History of the Wastewater Assessment of Polio and Non-Polio Enteroviruses in the Slovak Republic in 1963–2019
Abstract
1. Introduction
1.1. Background of the Slovak Polio Surveillance Program
1.2. Present Status
2. Materials and Methods
2.1. Development of the Methodology
2.2. Present Methodology
2.3. Data Analysis
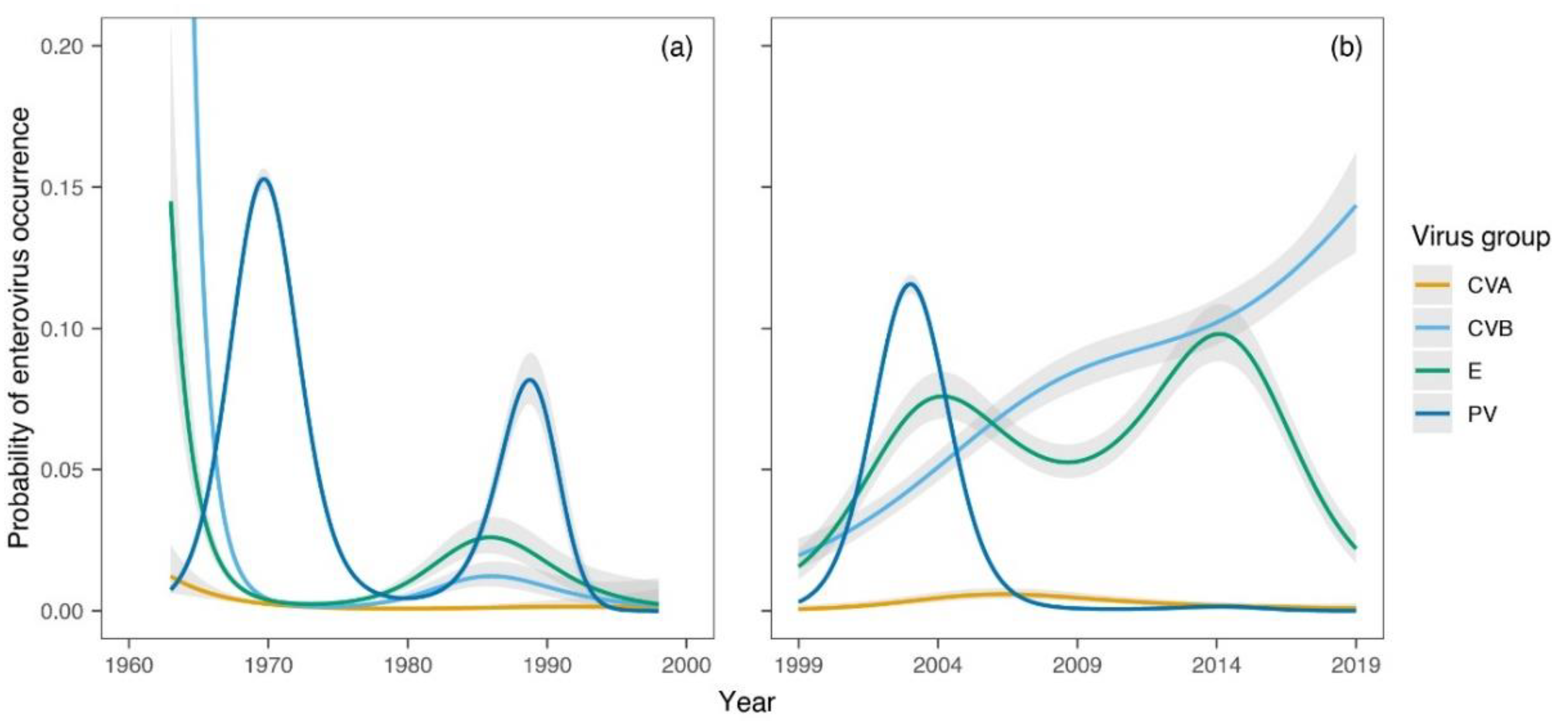
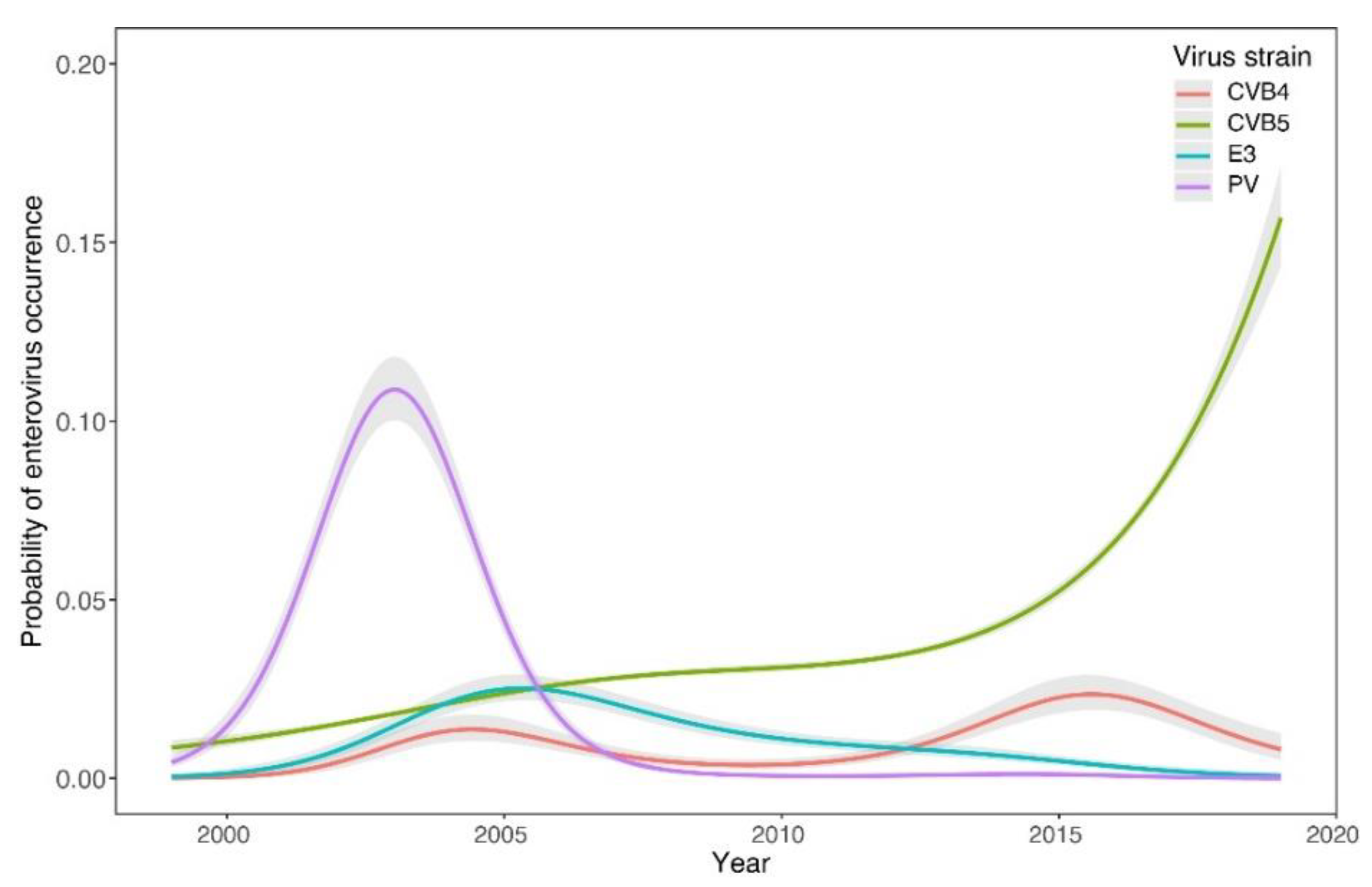
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Expert Committee on Poliomyelitis & World Health Organization. 1958. Available online: https://apps.who.int/iris/handle/10665/40404 (accessed on 1 June 2022).
- ICTV Virus Taxonomy. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 1 May 2020).
- Rajcani, J.; Ciampor, F. Medical Virology, 1st ed.; VEDA: Bratislava, Slovak Republic, 2006; pp. 402–420. [Google Scholar]
- Picornaviridae. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/positive-sense-rna-viruses/w/picornaviridae (accessed on 2 June 2022).
- Our Progress Against Polio. Available online: https://www.cdc.gov/polio/progress/index.htm (accessed on 2 June 2022).
- Poliomyelitis. Available online: https://www.who.int/health-topics/poliomyelitis#tab=tab_1 (accessed on 2 June 2022).
- Polio Global Eradication Initiative. Available online: https://polioeradication.org/polio-today/polio-now/this-week/ (accessed on 2 June 2022).
- Hovi, T.; Shulman, L.M.; van der Avoort, H.; Deshpande, J.; Roivainen, M.; de Gourville, E.M. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol. Infect. 2012, 140, 1–13. [Google Scholar] [CrossRef]
- Annual reports of Medical Microbiology department of Regional Authority of Public Health in Banska Bystrica and National Reference Centre for Poliomyelitis Public Health Authority of the Slovak Republic in Bratislava, Years 1963–2019. Available online: https://www.uvzsr.sk/index.php?option=com_content&view=category&id=25:vyrona-sprava&layout=blog&Itemid=34 (accessed on 2 June 2022).
- Sutorisova-Stolzova, M. Aktívna imunizácia proti poliomyelitíde. Lek. Obz. 1958, 8, 718–723. [Google Scholar]
- Cervenka, J.; Straka, S. Epidemiollogická problematika poliomyelitídy a ochorení napodobňujúcich poliomyelitídu od začatia masívnej aktívnej imunizácie. Lek. Obz. 1965, 14, 477–488. [Google Scholar]
- WHO Expanded Programme on Immunization. Global Eradication of Poliomyelitis by the year 2000. Wkly. Epidemiol. Rec. 1988, 63, 161–162. [Google Scholar]
- World Health Organization. Laboratory Support for Poliomyelitis Eradication: Memorandum from a WHO Meeting. Bull. World Health Organ. 1989, 67, 365–367. Available online: https://apps.who.int/iris/handle/10665/264681 (accessed on 2 June 2022).
- Enterovirus Surveillance Guidelines. Guidelines for Enterovirus Surveillance in Support of the Polio Eradication Initiative. 2015. Available online: https://www.euro.who.int/en/publications/abstracts/enterovirus-surveillance-guidelines.-guidelines-for-enterovirus-surveillance-in-support-of-the-polio-eradication-initiative (accessed on 2 June 2022).
- World Health Organization. Guidelines for Environmental Surveillance of Poliovirus Circulation. 2003. Available online: https://apps.who.int/iris/handle/10665/67854 (accessed on 2 June 2022).
- Pertinacova, J.; Bohmova, E.; Sobotova, Z. Izolácia vysoko divergentného vakcinálneho poliovírusu na Slovensku. Epidemiol. Mikrobiol. Imunol. 2004, 53, 22–24. [Google Scholar]
- Klement, C.; Kissova, R.; Lengyelova, V.; Stipalova, D.; Sobotova, Z.; Galama, J.M.D.; Bopegamage, S. Human enterovirus surveillance in the Slovak Republic from 2001 to 2011. Epidemiol. Infect. 2013, 141, 2658–2662. [Google Scholar] [CrossRef][Green Version]
- Pastuchova, K.; Kissova, R.; Lengyelova, V. Full-Area Examination of Sewage Waters for the presence of Polioviruses and Other Enteroviruses in the External Environment in the Slovak Republic. In Proceedings of the Conference of the Polio Labor-Atory Network, National Poliovirus Containment Coordinators, National Authorities for Containment, Copenhagen, Denmark, 24–26 September 2019. [Google Scholar]
- Riordan, J.T. Isolation of enteroviruses from sewage before and after vaccine administration. Yale J. Biol. Med. 1962, 34, 512–521. [Google Scholar]
- Matrajt, G.; Naughton, B.; Bandyopadhyay, A.S.; Meschke, J.S. A Review of the Most Commonly Used Methods for Sample Collection in Environmental Surveillance of Poliovirus. Clin. Infect. Dis. 2018, 67 (Suppl. 1), S90–S97. [Google Scholar] [CrossRef]
- Moore, B. The detection of enteric carriers in towns by means of sewage examination. J. R. Sanit. Inst. 1951, 71, 57–60. [Google Scholar] [CrossRef]
- Sattar, S.A.; Westwood, J.C. Isolation of apparently wild strains of poliovirus type 1 from sewage in the Ottawa area. Can. Med. Assoc. J. 1977, 116, 25–27. [Google Scholar] [PubMed]
- Wallis, C.; Melnick, J.L. Concentration of viruses on aluminum and calcium salts. Am. J. Epidemiol. 1967, 85, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Simkova, A. The Bulletin of the Czechoslovak Society of Microbiology at CSAS No. 1977; Czechoslovak Society of Microbiology: Prague, Czech Republic, 1977. [Google Scholar]
- Methodology for the control of water contamination by enteroviruses. Acta Hyg. Epidemiol. Microbiol. 1986, Suppl. 20, S1–S12.
- World Health Organization; WHO Expanded Programme on Im-munization & World Health Organization; Division of Communicable Diseases. Manual for the Virological Investigation of Poliomyelitis. 1990. Available online: https://apps.who.int/iris/handle/10665/62186 (accessed on 3 June 2022).
- World Health Organization. Polio Laboratory Manual, 4th ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/handle/10665/68762 (accessed on 3 June 2022).
- Zdrazilek, J. Citlivost lidských embryonálních plicních fibroblastů pro isolaci echovirů z odpadní vody. Acta Hyg. Epidemiol. Microbiol. 1972, 5, 107–112. [Google Scholar]
- Hayflick, L.; Moorhead, P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961, 25, 585–621. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; Chapman and Hall: New York, NY, USA; CRC: New York, NY, USA, 2017. [Google Scholar]
- Wood, S.N. On p-values for smooth components of an extended generalized additive model. Biometrika 2013, 100, 221–228. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F. Canonical correspondence analysis: A new eigenvector technique for multivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Update Notes: CANOCO Version 3.1. Wageningen: Agricultural Mathematics Group. 1990. Available online: https://edepot.wur.nl/250652 (accessed on 3 June 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 1990. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; R Package Version 2.5–6; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://CRAN.Rproject.org/package=vegan (accessed on 19 December 2019).
- Bubba, L.; Broberg, E.K.; Jasir, A.; Simmonds, P.; Harvala, H. Circulation of Non-Polio Enteroviruses in 24 EU and EEA Countries between 2015 and 2017: A Retrospective Surveillance Study. Lancet Infect. Dis. 2020, 20, 350–361. [Google Scholar] [CrossRef]
- Bisseux, M.; Debroas, D.; Mirand, A.; Archimbaud, C.; Peigue-Lafeuille, H.; Bailly, J.-L.; Henquell, C. Monitoring of enterovirus diversity in wastewater by ultra-deep sequencing: An effective complementary tool for clinical enterovirus surveillance. Water Res. 2020, 163, 115246. [Google Scholar] [CrossRef]
- Stefanelli, P.; Buttinelli, G.; Fiore, S.; Amato, C.; D’Alberto, A.; Fontana, S. Environmental and Enterovirus Surveillance activities in Suppert of Poliovirus Surveillance the Italian Experience, 2005–2018. In Proceedings of the Conference of the Polio Laboratory Network, National Poliovirus Containment Coordinators, National Authorities for Containment, Copenhagen, Denmark, 24–26 September 2019. [Google Scholar] [CrossRef]
- Palansch, M.A.; Oberste, M.S.; Whitton, J.L. Enteroviruses: Polioviruses, Coxsackieviruses, Echoviruses and Newer Entero-Viruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Cohen, J.I., Griffin, D.E., Lamb, R.A., Martin, M.A., Racaniello, V.R., Roizman, B., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 1. [Google Scholar]
- Pellegrinelli, L.; Binda, S.; Chiaramonte, I.; Primache, V.; Fiore, L.; Battistone, A.; Fiore, S.; Gambino, M.; Bubba, L.; Barbi, M. Detection and distribution of culturable Human Enteroviruses through environmental surveillance in Milan, Italy. J. Appl. Microbiol. 2013, 115, 1231–1239. [Google Scholar] [CrossRef]
- Apostol, M.; Ghidirim, V.; Spinu, C. Monitoring and Assessment of Enterovirus Circulation in the Human population and the Environment in the Republic of Moldova. In Proceedings of the Conference of the Polio Laboratory Network, National Po-Liovirus Containment Coordinators, National Authorities for Containment, Copenhagen, Denmark, 24–26 September 2019. [Google Scholar]
- Brinkman, N.E.; Fout, G.S.; Keely, S. Retrospective Surveillance of Wastewater To Examine Seasonal Dynamics of Enterovirus Infections. mSphere 2017, 2, e00099-17. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Dhole, T.N. Assessment of enteroviruses from sewage water and clinical samples during eradication phase of polio in North India. Virol. J. 2018, 15, 157. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, O.E.; Yarmolskaya, M.S.; Eremeeva, T.P.; Babkina, G.M.; Baykova, O.Y.; Akhmadishina, L.V.; Krasota, A.Y.; Kozlovskaya, L.I.; Lukashev, A.N. Environmental Surveillance for Poliovirus and Other Enteroviruses: Long-Term Experience in Moscow, Russian Federation, 2004–2017. Viruses 2019, 11, 424. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kissova, R.; Pastuchova, K.; Lengyelova, V.; Svitok, M.; Mikas, J.; Klement, C.; Bopegamage, S. History of the Wastewater Assessment of Polio and Non-Polio Enteroviruses in the Slovak Republic in 1963–2019. Viruses 2022, 14, 1599. https://doi.org/10.3390/v14081599
Kissova R, Pastuchova K, Lengyelova V, Svitok M, Mikas J, Klement C, Bopegamage S. History of the Wastewater Assessment of Polio and Non-Polio Enteroviruses in the Slovak Republic in 1963–2019. Viruses. 2022; 14(8):1599. https://doi.org/10.3390/v14081599
Chicago/Turabian StyleKissova, Renata, Katarina Pastuchova, Viera Lengyelova, Marek Svitok, Jan Mikas, Cyril Klement, and Shubhada Bopegamage. 2022. "History of the Wastewater Assessment of Polio and Non-Polio Enteroviruses in the Slovak Republic in 1963–2019" Viruses 14, no. 8: 1599. https://doi.org/10.3390/v14081599
APA StyleKissova, R., Pastuchova, K., Lengyelova, V., Svitok, M., Mikas, J., Klement, C., & Bopegamage, S. (2022). History of the Wastewater Assessment of Polio and Non-Polio Enteroviruses in the Slovak Republic in 1963–2019. Viruses, 14(8), 1599. https://doi.org/10.3390/v14081599





