Serological Investigation and Genetic Characteristics of Pseudorabies Virus between 2019 and 2021 in Henan Province of China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Serological Detection
2.3. Virus Detection and Isolation
2.4. Sequencing and Phylogenetic Analysis
3. Results
3.1. Seroprevalence of PRV in Henan Province
3.2. The Results of PRV Isolation
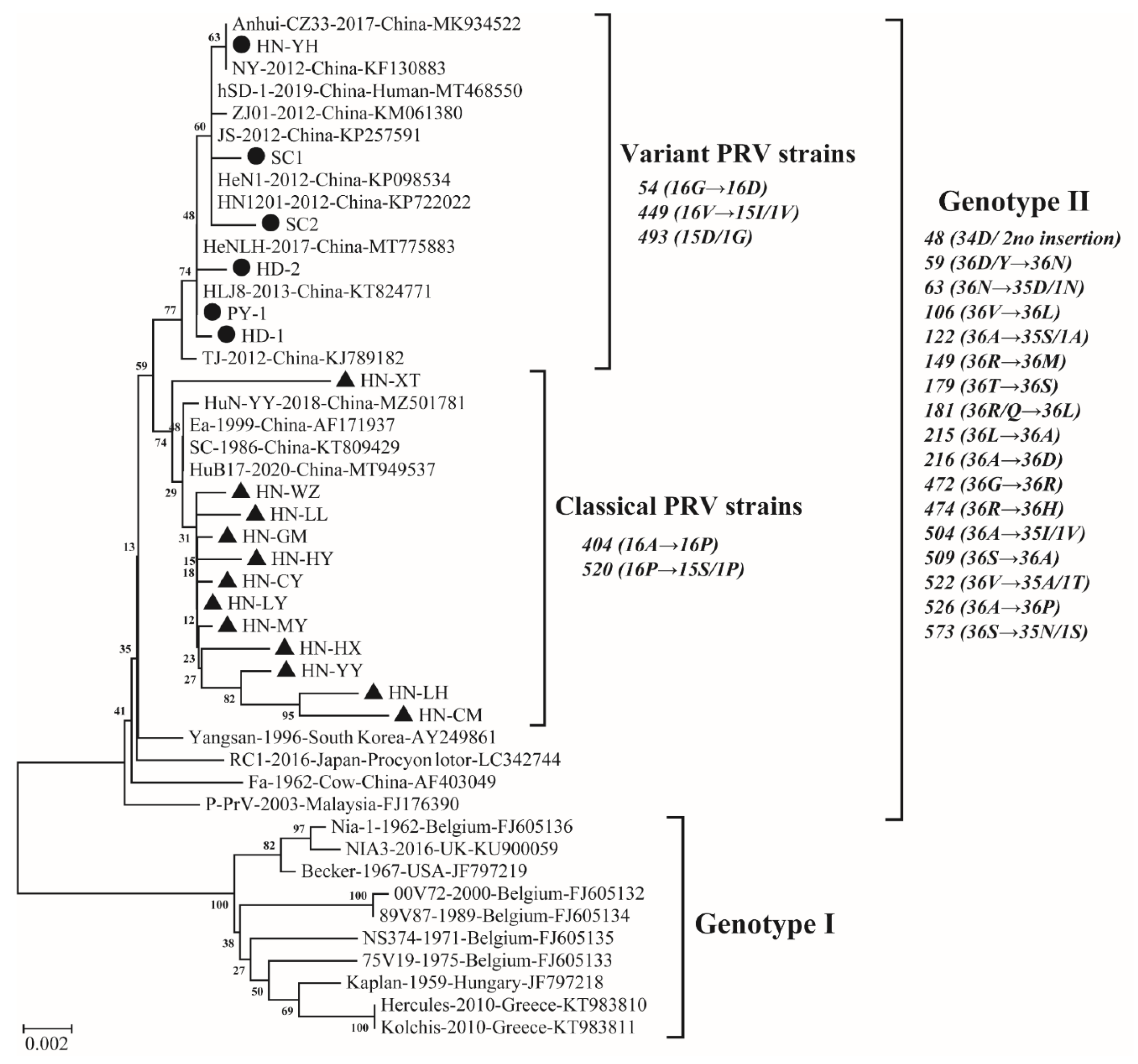
3.3. Genetic Analysis Results Based on gE
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauwynck, H.J. Functional aspects of Aujeszky’s disease (pseudorabies) viral proteins with relation to invasion, virulence and immunogenicity. Vet. Microbiol. 1997, 55, 3–11. [Google Scholar] [CrossRef]
- Mettenleiter, T.C. Aujeszky’s disease (pseudorabies) virus: The virus and molecular pathogenesis—State of the art, June 1999. Vet. Res. 2000, 31, 99–115. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691–1705. [Google Scholar] [CrossRef] [PubMed]
- Hahn, E.C.; Fadl-Alla, B.; Lichtensteiger, C.A. Variation of Aujeszky’s disease viruses in wild swine in USA. Vet. Microbiol. 2010, 143, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenner, F.; Bachman, P.A.; Gibbs, E.J.P. Pseudorabies. In Veterinary Virology; Fenner, F., Bachman, P.A., Gibbs, E.J.P., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1987; pp. 353–356. [Google Scholar]
- Marcaccini, A.; López Peña, M.; Quiroga, M.I.; Bermúdez, R.; Nieto, J.M.; Alemañ, N. Pseudorabies virus infection in mink: A host-specific pathogenesis. Vet. Immunol. Immunopathol. 2008, 124, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Pensaert, M.B.; Kluge, P. Pseudorabies Virus (Aujeszky’s Disease). In Virus Infections of Porcines; Pensaert, M.B., Ed.; Elsevier Science Publishers: New York, NY, USA, 1989; pp. 39–64. [Google Scholar]
- Ai, J.W.; Weng, S.S.; Cheng, Q.; Cui, P.; Li, Y.J.; Wu, H.L.; Zhu, Y.M.; Xu, B.; Zhang, W.H. Human Endophthalmitis Caused by Pseudorabies Virus Infection, China, 2017. Emerg. Infect. Dis. 2018, 24, 1087–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.J.; Lindsay, D.S.; Sriranganathan, N. Wild boars as sources for infectious diseases in livestock and humans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Tao, X.; Fei, M.; Chen, J.; Guo, W.; Li, P.; Wang, J. Human encephalitis caused by pseudorabies virus infection: A case report. J. Neurovirol. 2020, 26, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Han, H.; Wang, H.; Cui, Y.; Liu, H.; Ding, S. A Case of Human Viral Encephalitis Caused by Pseudorabies Virus Infection in China. Front. Neurol. 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Wang, X.; Xie, C.; Ding, S.; Yang, H.; Guo, S.; Li, J.; Qin, L.; Ban, F.; Wang, D.; et al. A Novel Human Acute Encephalitis Caused by Pseudorabies Virus Variant Strain. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e3690–e3700. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Chen, H.C. Pseudorabies epidemic status and control measures in China. Chin. J. Vet. Sci. 1999, 19, 1–2. [Google Scholar]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liang, W.; Liu, Q.; Zhao, T.; Zhu, H.; Hua, L.; Peng, Z.; Tang, X.; Stratton, C.W.; Zhou, D.; et al. Epidemiological and genetic characteristics of swine pseudorabies virus in mainland China between 2012 and 2017. PeerJ 2018, 6, e5785. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.; Liu, F.; Zheng, H.; Liang, C.; Zhou, Y.J.; Jiang, Y.F.; Shan, T.L.; Gao, F.; Li, G.X.; Tong, G.Z. Emergence of a Pseudorabies virus variant with increased virulence to piglets. Vet. Microbiol. 2015, 181, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Bai, C.; Sun, J.; Chang, S.; Zhang, X. Emergence of virulent pseudorabies virus infection in northern China. J. Vet. Sci. 2013, 14, 363–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Qiao, S.; Li, X.; Xie, W.; Guo, J.; Li, Q.; Liu, X.; Hou, J.; Xu, Y.; Wang, L.; et al. Molecular epidemiology of outbreak-associated pseudorabies virus (PRV) strains in central China. Virus Genes 2015, 50, 401–409. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, Z.; Hu, D.; Zhang, Q.; Han, T.; Li, X.; Gu, X.; Yuan, L.; Zhang, S.; Wang, B.; et al. Pathogenic pseudorabies virus, China, 2012. Emerg. Infect. Dis. 2014, 20, 102–104. [Google Scholar] [CrossRef] [Green Version]
- Ye, P.G. New trends of the pseudorabies infection in China. Swine Indus 2013, 6, 23. [Google Scholar]
- Zheng, H.H.; Jin, Y.; Hou, C.Y.; Li, X.S.; Zhao, L.; Wang, Z.Y.; Chen, H.Y. Seroprevalence investigation and genetic analysis of pseudorabies virus within pig populations in Henan province of China during 2018–2019. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2021, 92, 104835. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.Q.; Zheng, H.H.; Yang, Y.R.; Liu, F.; Zheng, L.L.; Jin, Y.; Chen, H.Y. Construction and immunogenicity of a gE/gI/TK-deleted PRV based on porcine pseudorabies virus variant. Mol. Cell. Probes 2020, 53, 101605. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Luo, Y.; Wang, C.H.; Yuan, J.; Li, N.; Song, K.; Qiu, H.J. Control of swine pseudorabies in China: Opportunities and limitations. Vet. Microbiol. 2016, 183, 119–124. [Google Scholar] [CrossRef] [PubMed]
- van Oirschot, J.T. Diva vaccines that reduce virus transmission. J. Biotechnol. 1999, 73, 195–205. [Google Scholar] [CrossRef]
- Ren, Q.; Ren, H.; Gu, J.; Wang, J.; Jiang, L.; Gao, S. The Epidemiological Analysis of Pseudorabies Virus and Pathogenicity of the Variant Strain in Shandong Province. Front. Vet. Sci. 2022, 9, 806824. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, W.; Chi, J.; Lu, C.; Li, X.; Li, C.; Jiang, S.; Tian, X.; Li, F.; Wang, L.; et al. Epidemiology of Porcine Pseudorabies from 2010 to 2018 in Tianjin, China. Viral Immunol. 2021, 34, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Pan, Y.; Liu, M.; Han, Z. Prevalence of Porcine Pseudorabies Virus and Its Coinfection Rate in Heilongjiang Province in China from 2013 to 2018. Viral Immunol. 2020, 33, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Tan, L.; Wang, C.; He, S.; Fang, L.; Wang, Z.; Zhong, Y.; Zhang, K.; Liu, D.; Yang, Q.; et al. Serological Investigation and Genetic Characteristics of Pseudorabies Virus in Hunan Province of China From 2016 to 2020. Front. Vet. Sci. 2021, 8, 762326. [Google Scholar] [CrossRef]
- Yang, H.C. Epidemiological situation of swine diseases in 2014 and the epidemiological trend and control strategies in 2015. Swine Ind. Sci. 2015, 32, 38–40. (In Chinese) [Google Scholar]
- Liu, Y.; Zhang, S.; Xu, Q.; Wu, J.; Zhai, X.; Li, S.; Wang, J.; Ni, J.; Yuan, L.; Song, X.; et al. Investigation on pseudorabies prevalence in Chinese swine breeding farms in 2013-2016. Trop. Anim. Health Prod. 2018, 50, 1279–1285. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, M.; Liu, Z.; Meng, F.; Wang, H.; Cao, L.; Li, Y.; Jiao, Q.; Han, Z.; Liu, S. Epidemiological investigation of porcine circovirus type 2 and its coinfection rate in Shandong province in China from 2015 to 2018. BMC Vet. Res. 2021, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Sabó, A. Analysis of reactivation of latent pseudorabies virus infection in tonsils and Gasserian ganglia of pigs. Acta Virolog. 1985, 29, 393–402. [Google Scholar]
- Zhou, Q.; Zhang, L.; Liu, H.; Ye, G.; Huang, L.; Weng, C. Isolation and Characterization of Two Pseudorabies Virus and Evaluation of Their Effects on Host Natural Immune Responses and Pathogenicity. Viruses 2022, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; Longobardi, C.; D’Ambrosi, F.; Amoroso, M.G.; D’Alessio, N.; Damiano, S.; Ciarcia, R.; Iovane, V.; Iovane, G.; Pagnini, U.; et al. Aujeszky’s Disease in South-Italian Wild Boars (Sus scrofa): A Serological Survey. Animals 2021, 11, 3298. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Sozzi, E.; Grilli, G.; Gibelli, L.R.; Gelmetti, D.; Lelli, D.; Chiari, M.; Prati, P.; Alborali, G.L.; Boniotti, M.B.; et al. Detection and molecular analysis of Pseudorabies virus strains isolated from dogs and a wild boar in Italy. Vet. Microbiol. 2015, 177, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Kong, Z.; Liu, P.; Fu, Z.; Zhang, J.; Liu, M.; Shang, Y. Natural infection of a variant pseudorabies virus leads to bovine death in China. Transbound. Emerg. Dis. 2020, 67, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Klupp, B.G.; Hengartner, C.J.; Mettenleiter, T.C.; Enquist, L.W. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 2004, 78, 424–440. [Google Scholar] [CrossRef] [Green Version]
- Kimman, T.G.; de Wind, N.; Oei-Lie, N.; Pol, J.M.; Berns, A.J.; Gielkens, A.L. Contribution of single genes within the unique short region of Aujeszky’s disease virus (suid herpesvirus type 1) to virulence, pathogenesis and immunogenicity. J. Gen. Virol. 1992, 73 Pt 2, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current Status and Challenge of Pseudorabies Virus Infection in China. Virolog. Sinic. 2021, 36, 588–607. [Google Scholar] [CrossRef]
- Wang, X.; Wu, C.X.; Song, X.R.; Chen, H.C.; Liu, Z.F. Comparison of pseudorabies virus China reference strain with emerging variants reveals independent virus evolution within specific geographic regions. Virology 2017, 506, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.; Meloen, R.H.; Gielkens, A.L.; van Oirschot, J.T. Epitope analysis of glycoprotein I of pseudorabies virus. J. Gen. Virol. 1990, 71 Pt 4, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, N.; Cong, X.; Wang, C.H.; Du, M.; Li, L.; Zhao, B.; Yuan, J.; Liu, D.D.; Li, S.; et al. Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet. Microbiol. 2014, 174, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Y.; Sun, Z.; Tan, F.F.; Guo, L.H.; Wang, Y.Z.; Wang, J.; Wang, Z.Y.; Wang, L.L.; Li, X.D.; Xiao, Y.; et al. Pathogenicity of a currently circulating Chinese variant pseudorabies virus in pigs. World J. Virol. 2016, 5, 23–30. [Google Scholar] [CrossRef] [PubMed]
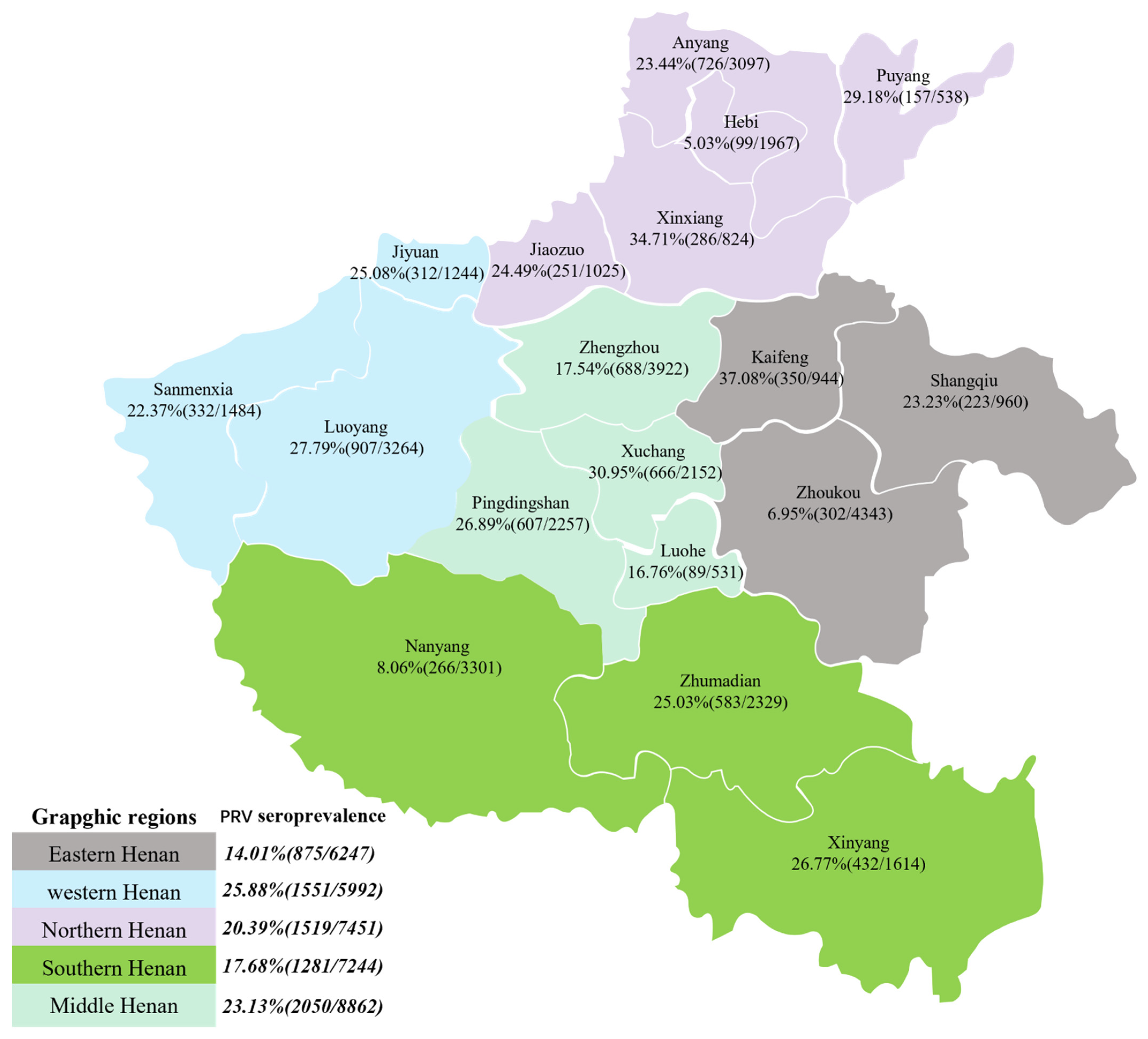
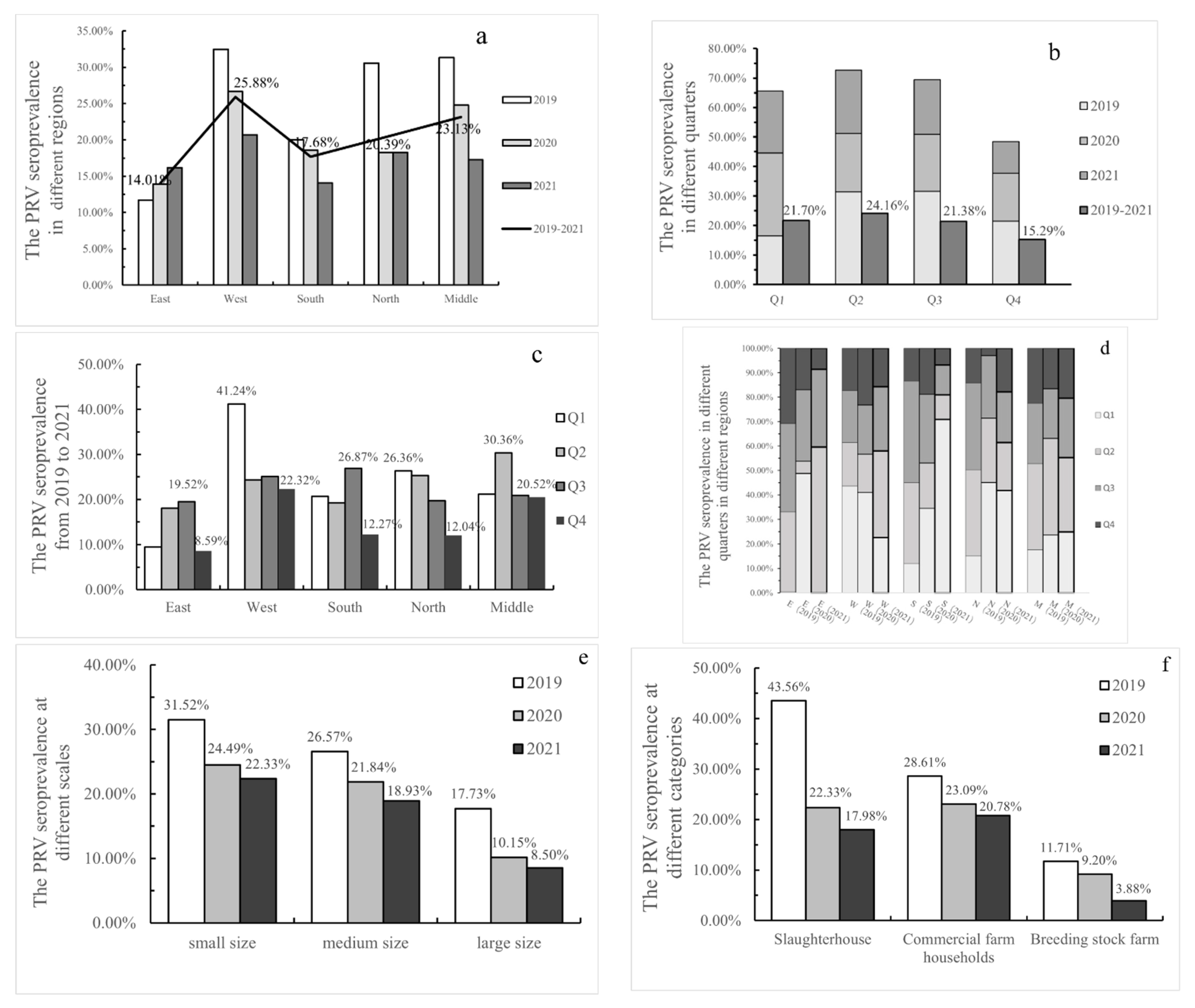

| Time | The Regions of Henan Province, China | The Categories of Pig Farms | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Eastern Henan | Western Henan | Southern Henan | Northern Henan | Middle Henan | Slaughterhouse | Commercial Farm Households | Breeding Stock Farm | ||
| January–March in 2019 (Q1) | 0.18% (1/570) | 61.92% (161/260) | 11.40% (107/939) | 18.78% (74/394) | 22.13% (52/235) | 62.50% (50/80) | 27.50% (327/1189) | 1.59% (18/1129) | 16.47% (395/2398) |
| April–June in 2019 (Q2) | 16.13% (121/750) | 25.45% (112/440) | 31.22% (153/490) | 44.03% (295/670) | 44.25% (200/452) | 65.00% (195/300) | 29.90% (572/1913) | 19.35% (114/589) | 31.44% (881/2802) |
| July–September in 2019(Q3) | 17.79% (71/399) | 30.11% (137/455) | 39.21% (149/380) | 44.13% (124/281) | 31.12% (164/527) | 46.86% (112/239) | 27.96% (402/1438) | 35.89% (131/365) | 31.59% (645/2042) |
| October–December in 2019 (Q4) | 15.22% (35/230) | 24.67% (111/450) | 12.69% (82/646) | 17.76% (114/642) | 28.34% (333/1175) | 19.22% (69/359) | 28.48% (508/1784) | 9.80% (98/1000) | 21.48% (675/3143) |
| Total for 2019 | 11.70% (228/1949) | 32.46% (521/1605) | 20.00% (491/2455) | 30.55% (607/1987) | 31.35% (749/2389) | 43.56% (426/978) | 28.61% (1809/6324) | 11.71% (361/3083) | 25.00% (2596/10,385) |
| January–March in 2020 (Q1) | 24.42% (138/565) | 52.94% (63/119) | 26.96% (86/319) | 29.97% (205/684) | 24.11% (102/423) | 33.47% (159/475) | 29.45% (415/1409) | 19.06% (61/320) | 28.15% (594/2110) |
| April–June in 2020 (Q2) | 2.56% (10/390) | 20.05% (149/743) | 14.53% (100/688) | 17.59% (57/324) | 40.00% (212/530) | 14.76% (31/210) | 25.76% (448/1739) | 6.75% (49/726) | 19.74% (528/2675) |
| July–September in 2020(Q3) | 14.63% (109/745) | 26.06% (135/518) | 22.00% (187/850) | 17.01% (272/1599) | 20.64% (219/1061) | 18.97% (184/970) | 22.87% (705/3082) | 4.58% (33/721) | 19.32% (922/4773) |
| October–December in 2020 (Q4) | 8.46% (33/390) | 29.82% (201/674) | 14.65% (103/703) | 1.98% (7/353) | 16.75% (70/418) | 17.78% (16/90) | 17.27% (328/1899) | 12.75% (70/549) | 16.31% (414/2538) |
| Total for 2020 | 13.88% (290/2090) | 26.68% (548/2054) | 18.59% (476/2560) | 18.28% (541/2960) | 24.79% (603/2432) | 22.33% (383/1715) | 23.09% (1862/8065) | 9.20% (213/2316) | 20.32% (2458/12,096) |
| January–March in 2021 (Q1) | 0.00% (0/330) | 18.67% (56/300) | 81.82% (90/110) | 27.89% (94/337) | 17.20% (65/378) | 18.64% (11/59) | 21.88% (258/1179) | 16.59% (36/217) | 20.96% (305/1455) |
| April–June in 2021 (Q2) | 49.17% (118/240) | 29.12% (166/570) | 11.71% (41/350) | 13.16% (109/828) | 20.91% (252/1205) | 14.11% (59/418) | 25.28% (565/2235) | 11.48% (62/540) | 21.48% (686/3193) |
| July–September in 2021(Q3) | 26.29% (168/639) | 21.65% (176/813) | 14.04% (99/705) | 13.84% (62/448) | 16.82% (215/1278) | 18.67% (84/450) | 23.90% (625/2615) | 1.34% (11/818) | 18.54% (720/3883) |
| October–December in 2021 (Q4) | 7.11% (71/999) | 12.92% (84/650) | 7.89% (84/1064) | 11.90% (106/891) | 14.07% (166/1180) | 20.42% (106/519) | 13.95% (398/2854) | 0.61% (7/1141) | 10.68% (511/4784) |
| Total for 2021 | 16.17% (357/2208) | 20.66% (482/2333) | 14.09% (314/2229) | 18.28% (371/2504) | 17.27% (698/4041) | 17.98% (260/1446) | 20.78% (1846/8883) | 3.88% (116/2986) | 16.69% (2222/13,315) |
| Total for 2019–2021 | 14.01% (875/6247) | 25.88% (1551/5992) | 17.68% (1281/7244) | 20.39% (1519/7451) | 23.13% (2050/8862) | 25.83% (1069/4139) | 23.80% (5517/23,177) | 8.14% (690/8480) | 20.33% (7276/35,796) |
| Year | Prevalence on Pig Farms of Different Sizes | Prevalence of Serum Samples on Pig Farms of Different Sizes | ||||||
|---|---|---|---|---|---|---|---|---|
| Small (15∼29) | Medium (30) | Large (31∼200) | Total | Small | Medium | Large | Total | |
| 2019 | 73.33% (22/30) | 48.81% (123/252) | 42.86% (18/42) | 50.31% (163/324) | 31.52% (197/625) | 26.57% (2009/7560) | 17.73% (390/2200) | 25.00% (2596/10,385) |
| 2020 | 69.23% (27/39) | 49.20% (154/313) | 42.42% (14/33) | 50.65% (195/385) | 24.49% (226/923) | 21.84% (2051/9390) | 10.15% (181/1783) | 20.32% (2458/12,096) |
| 2021 | 65.52% (19/29) | 48.42% (153/316) | 50.00% (18/36) | 49.87% (190/381) | 22.33% (163/730) | 18.93% (1795/9480) | 8.50% (264/3105) | 16.69% (2222/13,315) |
| Total | 69.39% (68/98) | 48.81% (430/881) | 45.05% (50/111) | 50.28% (548/1090) | 25.72% (586/2278) | 22.15% (5855/26,430) | 11.78% (835/7088) | 20.33% (7276/35,796) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, H.; Zhu, Q.; Chen, H.; Wang, Z.; Zheng, L.; Liu, F.; Wei, Z. Serological Investigation and Genetic Characteristics of Pseudorabies Virus between 2019 and 2021 in Henan Province of China. Viruses 2022, 14, 1685. https://doi.org/10.3390/v14081685
Chen X, Li H, Zhu Q, Chen H, Wang Z, Zheng L, Liu F, Wei Z. Serological Investigation and Genetic Characteristics of Pseudorabies Virus between 2019 and 2021 in Henan Province of China. Viruses. 2022; 14(8):1685. https://doi.org/10.3390/v14081685
Chicago/Turabian StyleChen, Ximeng, Hongxuan Li, Qianlei Zhu, Hongying Chen, Zhenya Wang, Lanlan Zheng, Fang Liu, and Zhanyong Wei. 2022. "Serological Investigation and Genetic Characteristics of Pseudorabies Virus between 2019 and 2021 in Henan Province of China" Viruses 14, no. 8: 1685. https://doi.org/10.3390/v14081685
APA StyleChen, X., Li, H., Zhu, Q., Chen, H., Wang, Z., Zheng, L., Liu, F., & Wei, Z. (2022). Serological Investigation and Genetic Characteristics of Pseudorabies Virus between 2019 and 2021 in Henan Province of China. Viruses, 14(8), 1685. https://doi.org/10.3390/v14081685






