Gamma-Delta T-Cell Phenotype and Function in DAA-Treated HIV-HCV Co-Infected and HCV-Mono-Infected Subjects
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Immune Studies
2.3. Γ-. Globulins Quantification
2.4. Statistics
3. Results
3.1. Study Population
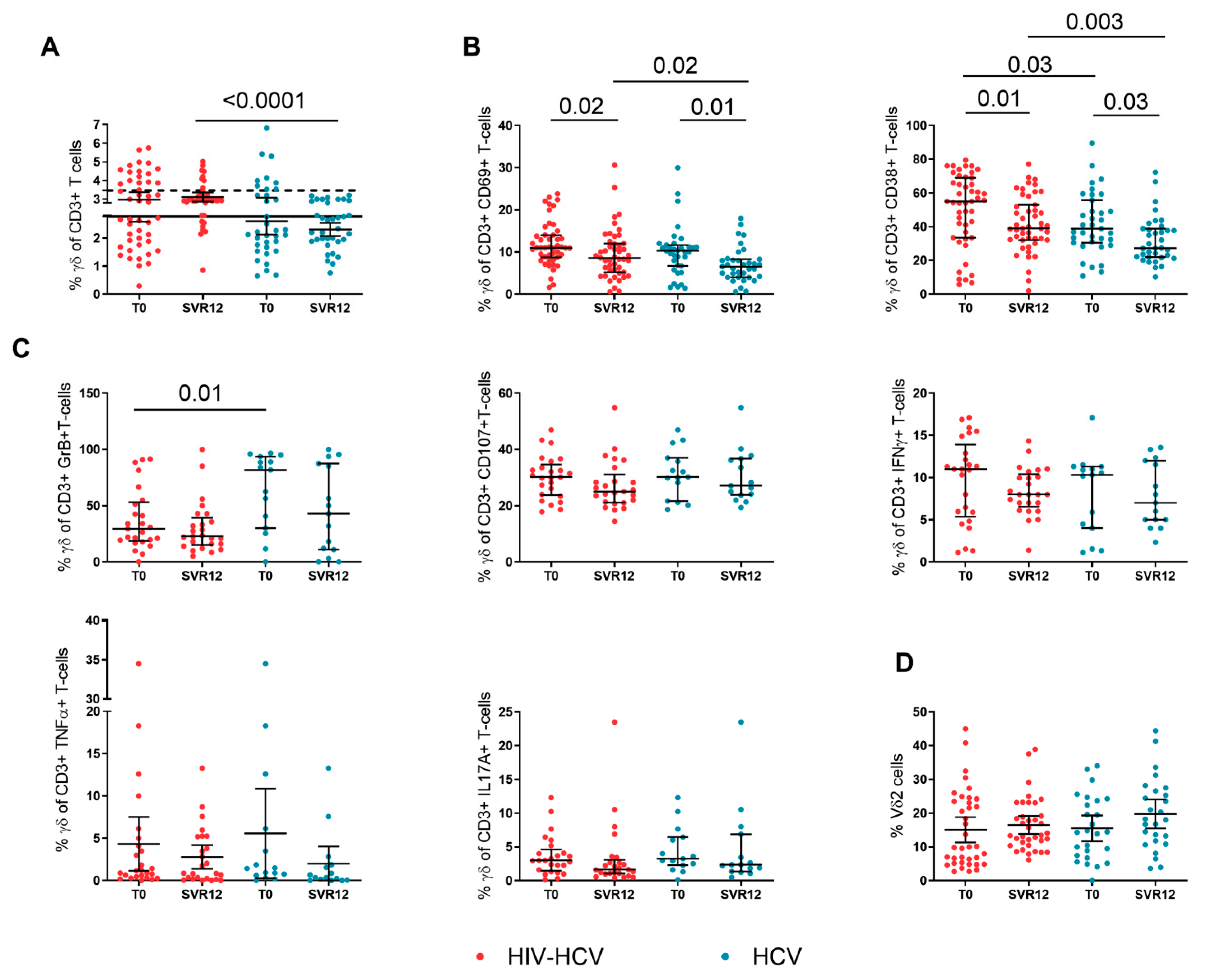
3.2. Γδ T-Cell Activation and Function during DAA Treatment
3.3. Treg and Th17-Cells during DAA Treatment
3.4. B-Cell Activation and γ-Globulins during DAA Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chrysanthidis, T.; Loli, G.; Metallidis, S.; Germanidis, G. Mechanisms of Accelerated Liver Fibrosis in HIV-HCV Coinfection. Aids Rev. 2017, 19, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, P.; Cima, S.; Zuccaro, V.; Columpsi, P.; Sarda, C.; Mariani, M.; Puoti, M.; Bruno, R. Understanding the Mechanisms of Fibrogenesis in HIV/HCV-Coinfected Patients: Implications for Clinical Practice. Aids Rev. 2015, 17, 159–170. [Google Scholar] [PubMed]
- Marchetti, G.; Nasta, P.; Bai, F.; Gatti, F.; Bellistrì, G.M.; Tincati, C.; Borghi, F.; Carosi, G.; Puoti, M.; Monforte, A.d. Circulating sCD14 is associated with virological response to pegylated-interferon-alpha/ribavirin treatment in HIV/HCV co-infected patients. PLoS ONE 2012, 7, e32028. [Google Scholar]
- Nyström, J.; Stenkvist, J.; Häggblom, A.; Weiland, O.; Nowak, P. Low levels of microbial translocation marker LBP are associated with sustained viral response after anti-HCV treatment in HIV-1/HCV co-infected patients. PLoS ONE 2015, 10, e0118643. [Google Scholar]
- Judge, C.J.; Sandberg, J.; Funderburg, N.; Sherman, K.E.; Butt, A.A.; Kang, M.; Landay, A.L.; Lederman, M.M.; Anthony, D.D. Brief Report: CD14brightCD16− monocytes and sCD14 level negatively associate with CD4-memory T-cell frequency and predict HCV-decline on therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2016, 73, 258–262. [Google Scholar] [CrossRef] [Green Version]
- Kared, H.; Saeed, S.; Klein, M.; Shoukry, N.H. CD127 Expression, Exhaustion Status and Antigen Specific Proliferation Predict Sustained Virologic Response to IFN in HCV/HIV Co-Infected Individuals. PLoS ONE 2014, 9, e101441. [Google Scholar] [CrossRef]
- Grubczak, K.; Grzeszczuk, A.; Groth, M.; Hryniewicz, A.; Kretowska-Grunwald, A.; Flisiak, R.; Moniuszko, M. Effects of Pegylated Interferon Alpha and Ribavirin (pegIFN-α/RBV) Therapeutic Approach on Regulatory T Cells in HCV-Monoinfected and HCV/HIV-Coinfected Patients. Viruses 2021, 13, 1448. [Google Scholar] [CrossRef]
- Kushner, L.E.; Wendelboe, A.M.; Lazzeroni, L.C.; Chary, A.; Winters, M.A.; Osinusi, A.; Kottilil, S.; Polis, M.A.; Holodniy, M. Immune Biomarker Differences and Changes Comparing HCV Mono-Infected, HIV/HCV Co-Infected, and HCV Spontaneously Cleared Patients. PLoS ONE 2013, 8, e60387. [Google Scholar] [CrossRef] [Green Version]
- Arizcorreta, A.; Márquez, M.; Fernández-Gutiérrez, C.; Guzmán, E.P.; Brun, F.; Rodríguez-Iglesias, M.; Girón-González, J.A. T cell receptor excision circles (TRECs), CD4+, CD8+ and their CD45RO+ and CD45RA+ subpopulations in hepatitis C virus (HCV)-HIV-co-infected patients during treatment with interferon alpha plus ribavirin: Analysis in a population on effective antiretroviral therapy. Clin. Exp. Immunol. 2006, 146, 270–277. [Google Scholar] [CrossRef]
- Brochado, Ó.; the GESIDA Study Group; Martínez, I.; Berenguer, J.; Medrano, L.; González-García, J.; Jiménez-Sousa, M..; Carrero, A.; Hontañón, V.; Navarro, J.; et al. HCV eradication with IFN-based therapy does not completely restore gene expression in PBMCs from HIV/HCV-coinfected patients. J. Biomed. Sci. 2021, 28, 1–14. [Google Scholar] [CrossRef]
- Wedemeyer, H.; Khera, T.; Strunz, B.; Björkström, N.K. Reversal of Immunity After Clearance of Chronic HCV Infection-All Reset? Front. Immunol. 2020, 11, 571166. [Google Scholar] [CrossRef] [PubMed]
- Hengst, J.; Falk, C.S.; Schlaphoff, V.; Deterding, K.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Direct-Acting Antiviral–Induced Hepatitis C Virus Clearance Does Not Completely Restore the Altered Cytokine and Chemokine Milieu in Patients with Chronic Hepatitis C. J. Infect. Dis. 2016, 214, 1965–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Hausen, A.; Dold, L.; van Heteren, P.; Huneburg, R.; Natterman, J.; Strassburg, C.P.; Spengler, U. Increased peripheral CD4 + regulatory T cells persist after successful direct-acting antiviral treatment of chronic hepatitis C. J Hepatol. 2017, 66, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Mondal, R.K.; Romani, S.; Bagchi, S.; Cairo, C.; Pauza, C.D.; Kottilil, S.; Poonia, B. Persistent gamma delta T-cell dysfunction in chronic HCV infection despite direct-acting antiviral therapy induced cure. J. Viral Hepat. 2019, 26, 1105–1116. [Google Scholar] [CrossRef]
- Ravens, S.; Hengst, J.; Schlapphoff, V.; Deterding, K.; Dhingra, A.; Schultze-Florey, C.; Koenecke, C.; Cornberg, M.; Wedemeyer, H.; Prinz, I. Human γδ T Cell Receptor Repertoires in Peripheral Blood Remain Stable Despite Clearance of Persistent Hepatitis C Virus Infection by Direct-Acting Antiviral Drug Therapy. Front. Immunol. 2018, 9, 510. [Google Scholar] [CrossRef] [Green Version]
- Sturm, N.; Thélu, M.-A.; Camous, X.; Dimitrov, G.; Ramzan, M.; Dufeu-Duchesne, T.; Bonorino, P.; Guillermet, C.; Brambilla, E.; Arvers, P.; et al. Characterization and role of intra-hepatic regulatory T cells in chronic hepatitis C pathogenesis. J. Hepatol. 2010, 53, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Rios, D.A.; Valva, P.; Casciato, P.C.; Frias, S.; Soledad Caldirola, M.; Gaillard, M.I.; Bezrodnik, L.; Bandi, J.; Galdame, O.; Ameigeiras, B.; et al. Chronic hepatitis C liver microenvironment: Role of the Th17/Treg interplay related to fibrogenesis. Sci. Rep. 2017, 7, 13283. [Google Scholar] [CrossRef] [Green Version]
- Ikeno, Y.; Ohara, D.; Takeuchi, Y.; Watanabe, H.; Kondoh, G.; Taura, K.; Uemoto, S.; Hirota, K. Foxp3+ Regulatory T Cells Inhibit CCl4-Induced Liver Inflammation and Fibrosis by Regulating Tissue Cellular Immunity. Front. Immunol. 2020, 11, 584048. [Google Scholar] [CrossRef]
- Liu, M.; Hu, Y.; Yuan, Y.; Tian, Z.; Zhang, C. γδT Cells Suppress Liver Fibrosis via Strong Cytolysis and Enhanced NK Cell-Mediated Cytotoxicity Against Hepatic Stellate Cells. Front. Immunol. 2019, 10, 477. [Google Scholar] [CrossRef] [Green Version]
- Agrati, C.; D’Offizi, G.; Narciso, P.; Selva, C.; Pucillo, L.P.; Ippolito, G.; Poccia, F. Gammadelta T cell activation by chronic HIV infection may contribute to intrahepatic vdelta1 compartmentalization and hepatitis C virus disease progression independent of highly active antiretroviral therapy. AIDS Res. Hum. Retrovir. 2001, 17, 1357–1363. [Google Scholar] [CrossRef]
- Cacoub, P.; Saadoun, D. Extrahepatic Manifestations of Chronic HCV Infection. N. Engl. J. Med. 2021, 384, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Cimini, E.; Sacchi, A.; Grassi, G.; Casetti, R.; Notari, S.; Bordoni, V.; Forini, O.; Grilli, E.; Vergori, A.; Capobianchi, M.R.; et al. Persistent gamma delta T-cell dysfunction in HCV/HIV co-infection despite direct-acting antiviral therapy-induced cure. J. Viral Hepat. 2020, 27, 754–756. [Google Scholar] [CrossRef]
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [Green Version]
- Marchetti, G.; for the ICONA Foundation Study Group; Cozzi-Lepri, A.; Tincati, C.; Calcagno, A.; Ceccherini-Silberstein, F.; De Luca, A.; Antinori, A.; Castagna, A.; Puoti, M.; et al. Immune activation and microbial translocation in liver disease progression in HIV/hepatitis co-infected patients: Results from the Icona Foundation study. BMC Infect. Dis. 2014, 14, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieland, D.; Kemming, J.; Schuch, A.; Emmerich, F.; Knolle, P.; Neumann-Haefelin, C.; Held, W.; Zehn, D.; Hofmann, M.; Thimme, R. TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat. Commun. 2017, 8, 15050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serti, E.; Chepa-Lotrea, X.; Kim, Y.J.; Keane, M.; Fryzek, N.; Liang, T.J.; Ghany, M.; Rehermann, B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015, 149, 190–200.e2. [Google Scholar] [CrossRef] [Green Version]
- Cannizzo, E.S.; Cerrone, M.; Merlini, E.; Van Wilgenburg, B.; Swadling, L.; Ancona, G.; De Bona, A.; Monforte, A.D.; Klenerman, P.; Marchetti, G. Successful direct-acting antiviral therapy in HIV/HCV co-infected patients fails to restore circulating mucosal-associated invariant T cells. Eur. J. Immunol. 2019, 49, 1127–1129. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, J.Y.; Huang, A.; Li, Y.Y.; Zhang, S.; Wei, J.; Xia, S.; Wan, Y.; Chen, W.; Yin, Z. Decreased Vδ2 γδ T cells associated with liver damage by regulation of Th17 response in patients with chronic hepatitis B. J. Infect. Dis. 2013, 208, 1294–1304. [Google Scholar] [CrossRef]
- Farcomeni, S.; Moretti, S.; Fimiani, C.; Sulekova, L.F.; Vescio, F.; Sernicola, L.; Maggiorella, M.T.; Remoli, A.L.; Picconi, O.; Mosca, L.; et al. Short- and Long-Term Immunological Responses in Chronic HCV/HIV Co-Infected Compared to HCV Mono-Infected Patients after DAA Therapy. Pathogens 2021, 10, 1488. [Google Scholar] [CrossRef]
- Amele, S.; Sandri, A.K.; Rodger, A.; Vandekerckhove, L.; Benfield, T.; Milinkovic, A.; Duvivier, C.; Stellbrink, H.; Sambatakou, H.; Chkhartishvili, N.; et al. HCV reinfection after HCV therapy among HIV/HCV-coinfected individuals in Europe. HIV Med. 2021, 23, 684–692. [Google Scholar] [CrossRef]


| HIV-HCV | HCV | p-Value | |
|---|---|---|---|
| (N = 47) | (N = 35) | ||
| Male, n (%) | 37 (79) | 22 (63) | 0.13 |
| Age (years), median (IQR) | 52 (48–57) | 54 (47–62) | 0.66 |
| Time since HCV diagnosis, years (IQR) | 12 (4–23) | 14 (2–20) | 0.22 |
| HCV genotype, n (%) | 0.8 | ||
| 1 | 23 (49) | 17 (48) | |
| 2 | 2 (4) | 5 (14) | |
| 3 | 15 (32) | 4 (11) | |
| 4 | 7 (15) | 8 (23) | |
| 5 | 0 | 1 (3) | |
| Liver fibrosis | 0.7 | ||
| Stiffness < 9.5 KPa (F0–F2) | 35 (74) | 28 (80) | |
| Stiffness ≥ 9.5 KPa (F3–F4) | 11 (23) | 7 (20) | |
| DAA regimen, n (%) | 0.02 | ||
| ombitasvir/paritaprevir/rtv | 1 (2.1) | 2 (5.7) | |
| ombitasvir/paritaprevir/rtv + dasabuvir | 2 (4.2) | 3 (8.6) | |
| glecaprevir/pibrentasvir | 9 (19.1) | 17 (48.6) | |
| ledipasvir/sofusbuvir + rbv | 4 (3.5) | 0 (0) | |
| sofusbuvir/daclatasvir | 6 (12.8) | 2 (5.7) | |
| sofusbuvir/velpatasvir | 18 (38.3) | 5 (14.3) | |
| elbasvir/grazoprevir | 5 (10.7) | 5 (14.3) | |
| ombitasvir + rbv | 2 (4.2) | 0 (0) | |
| sofusbuvir/daclatasvir + rbv | 0 (0) | 1 (2.9) | |
| DAA duration, n (%) | 0.2 | ||
| 8 weeks | 9 (19) | 12 (34) | |
| 12 weeks | 34 (72) | 21 (30) | |
| 24 weeks | 4 (8) | 2 (6) | |
| Liver transaminases at SVR12 | |||
| AST, IU/L (median, IQR) | 25 (20–30) | 21 (17–28) | 0.2 |
| ALT, IU/L (median, IQR) | 24 (15–32) | 20 (13–26) | 0.2 |
| HIV/HCV | |
|---|---|
| (N = 47) | |
| Time since HIV diagnosis, years (IQR) | 17 (11–25) |
| CD4% at T0, median (IQR) | 28 (22–34) |
| CD4, cell/mmc at T0, (IQR) | 612 (327–831) |
| CD4/CD8 ratio, median (IQR) | 0.66 (0.49–0.95) |
| Patients with HIV/RNA < 40 cp/mL, n (%) | 47 (100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bono, V.; Tincati, C.; Van Den Bogaart, L.; Cannizzo, E.S.; Rovito, R.; Augello, M.; De Bona, A.; D’Arminio Monforte, A.; Milazzo, L.; Marchetti, G. Gamma-Delta T-Cell Phenotype and Function in DAA-Treated HIV-HCV Co-Infected and HCV-Mono-Infected Subjects. Viruses 2022, 14, 1594. https://doi.org/10.3390/v14081594
Bono V, Tincati C, Van Den Bogaart L, Cannizzo ES, Rovito R, Augello M, De Bona A, D’Arminio Monforte A, Milazzo L, Marchetti G. Gamma-Delta T-Cell Phenotype and Function in DAA-Treated HIV-HCV Co-Infected and HCV-Mono-Infected Subjects. Viruses. 2022; 14(8):1594. https://doi.org/10.3390/v14081594
Chicago/Turabian StyleBono, Valeria, Camilla Tincati, Lorena Van Den Bogaart, Elvira Stefania Cannizzo, Roberta Rovito, Matteo Augello, Anna De Bona, Antonella D’Arminio Monforte, Laura Milazzo, and Giulia Marchetti. 2022. "Gamma-Delta T-Cell Phenotype and Function in DAA-Treated HIV-HCV Co-Infected and HCV-Mono-Infected Subjects" Viruses 14, no. 8: 1594. https://doi.org/10.3390/v14081594







