. Brain Pathol. 1995, 5, 223–230. [Google Scholar] [CrossRef]
- Stitz, L.; Nöske, K.; Planz, O.; Furrer, E.; Lipkin, W.I.; Bilzer, T. A Functional Role for Neutralizing Antibodies in Borna Disease: Influence on Virus Tropism outside the Central Nervous System. J. Virol. 1998, 72, 8884–8892. [Google Scholar] [CrossRef]
- Herzog, S.; Kompter, C.; Frese, K.; Rott, R. Replication of Borna disease virus in rats: Age-dependent differences in tissue distribution. Med. Microbiol. Immunol. 1984, 173, 171–177. [Google Scholar] [CrossRef]
- Enbergs, T.W.V.H.K.; Vahlenkamp, T.W.; Kipar, A.; Müller, H. Experimental infection of mice with Borna disease virus (BDV): Replication and distribution of the virus after intracerebral infection. J. NeuroVirol. 2001, 7, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.A.; Herzog, S.; Kompter, C.; Frese, K.; Rott, R. Axonal transport of Borna disease virus along olfactory pathways in spontaneously and experimentally infected rats. Med. Microbiol. Immunol. 1988, 177, 51–68. [Google Scholar] [CrossRef]
- Carbone, K.M.; Duchala, C.S.; Griffin, J.W.; Kincaid, A.L.; Narayan, O. Pathogenesis of Borna disease in rats: Evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J. Virol. 1987, 61, 3431–3440. [Google Scholar] [CrossRef] [PubMed]
- Krey, H.; Ludwig, H.; Rott, R. Spread of infectious virus along the optic nerve into the retina in Borna disease virus-infected rabbits. Arch. Virol. 1979, 61, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, A.; Guelzow, T.; Staeheli, P.; Schneider, U.; Heimrich, B. Visualizing Viral Dissemination in the Mouse Nervous System, Using a Green Fluorescent Protein-Expressing Borna Disease Virus Vector. J. Virol. 2010, 84, 5438–5442. [Google Scholar] [CrossRef][Green Version]
- Stitz, L.; Schilken, D.; Frese, K. Atypical dissemination of the highly neurotropic Borna disease virus during persistent infection in cyclosporine A-treated, immunosuppressed rats. J. Virol. 1991, 65, 457–460. [Google Scholar] [CrossRef]
- Sauder, C.; Staeheli, P. Rat Model of Borna Disease Virus Transmission: Epidemiological Implications. J. Virol. 2003, 77, 12886–12890. [Google Scholar] [CrossRef]
- Puorger, M.E.; Hilbe, M.; Müller, J.-P.; Kolodziejek, J.; Nowotny, N.; Zlinszky, K.; Ehrensperger, F. Distribution of Borna Disease Virus Antigen and RNA in Tissues of Naturally Infected Bicolored White-Toothed Shrews, Crocidura leucodon, Supporting Their Role as Reservoir Host Species. Vet. Pathol. 2010, 47, 236–244. [Google Scholar] [CrossRef]
- Dürrwald, R.; Kolodziejek, J.; Weissenböck, H.; Nowotny, N. The Bicolored White-Toothed Shrew Crocidura leucodon (HERMANN 1780) Is an Indigenous Host of Mammalian Borna Disease Virus. PLoS ONE 2014, 9, e93659. [Google Scholar] [CrossRef]
- Nobach, D.; Bourg, M.; Herzog, S.; Lange-Herbst, H.; Encarnação, J.A.; Eickmann, M.; Herden, C. Shedding of Infectious Borna Disease Virus-1 in Living Bicolored White-Toothed Shrews. PLoS ONE 2015, 10, e0137018. [Google Scholar] [CrossRef]
- Schlottau, K.; Jenckel, M.; Brand, J.V.D.; Fast, C.; Herden, C.; Höper, D.; Homeier-Bachmann, T.; Thielebein, J.; Mensing, N.; Diender, B.; et al. Variegated Squirrel Bornavirus 1 in Squirrels, Germany and the Netherlands. Emerg. Infect. Dis. 2017, 23, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Wünschmann, A.; Honkavuori, K.; Briese, T.; Lipkin, W.I.; Shivers, J.; Armien, A.G. Antigen tissue distribution of Avian bornavirus (ABV) in psittacine birds with natural spontaneous proventricular dilatation disease and ABV genotype 1 infection. J. Vet. Diagn. Investig. 2011, 23, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Raghav, R.; Taylor, M.; DeLay, J.; Ojkic, D.; Pearl, D.L.; Kistler, A.L.; DeRisi, J.L.; Ganem, D.; Smith, D.A. Avian Bornavirus is Present in Many Tissues of Psittacine Birds with Histopathologic Evidence of Proventricular Dilatation Disease. J. Vet. Diagn. Investig. 2010, 22, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Heatley, J.; Villalobos, A.R. Avian bornavirus in the urine of infected birds. Vet. Med. Res. Rep. 2012, 3, 19–23. [Google Scholar] [CrossRef][Green Version]
- Ouyang, N.; Storts, R.; Tian, Y.; Wigle, W.; Villanueva, I.; Mirhosseini, N.; Payne, S.; Gray, P.; Tizard, I. Histopathology and the detection of avian bornavirus in the nervous system of birds diagnosed with proventricular dilatation disease. Avian Pathol. 2009, 38, 393–401. [Google Scholar] [CrossRef]
- Weissenböck, H.; Bago, Z.; Kolodziejek, J.; Hager, B.; Palmetzhofer, G.; Dürrwald, R.; Nowotny, N. Infections of horses and shrews with bornaviruses in Upper Austria: A novel endemic area of Borna disease. Emerg. Microbes Infect. 2017, 6, e52. [Google Scholar] [CrossRef]
- De Araujo, J.L.; Rodrigues-Hoffmann, A.; Giaretta, P.R.; Guo, J.; Heatley, J.; Tizard, I.; Rech, R.R. Distribution of Viral Antigen and Inflammatory Lesions in the Central Nervous System of Cockatiels (Nymphicus hollandicus) Experimentally Infected with Parrot Bornavirus 2. Vet. Pathol. 2018, 56, 106–117. [Google Scholar] [CrossRef]
- Högemann, C.; Richter, R.; Korbel, R.; Rinder, M. Plasma protein, haematologic and blood chemistry changes in African grey parrots (Psittacus erithacus) experimentally infected with bornavirus. Avian Pathol. 2017, 46, 556–570. [Google Scholar] [CrossRef]
- Rall, I.; Herden, C.; Amann, R.; Malberg, S.; Rubbenstroth, D. Recombinant Modified Vaccinia Virus Ankara (MVA) Vaccines Efficiently Protect Cockatiels Against Parrot Bornavirus Infection and Proventricular Dilatation Disease. Viruses 2019, 11, 1130. [Google Scholar] [CrossRef]
- Hoppes, S.; Gray, P.L.; Payne, S.; Shivaprasad, H.L.; Tizard, I. The Isolation, Pathogenesis, Diagnosis, Transmission, and Control of Avian Bornavirus and Proventricular Dilatation Disease. Vet. Clin. North Am. Exot. Anim. Pract. 2010, 13, 495–508. [Google Scholar] [CrossRef]
- Fluck, A.; Enderlein, D.; Piepenbring, A.; Heffels-Redmann, U.; Herzog, S.; Pieper, K.; Herden, C.; Lierz, M. Correlation of avian bornavirus-specific antibodies and viral ribonucleic acid shedding with neurological signs and feather-damaging behaviour in psittacine birds. Vet. Rec. 2019, 184, 476. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, A.; Pees, M.; Schmidt, V.; Weber, M.; Krautwald-Junghanns, M.-E.; Oechtering, G. Blindness as a sign of proventricular dilatation disease in a grey parrot (Psittacus erithacus erithacus). J. Small Anim. Pract. 2008, 49, 660–662. [Google Scholar] [CrossRef] [PubMed]
- Sassa, Y.; Horie, M.; Fujino, K.; Nishiura, N.; Okazaki, S.; Furuya, T.; Nagai, M.; Omatsu, T.; Kojima, A.; Mizugami, M.; et al. Molecular epidemiology of avian bornavirus from pet birds in Japan. Virus Genes 2013, 47, 173–177. [Google Scholar] [CrossRef]
- Horie, M. Parrot bornavirus infection: Correlation with neurological signs and feather picking? Vet. Rec. 2019, 184, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Staeheli, P.; Rinder, M.; Kaspers, B. Avian Bornavirus Associated with Fatal Disease in Psittacine Birds. J. Virol. 2010, 84, 6269–6275. [Google Scholar] [CrossRef]
- De Araujo, J.L.; Hameed, S.; Tizard, I.; Escandon, P.; Giaretta, P.R.; Heatley, J.J.; Hoppes, S.; Rech, R.R. Cardiac Lesions of Natural and Experimental Infection by Parrot Bornaviruses. J. Comp. Pathol. 2020, 174, 104–112. [Google Scholar] [CrossRef]
- De Araújo, J.L.; Rech, R.R.; Rodrigues-Hoffmann, A.; Giaretta, P.R.; Cirqueira, C.; Wenceslau, R.R.; Tizard, I.; Diaz-Delgado, J. Immunophenotype of the inflammatory response in the central and enteric nervous systems of cockatiels (Nymphicus hollandicus) experimentally infected with parrot bornavirus 2. Vet. Pathol. 2022, 59, 493–497. [Google Scholar] [CrossRef]
- Delnatte, P.; Berkvens, C.; Kummrow, M.; Smith, D.A.; Campbell, D.; Crawshaw, G.; Ojkic, D.; DeLay, J. New genotype of avian bornavirus in wild geese and trumpeter swans in Canada. Vet. Rec. 2011, 169, 108. [Google Scholar] [CrossRef]
- Stitz, L.; Bilzer, T.; Planz, O. The immunopathogenesis of Borna disease virus infection. Front Biosci. 2002, 7, d541–d555. [Google Scholar] [CrossRef]
- Niller, H.H.; Angstwurm, K.; Rubbenstroth, D.; Schlottau, K.; Ebinger, A.; Giese, S.; Wunderlich, S.; Banas, B.; Forth, L.F.; Hoffmann, D.; et al. Zoonotic spillover infections with Borna disease virus 1 leading to fatal human encephalitis, 1999–2019: An epidemiological investigation. Lancet Infect. Dis. 2020, 20, 467–477. [Google Scholar] [CrossRef]
- Herzog, S.; Rott, R. Replication of Borna disease virus in cell cultures. Med. Microbiol. Immunol. 1980, 168, 153–158. [Google Scholar] [CrossRef]
- Danner, K.; Heubeck, D.; Mayr, A. In vitro studies on Borna virus. I. The use of cell cultures for the demonstration, titration and production of Borna virus. Arch. Virol. 1978, 57, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Stitz, L.; Soeder, D.; Deschl, U.; Frese, K.; Rott, R. Inhibition of immune-mediated meningoencephalitis in persistently Borna disease virus-infected rats by cyclosporine A. J. Immunol. 1989, 143, 4250–4256. [Google Scholar] [PubMed]
- Richt, J.A.; Stitz, L.; Wekerle, H.; Rott, R. Borna disease, a progressive meningoencephalomyelitis as a model for CD4+ T cell-mediated immunopathology in the brain. J. Exp. Med. 1989, 170, 1045–1050. [Google Scholar] [CrossRef]
- Herzog, S.; Wonigeit, K.; Frese, K.; Hedrich, H.J.; Rott, R. Effect of Borna Disease Virus Infection on Athymic Rats. J. Gen. Virol. 1985, 66 Pt 3, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Narayan, O.; Herzog, S.; Frese, K.; Scheefers, H.; Rott, R. Behavioral Disease in Rats Caused by Immunopathological Responses to Persistent Borna Virus in the Brain. Science 1983, 220, 1401–1403. [Google Scholar] [CrossRef]
- Gierend, M.; Ludwig, H. Influence of immunosuppressive treatment on Borna Disease in rabbits. Arch. Virol. 1981, 67, 217–228. [Google Scholar] [CrossRef]
- Planz, O.; Bilzer, T.; Stitz, L. Immunopathogenic role of T-cell subsets in Borna disease virus-induced progressive encephalitis. J. Virol. 1995, 69, 896–903. [Google Scholar] [CrossRef]
- Hirano, N.; Kao, M.; Ludwig, H. Persistent, Tolerant or Subacute Infection in Borna Disease Virus-infected Rats. J. Gen. Virol. 1983, 64 Pt 7, 1521–1530. [Google Scholar] [CrossRef]
- Carbone, K.M.; Park, S.W.; Rubin, S.A.; Waltrip, R.W., 2nd; Vogelsang, G.B. Borna disease: Association with a maturation defect in the cellular immune response. J. Virol. 1991, 65, 6154–6164. [Google Scholar] [CrossRef]
- Sobbe, M.; Bilzer, T.; Gommel, S.; Nöske, K.; Planz, O.; Stitz, L. Induction of degenerative brain lesions after adoptive transfer of brain lymphocytes from Borna disease virus-infected rats: Presence of CD8+ T cells and perforin mRNA. J. Virol. 1997, 71, 2400–2407. [Google Scholar] [CrossRef] [PubMed]
- Batra, A.; Planz, O.; Bilzer, T.; Stitz, L. Precursors of Borna Disease Virus-Specific T Cells in Secondary Lymphatic Tissue of Experimentally Infected Rats. J. NeuroVirol. 2003, 9, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Hallensleben, W.; de la Torre, J.C.; Pagenstecher, A.; Zimmermann, C.; Pircher, H.; Staeheli, P. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc. Natl. Acad. Sci. USA 1999, 96, 9769–9774. [Google Scholar] [CrossRef]
- Hallensleben, W.; Schwemmle, M.; Hausmann, J.; Stitz, L.; Volk, B.; Pagenstecher, A.; Staeheli, P. Borna Disease Virus-Induced Neurological Disorder in Mice: Infection of Neonates Results in Immunopathology. J. Virol. 1998, 72, 4379–4386. [Google Scholar] [CrossRef] [PubMed]
- Bilzer, T.; Stitz, L. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J. Immunol. 1994, 153, 818–823. [Google Scholar] [PubMed]
- Planz, O.; Bilzer, T.; Sobbe, M.; Stitz, L. Lysis of major histocompatibility complex class I-bearing cells in Borna disease virus-induced degenerative encephalopathy. J. Exp. Med. 1993, 178, 163–174. [Google Scholar] [CrossRef]
- Stitz, L.; Sobbe, M.; Bilzer, T. Preventive effects of early anti-CD4 or anti-CD8 treatment on Borna disease in rats. J. Virol. 1992, 66, 3316–3323. [Google Scholar] [CrossRef]
- Richt, J.; Stitz, L.; Deschl, U.; Frese, K.; Rott, R. Borna disease virus-induced meningoencephalomyelitis caused by a virus-specific CD4+ T cell-mediated immune reaction. J. Gen. Virol. 1990, 71 Pt 11, 2565–2573. [Google Scholar] [CrossRef]
- Richt, J.A.; Schmeel, A.; Frese, K.; Carbone, K.M.; Narayan, O.; Rott, R. Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J. Exp. Med. 1994, 179, 1467–1473. [Google Scholar] [CrossRef]
- Richter, K.; Baur, K.; Ackermann, A.; Schneider, U.; Hausmann, J.; Staeheli, P. Pathogenic Potential of Borna Disease Virus Lacking the Immunodominant CD8 T-Cell Epitope. J. Virol. 2007, 81, 11187–11194. [Google Scholar] [CrossRef][Green Version]
- Schamel, K.; Staeheli, P.; Hausmann, J. Identification of the Immunodominant H-2Kk -Restricted Cytotoxic T-Cell Epitope in the Borna Disease Virus Nucleoprotein. J. Virol. 2001, 75, 8579–8588. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fassnacht, U.; Ackermann, A.; Staeheli, P.; Hausmann, J. Immunization with dendritic cells can break immunological ignorance toward a persisting virus in the central nervous system and induce partial protection against intracerebral viral challenge. J. Gen. Virol. 2004, 85 Pt 8, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Rauer, M.; Gotz, J.; Schuppli, D.; Staeheli, P.; Hausmann, J. Transgenic Mice Expressing the Nucleoprotein of Borna Disease Virus in either Neurons or Astrocytes: Decreased Susceptibility to Homotypic Infection and Disease. J. Virol. 2004, 78, 3621–3632. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Sauder, C.; Wasmer, M.; Lu, B.; Staeheli, P. Neurological disorder after Borna disease virus infection in the absence of either interferon-gamma, Fas, inducible NO synthase, or chemokine receptor CXCR3. Viral Immunol. 2004, 17, 79–85. [Google Scholar] [CrossRef]
- Hausmann, J.; Schamel, K.; Staeheli, P. CD8+ T Lymphocytes Mediate Borna Disease Virus-Induced Immunopathology Independently of Perforin. J. Virol. 2001, 75, 10460–10466. [Google Scholar] [CrossRef]
- Stitz, L.; Planz, O.; Bilzer, T.; Frei, K.; Fontana, A. Transforming growth factor-beta modulates T cell-mediated encephalitis caused by Borna disease virus. Pathogenic importance of CD8+ cells and suppression of antibody formation. J. Immunol. 1991, 147, 3581–3586. [Google Scholar]
- Herzog, S.; Frese, K.; Rott, R. Studies on the genetic control of resistance of black hooded rats to Borna disease. J. Gen. Virol. 1991, 72 Pt 3, 535–540. [Google Scholar] [CrossRef]
- Wu, Y.-J.; Schulz, H.; Lin, C.-C.; Saar, K.; Patone, G.; Fischer, H.; Hübner, N.; Heimrich, B.; Schwemmle, M. Borna disease virus-induced neuronal degeneration dependent on host genetic background and prevented by soluble factors. Proc. Natl. Acad. Sci. USA 2013, 110, 1899–1904. [Google Scholar] [CrossRef]
- Hornig, M.; Weissenböck, H.; Horscroft, N.; Lipkin, W.I. An infection-based model of neurodevelopmental damage. Proc. Natl. Acad. Sci. USA 1999, 96, 12102–12107. [Google Scholar] [CrossRef]
- Weissenböck, H.; Hornig, M.; Hickey, W.F.; Lipkin, W.I. Microglial Activation and Neuronal Apoptosis in Bornavirus Infected Neonatal Lewis Rats. Brain Pathol. 2000, 10, 260–272. [Google Scholar] [CrossRef]
- Dittrich, W.; Bode, L.; Ludwig, H.; Kao, M.; Schneider, K. Learning deficiencies in borna disease virus-infected but clinically healthy rats. Biol. Psychiatry 1989, 26, 818–828. [Google Scholar] [CrossRef]
- Jie, J.; Xu, X.; Xia, J.; Tu, Z.; Guo, Y.; Li, C.; Zhang, X.; Wang, H.; Song, W.; Xie, P. Memory Impairment Induced by Borna Disease Virus 1 Infection is Associated with Reduced H3K9 Acetylation. Cell. Physiol. Biochem. 2018, 49, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Nishino, Y.; Kobasa, D.; Rubin, S.A.; Pletnikov, M.V.; Carbone, K.M. Enhanced Neurovirulence of Borna Disease Virus Variants Associated with Nucleotide Changes in the Glycoprotein and L Polymerase Genes. J. Virol. 2002, 76, 8650–8658. [Google Scholar] [CrossRef]
- Kao, M.; Ludwig, H.; Gosztonyi, G. Adaptation of Borna Disease Virus to the Mouse. J. Gen. Virol. 1984, 65 Pt 10, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, A.; Staeheli, P.; Schneider, U. Adaptation of Borna Disease Virus to New Host Species Attributed to Altered Regulation of Viral Polymerase Activity. J. Virol. 2007, 81, 7933–7940. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, A.; Kugel, D.; Schneider, U.; Staeheli, P. Enhanced polymerase activity confers replication competence of Borna disease virus in mice. J. Gen. Virol. 2007, 88 Pt 11, 3130–3132. [Google Scholar] [CrossRef]
- Tizard, I.; Shivaprasad, H.L.; Guo, J.; Hameed, S.; Ball, J.; Payne, S. The pathogenesis of proventricular dilatation disease. Anim. Health Res. Rev. 2017, 17, 110–126. [Google Scholar] [CrossRef]
- Rossi, G.; Crosta, L.; Pesaro, S. Parrot proventricular dilation disease. Vet. Rec. Exot Anim Pract. 2008, 163, 310. [Google Scholar] [CrossRef]
- Rossi, G.; Dahlhausen, R.D.; Galosi, L.; Orosz, S.E. Avian ganglioneuritis in clinical practice. Vet. Clin. N. Am. 2018, 21, 33–67. [Google Scholar] [CrossRef]
- Leal de Araujo, J.; Tizard, I.; Guo, J.; Heatley, J.J.; Rodrigues Hoffmann, A.; Rech, R.R. Are anti-ganglioside antibodies associated with proventricular dilatation disease in birds? PeerJ 2017, 5, e3144. [Google Scholar] [CrossRef]
- Hausmann, J.; Baur, K.; Engelhardt, K.R.; Fischer, T.; Rziha, H.-J.; Staeheli, P. Vaccine-induced protection against Borna disease in wild-type and perforin-deficient mice. J. Gen. Virol. 2005, 86 Pt 2, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Henkel, M.; Planz, O.; Fischer, T.; Stitz, L.; Rziha, H.-J. Prevention of Virus Persistence and Protection against Immunopathology after Borna Disease Virus Infection of the Brain by a Novel Orf Virus Recombinant. J. Virol. 2005, 79, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, J.; Pagenstecher, A.; Baur, K.; Richter, K.; Rziha, H.-J.; Staeheli, P. CD8 T Cells Require Gamma Interferon to Clear Borna Disease Virus from the Brain and Prevent Immune System-Mediated Neuronal Damage. J. Virol. 2005, 79, 13509–13518. [Google Scholar] [CrossRef] [PubMed]
- Nöske, K.; Bilzer, T.; Planz, O.; Stitz, L. Virus-Specific CD4+ T Cells Eliminate Borna Disease Virus from the Brain via Induction of Cytotoxic CD8+ T Cells. J. Virol. 1998, 72, 4387–4395. [Google Scholar] [CrossRef]
- Haas, B.; Becht, H.; Rott, R. Purification and Properties of an Intranuclear Virus-specific Antigen from Tissue Infected with Borna Disease Virus. J. Gen. Virol. 1986, 67 Pt 2, 235–241. [Google Scholar] [CrossRef]
- Oldach, D.; Zink, M.C.; Pyper, J.M.; Herzog, S.; Rott, R.; Narayan, O.; Clements, J.E. Induction of protection against Borna disease by inoculation with high-dose-attenuated Borna disease virus. Virology 1995, 206, 426–434. [Google Scholar] [CrossRef][Green Version]
- Furrer, E.; Bilzer, T.; Stitz, L.; Planz, O. Neutralizing Antibodies in Persistent Borna Disease Virus Infection: Prophylactic Effect of gp94-Specific Monoclonal Antibodies in Preventing Encephalitis. J. Virol. 2001, 75, 943–951. [Google Scholar] [CrossRef]
- Lundgren, A.-L.; Johannisson, A.; Zimmermann, W.; Bode, L.; Rozell, B.; Muluneh, A.; Lindberg, R.; Ludwig, H. Neurological disease and encephalitis in cats experimentally infected with Borna disease virus. Acta Neuropathol. 1997, 93, 391–401. [Google Scholar] [CrossRef]
- De Kloet, A.H.; Kerski, A.; de Kloet, S.R. Diagnosis of Avian bornavirus infection in psittaciformes by serum antibody detection and reverse transcription polymerase chain reaction assay using feather calami. J. Vet. Diagn. Investig. 2011, 23, 421–429. [Google Scholar] [CrossRef]
- Delnatte, P.; Mak, M.; Ojkic, D.; Raghav, R.; DeLay, J.; Smith, D.A. Detection of Avian bornavirus in multiple tissues of infected psittacine birds using real-time reverse transcription polymerase chain reaction. J. Vet. Diagn. Investig. 2014, 26, 266–271. [Google Scholar] [CrossRef]
- Sigrist, B.; Geers, J.; Albini, S.; Rubbenstroth, D.; Wolfrum, N. A New Multiplex Real-Time RT-PCR for Simultaneous Detection and Differentiation of Avian Bornaviruses. Viruses 2021, 13, 1358. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Payne, S.; Zhang, S.; Turner, D.; Tizard, I.; Suchodolski, P. Avian Bornaviruses: Diagnosis, Isolation, and Genotyping. Curr. Protoc. Microbiol. 2014, 34, 15I.1.1–15I.1.33. [Google Scholar] [CrossRef]
- Schlottau, K.; Forth, L.; Angstwurm, K.; Höper, D.; Zecher, D.; Liesche, F.; Hoffmann, B.; Kegel, V.; Seehofer, D.; Platen, S.; et al. Fatal Encephalitic Borna Disease Virus 1 in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2018, 379, 1377–1379. [Google Scholar] [CrossRef] [PubMed]
- Herzog, S.; Enderlein, D.; Heffels-Redmann, U.; Piepenbring, A.; Neumann, D.; Kaleta, E.F.; Müller, H.; Lierz, M.; Herden, C. Indirect Immunofluorescence Assay for Intra Vitam Diagnosis of Avian Bornavirus Infection in Psittacine Birds. J. Clin. Microbiol. 2010, 48, 2282–2284. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J.M.; de Kloet, S.R. Discrepancy in the diagnosis of avian Borna disease virus infection of Psittaciformes by protein analysis of feather calami and enzyme-linked immunosorbent assay of plasma antibodies. J. Vet. Diagn. Investig. 2015, 27, 150–158. [Google Scholar] [CrossRef]
- McHugh, J.M.; de Kloet, S.R. The detection of bornavirus in birds, mammals and snakes by sandwich ELISA of bornaviral matrix protein. Arch. Vet. Sci. Med. 2021, 4, 85–102. [Google Scholar] [CrossRef]
- Horie, M.; Sassa, Y.; Iki, H.; Ebisawa, K.; Fukushi, H.; Yanai, T.; Tomonaga, K. Isolation of avian bornaviruses from psittacine birds using QT6 quail cells in Japan. J. Vet. Med. Sci. 2016, 78, 305–308. [Google Scholar] [CrossRef]
- Pham, P.H.; Leacy, A.; Deng, L.; Nagy, E.; Susta, L. Isolation of Ontario aquatic bird bornavirus 1 and characterization of its replication in immortalized avian cell lines. Virol. J. 2020, 17, 16. [Google Scholar] [CrossRef]
- Leacy, A.; Nagy, E.; Pham, P.H.; Susta, L. In Vitro and In Ovo Host Restriction of Aquatic Bird Bornavirus 1 in Different Avian Hosts. Viruses 2020, 12, 1272. [Google Scholar] [CrossRef]
- Hornig, M.; Briese, T.; Licinio, J.; Khabbaz, R.F.; Altshuler, L.L.; Potkin, S.G.; Schwemmle, M.; Siemetzki, U.; Mintz, J.; Honkavuori, K.; et al. Absence of evidence for bornavirus infection in schizophrenia, bipolar disorder and major depressive disorder. Mol. Psychiatry 2012, 17, 486–493. [Google Scholar] [CrossRef]
- Escandon, P.; Heatley, J.J.; Berghman, L.R.; Tizard, I.; Musser, J.M. Comparison Of Four Anti-Avian IgY Secondary Antibodies Used in Western Blot And Dot-Blot ELISA to Detect Avian Bornavirus Antibodies in Four Different Bird Species. Vet. Med. Res. Rep. 2019, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Musser, J.M.B.; Heatley, J.J.; Koinis, A.V.; Suchodolski, P.F.; Guo, J.; Escandon, P.; Tizard, I.R. Ribavirin Inhibits Parrot Bornavirus 4 Replication in Cell Culture. PLoS ONE 2015, 10, e0134080. [Google Scholar] [CrossRef] [PubMed]
- Reuter, A.; Horie, M.; Höper, D.; Ohnemus, A.; Narr, A.; Rinder, M.; Beer, M.; Staeheli, P.; Rubbenstroth, D. Synergistic antiviral activity of ribavirin and interferon-α against parrot bornaviruses in avian cells. J. Gen. Virol. 2016, 97, 2096–2103. [Google Scholar] [CrossRef]
- Jordan, I.; Briese, T.; Averett, D.R.; Lipkin, W.I. Inhibition of Borna Disease Virus Replication by Ribavirin. J. Virol. 1999, 73, 7903–7906. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Inagaki, H.; Araki, K.; Kariwa, H.; Arikawa, J.; Takashima, I. Inhibition of Borna disease virus replication by ribavirin in persistently infected cells. Arch. Virol. 1998, 143, 2039–2044. [Google Scholar] [CrossRef]
- Mizutani, T.; Inagaki, H.; Hayasaka, D.; Shuto, S.; Minakawa, N.; Matsuda, A.; Kariwa, H.; Takashima, I. Transcriptional control of Borna disease virus (BDV) in persistently BDV-infected cells. Arch. Virol. 1999, 144, 1937–1946. [Google Scholar] [CrossRef]
- Tokunaga, T.; Yamamoto, Y.; Sakai, M.; Tomonaga, K.; Honda, T. Antiviral activity of favipiravir (T-705) against mammalian and avian bornaviruses. Antivir. Res. 2017, 143, 237–245. [Google Scholar] [CrossRef]
- Lee, B.-J.; Matsunaga, H.; Ikuta, K.; Tomonaga, K. Ribavirin inhibits Borna disease virus proliferation and fatal neurological diseases in neonatally infected gerbils. Antivir. Res. 2008, 80, 380–384. [Google Scholar] [CrossRef]
- Solbrig, M.V.; Schlaberg, R.; Briese, T.; Horscroft, N.; Lipkin, W.I. Neuroprotection and Reduced Proliferation of Microglia in Ribavirin-Treated Bornavirus-Infected Rats. Antimicrob. Agents Chemother. 2002, 46, 2287–2291. [Google Scholar] [CrossRef]
- Hoppes, S.M.; Tizard, I.; Shivaprasad, H. Avian Bornavirus and Proventricular Dilatation Disease: Diagnostics, pathology, prevalence, and control. Vet. Clin. North Am. Exot. Anim. Pract. 2013, 16, 339–355. [Google Scholar] [CrossRef]
- Reuter, A.; Ackermann, A.; Kothlow, S.; Rinder, M.; Kaspers, B.; Staeheli, P. Avian Bornaviruses Escape Recognition by the Innate Immune System. Viruses 2010, 2, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Dietrich, D.E.; Stoyloff, R.; Emrich, H.M.; Ludwig, H. Amantadine and human Borna disease virus in vitro and in vivo in an infected patient with bipolar depression. Lancet 1997, 349, 178–179. [Google Scholar] [CrossRef]
- Cubitt, B.; De La Torre, J.J. Amantadine does not have antiviral activity against Borna disease virus. Arch. Virol. 1997, 142, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Hallensleben, W.; Zocher, M.; Staeheli, P. Borna disease virus is not sensitive to amantadine. Arch. Virol. 1997, 142, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Stitz, L.; Planz, O.; Bilzer, T. Lack of antiviral effect of amantadine in Borna disease virus infection. Med. Microbiol. Immunol. 1998, 186, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Lieb, K.; Hufert, F.T.; Bechter, K.; Bauer, J.; Kornhuber, J. Depression, Borna disease, and amantadine. Lancet 1997, 349, 958. [Google Scholar] [CrossRef]
- Huber, T.J.; Dietrich, D.E.; Emrich, H.M. Possible Use of Amantadine in Depression. Pharmacopsychiatry 1999, 32, 47–55. [Google Scholar] [CrossRef]
- Escandon, P.; Heatley, J.J.; Tizard, I.; Guo, J.; Shivaprasad, H.; Musser, J.M. Treatment with Nonsteroidal Anti-Inflammatory Drugs Fails to Ameliorate Pathology in Cockatiels Experimentally Infected with Parrot Bornavirus-2. Vet. Med. 2019, 10, 185–195. [Google Scholar] [CrossRef]
- Lewis, A.J.; Whitton, J.L.; Hatalski, C.G.; Weissenbock, H.L.; Lipkin, W.I. Effect of Immune Priming on Borna Disease. J. Virol. 1999, 73, 2541–2546. [Google Scholar] [CrossRef]
- Murray, O.; Turner, D.; Streeter, K.; Guo, J.; Shivaprasad, H.L.; Payne, S.; Tizard, I. Apparent resolution of parrot bornavirus infection in cockatiels (Nymphicus hollandicus). Vet. Med. Res. Rep. 2017, 8, 31–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).