Needs, Challenges and Countermeasures of SARS-CoV-2 Surveillance in Cold-Chain Foods and Packaging to Prevent Possible COVID-19 Resurgence: A Perspective from Advanced Detections
Abstract
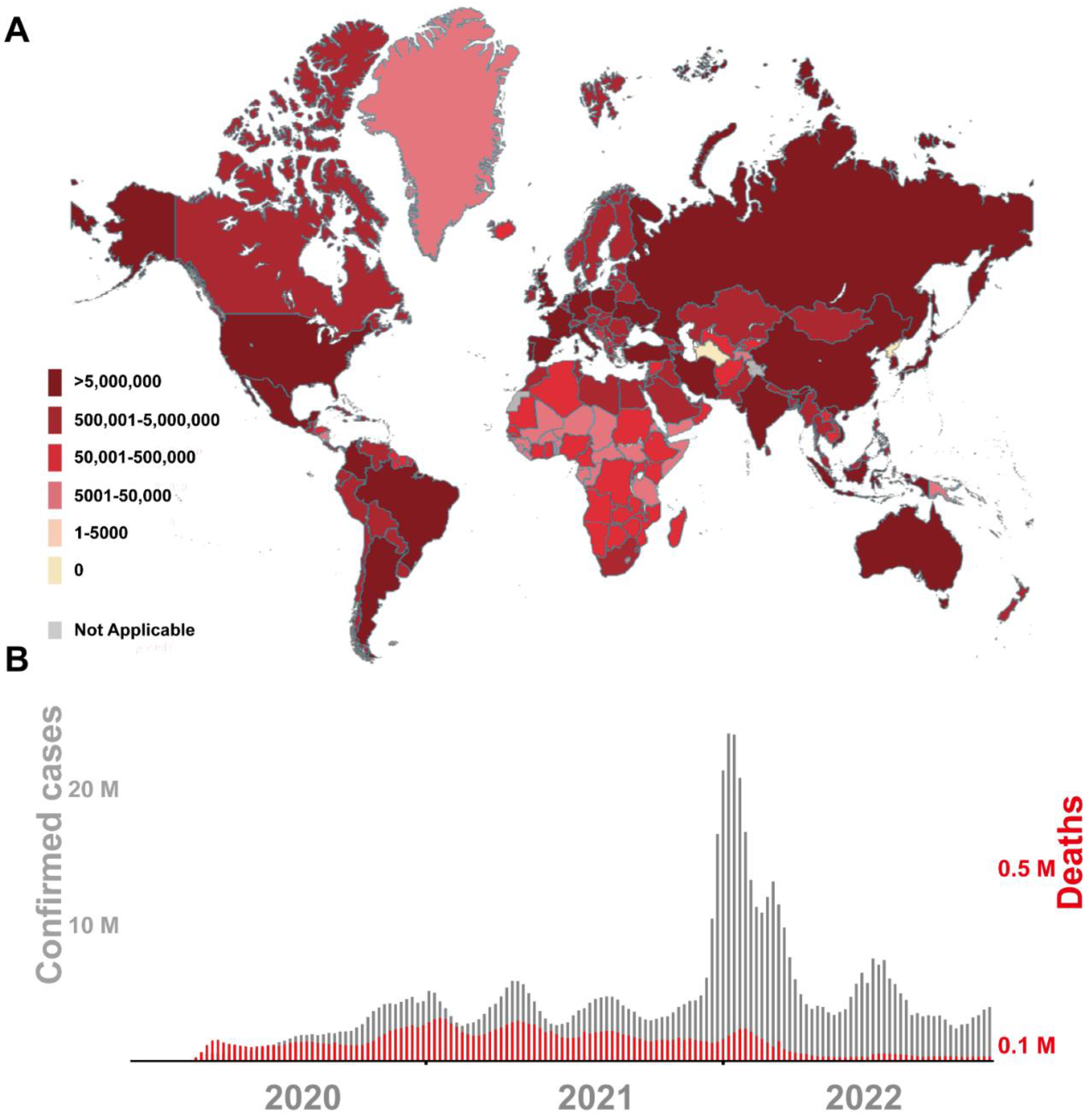
:1. Introduction
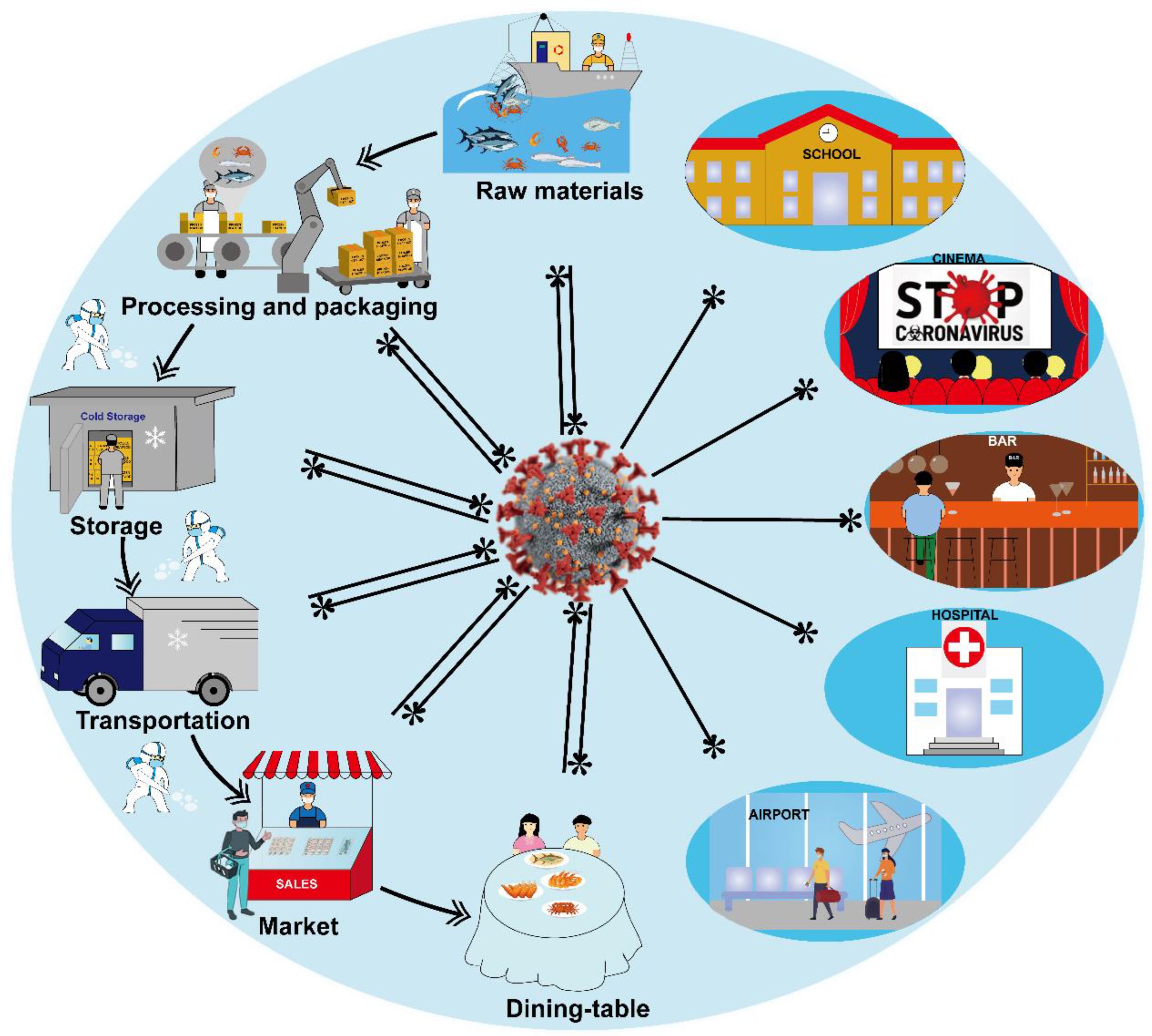
2. Transmission of SARS-CoV-2 in Cold-Chain Foods
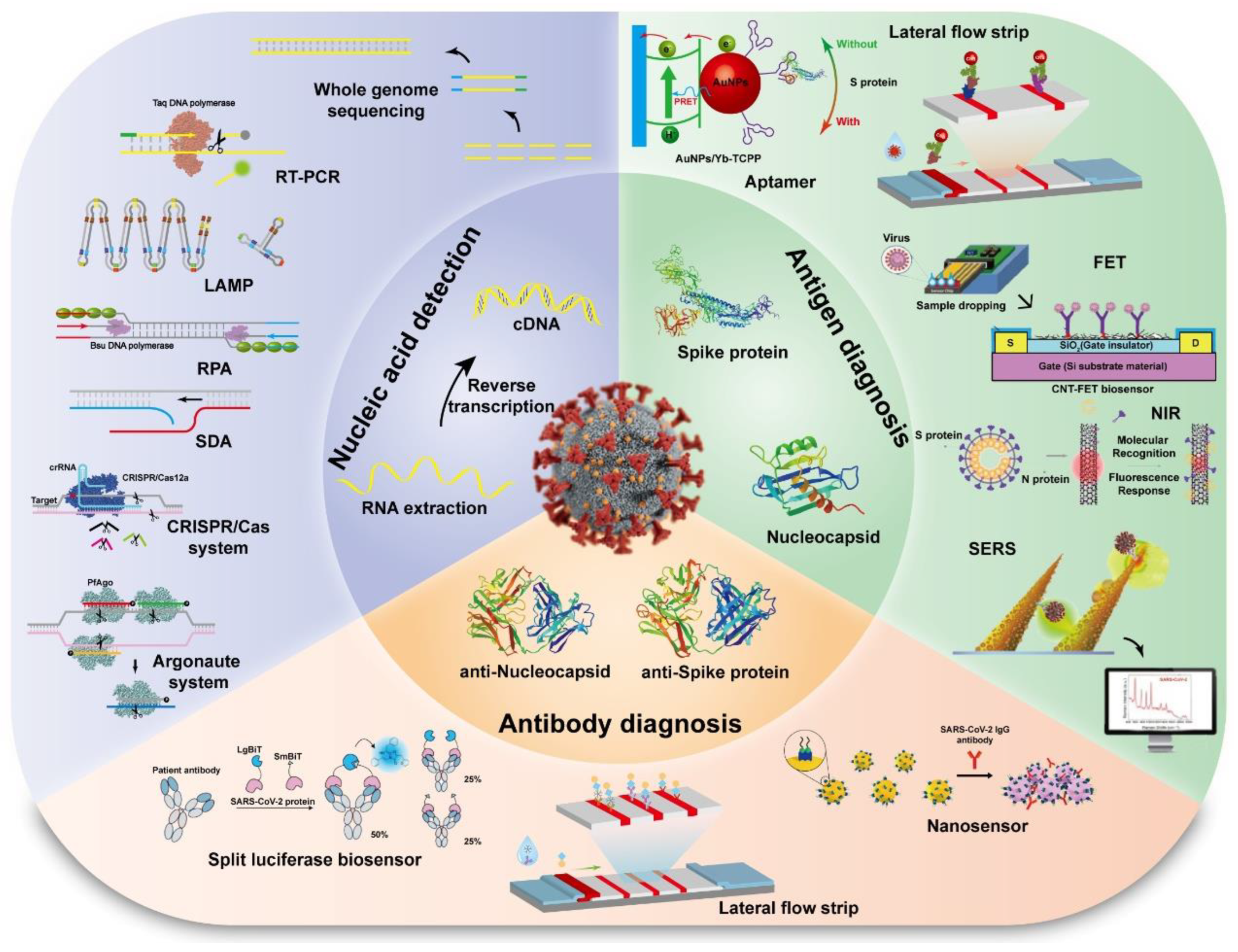
3. The Current Status of Detection Methods for SARS-CoV-2
3.1. Molecular Detection
3.2. CRISPR/Cas and Argonaute Based Detection
3.3. Virus Protein Detection/Antigen Detection
3.4. Other Detections
4. Challenges and Countermeasures
4.1. The Sample Pretreatment
4.2. Infectivity of Viruses
4.3. Quantitative PCR Detection Method
4.4. Safety Precautions in Cold-Chain Foods
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, Z.; Shu, Y.; Song, J.; Gao, G.F.; Tan, W.; Guo, D. A distinct name is needed for the new coronavirus. Lancet 2020, 395, 10228. [Google Scholar] [CrossRef] [PubMed]
- Behrmann, O.; Bachmann, I.; Spiegel, M.; Schramm, M.; Abd El Wahed, A.; Dobler, G.; Dame, G.; Hufert, F.T. Rapid detection of SARS-CoV-2 by low volume real-time single tube reverse transcription recombinase polymerase amplification using an Exo probe with an internally linked quencher (Exo-IQ). Clin. Chem. 2020, 66, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Nyaruaba, R.; Hong, W.; Li, X.; Yang, H.; Wei, H. Long-term preservation of SARS-CoV-2 RNA in silk for downstream RT-PCR tests. Anal. Chem. 2022, 94, 4522–4530. [Google Scholar] [CrossRef]
- Rizou, M.; Galanakis, I.M.; Aldawoud, T.M.S.; Galanakis, C.M. Safety of foods, food supply chain and environment within the COVID-19 pandemic. Trends Food Sci. Technol. 2020, 102, 293–299. [Google Scholar] [CrossRef]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Rodriguez-Morales, A.J. Coronavirus disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef]
- Chen, W.; Chen, C.L.; Cao, Q.; Chiu, C.H. Time course and epidemiological features of COVID-19 resurgence due to cold-chain food or packaging contamination. Biomed. J. 2022, 45, 432–438. [Google Scholar] [CrossRef]
- Sobolik, J.S.; Sajewski, E.T.; Jaykus, L.A.; Cooper, D.K.; Lopman, B.A.; Kraay, A.N.; Ryan, P.B.; Guest, J.L.; Webb-Girard, A.; Leon, J.S. Low risk of SARS-CoV-2 transmission via fomite, even in cold-chain. medRxiv 2021. [Google Scholar] [CrossRef]
- Pang, X.; Ren, L.; Wu, S.; Ma, W.; Yang, J.; Di, L.; Li, J.; Xiao, Y.; Kang, L.; Du, S.; et al. Cold-chain food contamination as the possible origin of COVID-19 resurgence in Beijing. Natl. Sci. Rev. 2020, 7, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Kung, Y.A.; Huang, P.N.; Chang, S.Y.; Gong, Y.N.; Han, Y.J.; Chiang, H.J.; Liu, K.T.; Lee, K.M.; Chang, C.Y.; et al. Stability of SARS-CoV-2 spike G614 variant surpasses that of the D614 variant after cold storage. mSphere 2021, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, Z.; Zhao, X.; Han, J.; Zhang, Y.; Wang, H.; Chen, C.; Wang, J.; Jiang, F.; Lei, J.; et al. Long distance transmission of SARS-CoV-2 from contaminated cold chain products to humans—Qingdao city, Shandong province, China, September 2020. China CDC Wkly. 2021, 3, 637–644. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Zhu, L.; Liu, G.; Wu, L. Food safety in post-COVID-19 pandemic: Challenges and countermeasures. Biosensors 2021, 11, 71. [Google Scholar] [CrossRef]
- Kong, J.; Li, W.; Hu, J.; Zhao, S.; Yue, T.; Li, Z.; Xia, Y. The safety of cold-chain food in post-COVID-19 pandemic: Precaution and quarantine. Foods 2022, 11, 1540. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Liu, H.X.; Xia, W.; Wong, G.W.K.; Xu, S.Q. Cold chain logistics: A possible mode of SARS-CoV-2 transmission? BMJ 2021, 375, e066129. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, M.; Zhao, X.; Guo, Y.; Wang, L.; Zhang, J.; Lei, W.; Han, W.; Jiang, F.; Liu, W.J.; et al. Cold-chain transportation in the frozen food industry may have caused a recurrence of COVID-19 cases in destination: Successful isolation of SARS-CoV-2 virus from the imported frozen cod package surface. Biosaf. Health 2020, 2, 199–201. [Google Scholar] [CrossRef]
- Sobolik, J.S.; Sajewski, E.T.; Jaykus, L.A.; Cooper, D.K.; Lopman, B.A.; Kraay, A.N.M.; Ryan, P.B.; Guest, J.L.; Webb-Girard, A.; Leon, J.S. Decontamination of SARS-CoV-2 from cold-chain food packaging provides no marginal benefit in risk reduction to food workers. Food Control 2022, 136, 108845. [Google Scholar] [CrossRef]
- Chen, C.; Feng, Y.; Chen, Z.; Xia, Y.; Zhao, X.; Wang, J.; Nie, K.; Niu, P.; Han, J.; Xu, W. SARS-CoV-2 cold-chain transmission: Characteristics, risks, and strategies. J. Med. Virol. 2022, 94, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Telenti, A.; Hodcroft, E.B.; Robertson, D.L. The evolution and biology of SARS-CoV-2 variants. Cold Spring Harb. Perspect. Med. 2022, 12, a041390. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta variant B.1.617.2 COVID-19. Clin. Pract. 2021, 11, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Spadaccini, M.; Canziani, L.; Aghemo, A.; Lleo, A.; Maselli, R.; Anderloni, A.; Carrara, S.; Fugazza, A.; Pellegatta, G.; Galtieri, P.A.; et al. What gastroenterologists should know about SARS-CoV 2 vaccine: World Endoscopy Organization perspective. United Eur. Gastroenterol. J. 2021, 9, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Jian, M.J.; Chang, C.K.; Lin, J.C.; Yeh, K.M.; Chen, C.W.; Chiu, S.K.; Wang, Y.H.; Liao, S.J.; Li, S.Y.; et al. Novel dual multiplex real-time RT-PCR assays for the rapid detection of SARS-CoV-2, influenza A/B, and respiratory syncytial virus using the BD MAX open system. Emerg. Microbes Infect. 2021, 10, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Niu, P.; Lu, R.; Zhao, L.; Wang, H.; Huang, B.; Ye, F.; Wang, W.; Tan, W. Three novel real-time RT-PCR assays for detection of COVID-19 virus. China CDC Wkly. 2020, 2, 453–457. [Google Scholar] [CrossRef]
- Das, D.; Lin, C.W.; Kwon, J.S.; Chuang, H.S. Rotational diffusometric sensor with isothermal amplification for ultra-sensitive and rapid detection of SARS-CoV-2 nsp2 cDNA. Biosens. Bioelectron. 2022, 210, 114293. [Google Scholar] [CrossRef]
- He, Y.; Xie, T.; Tong, Y. Rapid and highly sensitive one-tube colorimetric RT-LAMP assay for visual detection of SARS-CoV-2 RNA. Biosens. Bioelectron. 2021, 187, 113330. [Google Scholar] [CrossRef]
- Chaibun, T.; Puenpa, J.; Ngamdee, T.; Boonapatcharoen, N.; Athamanolap, P.; O’Mullane, A.P.; Vongpunsawad, S.; Poovorawan, Y.; Lee, S.Y.; Lertanantawong, B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Commun. 2021, 12, 802. [Google Scholar] [CrossRef]
- Bokelmann, L.; Nickel, O.; Maricic, T.; Paabo, S.; Meyer, M.; Borte, S.; Riesenberg, S. Point-of-care bulk testing for SARS-CoV-2 by combining hybridization capture with improved colorimetric LAMP. Nat. Commun. 2021, 12, 1467. [Google Scholar] [CrossRef]
- Sherrill-Mix, S.; Hwang, Y.; Roche, A.M.; Glascock, A.; Weiss, S.R.; Li, Y.; Haddad, L.; Deraska, P.; Monahan, C.; Kromer, A.; et al. Detection of SARS-CoV-2 RNA using RT-LAMP and molecular beacons. Genome Biol. 2021, 22, 169. [Google Scholar] [CrossRef]
- Zhang, C.; Belwal, T.; Luo, Z.; Su, B.; Lin, X. Application of nanomaterials in isothermal nucleic acid amplification. Small 2022, 18, e2102711. [Google Scholar] [CrossRef]
- Li, J.; Wu, D.; Yu, Y.; Li, T.; Li, K.; Xiao, M.M.; Li, Y.; Zhang, Z.Y.; Zhang, G.J. Rapid and unamplified identification of COVID-19 with morpholino-modified graphene field-effect transistor nanosensor. Biosens. Bioelectron. 2021, 183, 113206. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Recio, O.; Gutierrez-Rivas, M.; Peiro-Pastor, R.; Aguilera-Sepulveda, P.; Cano-Gomez, C.; Jimenez-Clavero, M.A.; Fernandez-Pinero, J. Sequencing of SARS-CoV-2 genome using different nanopore chemistries. Appl. Microbiol. Biotechnol. 2021, 105, 3225–3234. [Google Scholar] [CrossRef] [PubMed]
- Chiara, M.; D’Erchia, A.M.; Gissi, C.; Manzari, C.; Parisi, A.; Resta, N.; Zambelli, F.; Picardi, E.; Pavesi, G.; Horner, D.S.; et al. Next generation sequencing of SARS-CoV-2 genomes: Challenges, applications and opportunities. Brief. Bioinform. 2021, 22, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.R.; Dopp, J.L.; Wu, K.; Sadat Mousavi, P.; Jo, Y.R.; McNeley, C.E.; Lynch, Z.T.; Pardee, K.; Green, A.A.; Reuel, N.F. Toward mail-in-sensors for SARS-CoV-2 detection: Interfacing gel switch resonators with cell-free toehold switches. ACS Sens. 2022, 7, 806–815. [Google Scholar] [CrossRef]
- Shokr, A.; Pacheco, L.G.C.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Gandhi, J.; Kartik, D.; Silva, F.S.R.; Erdogmus, E.; Kandula, H.; Luo, S.; et al. Mobile health (mHealth) viral diagnostics enabled with adaptive adversarial learning. ACS Nano 2021, 15, 665–673. [Google Scholar] [CrossRef]
- Silva, F.S.R.; Erdogmus, E.; Shokr, A.; Kandula, H.; Thirumalaraju, P.; Kanakasabapathy, M.K.; Hardie, J.M.; Pacheco, L.G.C.; Li, J.Z.; Kuritzkes, D.R.; et al. SARS-CoV-2 RNA detection by a cellphone-based amplification-free system with CRISPR/Cas-dependent enzymatic (CASCADE) assay. Adv. Mater. Technol. 2021, 6, 2100602. [Google Scholar] [CrossRef]
- Tang, Y.; Qi, L.; Liu, Y.; Guo, L.; Zhao, R.; Yang, M.; Du, Y.; Li, B. CLIPON: A CRISPR-enabled strategy that turns commercial pregnancy test strips into general point-of-need test devices. Angew. Chem. 2022, 61, e202115907. [Google Scholar]
- Xiong, E.; Jiang, L.; Tian, T.; Hu, M.; Yue, H.; Huang, M.; Lin, W.; Jiang, Y.; Zhu, D.; Zhou, X. Simultaneous dual-gene diagnosis of SARS-CoV-2 based on CRISPR/Cas9-mediated lateral flow assay. Angew. Chem. 2021, 60, 5307–5315. [Google Scholar] [CrossRef]
- He, C.; Lin, C.; Mo, G.; Xi, B.; Li, A.A.; Huang, D.; Wan, Y.; Chen, F.; Liang, Y.; Zuo, Q.; et al. Rapid and accurate detection of SARS-CoV-2 mutations using a Cas12a-based sensing platform. Biosens. Bioelectron. 2022, 198, 113857. [Google Scholar] [CrossRef]
- Ma, L.; Yin, L.; Li, X.; Chen, S.; Peng, L.; Liu, G.; Ye, S.; Zhang, W.; Man, S. A smartphone-based visual biosensor for CRISPR-Cas powered SARS-CoV-2 diagnostics. Biosens. Bioelectron. 2022, 195, 113646. [Google Scholar] [CrossRef]
- Yin, L.; Man, S.; Ye, S.; Liu, G.; Ma, L. CRISPR-Cas based virus detection: Recent advances and perspectives. Biosens. Bioelectron. 2021, 193, 113541. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yang, J.; He, R.; Yu, X.; Chen, S.; Liu, Y.; Wang, L.; Li, A.; Liu, L.; Zhai, C.; et al. PfAgo-based detection of SARS-CoV-2. Biosens. Bioelectron. 2021, 177, 112932. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhou, H.; Guo, X.; Liu, D.; Li, Z.; Sun, J.; Huang, J.; Liu, T.; Zhao, P.; Xu, H.; et al. Argonaute-integrated isothermal amplification for rapid, portable, multiplex detection of SARS-CoV-2 and influenza viruses. Biosens. Bioelectron. 2022, 207, 114169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Man, S.; Ye, S.; Liu, G.; Ma, L. CRISPR-Cas-based detection for food safety problems: Current status, challenges, and opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3770–3798. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-based CRISPR/Cas assay on microfluidic paper analytical devices for supersensitive detection of pathogenic bacteria in foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar] [CrossRef]
- Shin, J.; Miller, M.; Wang, Y.C. Recent advances in CRISPR-based systems for the detection of foodborne pathogens. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3010–3029. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Liu, C. All-in-one dual CRISPR-Cas12a (AIOD-CRISPR) assay: A case for rapid, ultrasensitive and visual detection of novel coronavirus SARS-CoV-2 and HIV virus. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shi, Y.; Chen, Y.; Yang, Z.; Wu, H.; Zhou, Z.; Li, J.; Ping, J.; He, L.; Shen, H.; et al. Contamination-free visual detection of SARS-CoV-2 with CRISPR/Cas12a: A promising method in the point-of-care detection. Biosens. Bioelectron. 2020, 169, 112642. [Google Scholar] [CrossRef]
- Lu, S.; Tong, X.; Han, Y.; Zhang, K.; Zhang, Y.; Chen, Q.; Duan, J.; Lei, X.; Huang, M.; Qiu, Y.; et al. Fast and sensitive detection of SARS-CoV-2 RNA using suboptimal protospacer adjacent motifs for Cas12a. Nat. Biomed. Eng. 2022, 6, 286–297. [Google Scholar] [CrossRef]
- Patchsung, M.; Jantarug, K.; Pattama, A.; Aphicho, K.; Suraritdechachai, S.; Meesawat, P.; Sappakhaw, K.; Leelahakorn, N.; Ruenkam, T.; Wongsatit, T.; et al. Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020, 4, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, J.; Wu, G.; Weng, Z.; Song, Y.; Zhang, Y.; Vanegas, J.A.; Avery, L.; Gao, Z.; Sun, H.; et al. Amplification-free detection of SARS-CoV-2 and respiratory syncytial virus using CRISPR Cas13a and graphene field-effect transistors. Angew. Chem. 2022, 134, e202203826. [Google Scholar]
- Fozouni, P.; Son, S.; Diaz de Leon Derby, M.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell 2021, 184, 323–333.e9. [Google Scholar] [CrossRef]
- de Puig, H.; Lee, R.A.; Najjar, D.; Tan, X.; Soeknsen, L.R.; Angenent-Mari, N.M.; Donghia, N.M.; Weckman, N.E.; Ory, A.; Ng, C.F.; et al. Minimally instrumented SHERLOCK (miSHERLOCK) for CRISPR-based point-of-care diagnosis of SARS-CoV-2 and emerging variants. Sci. Adv. 2021, 7, eabh2944. [Google Scholar] [CrossRef]
- Brogan, D.J.; Chaverra-Rodriguez, D.; Lin, C.P.; Smidler, A.L.; Yang, T.; Alcantara, L.M.; Antoshechkin, I.; Liu, J.; Raban, R.R.; Belda-Ferre, P.; et al. Development of a rapid and sensitive CasRx-based diagnostic assay for SARS-CoV-2. ACS Sens. 2021, 6, 3957–3966. [Google Scholar] [CrossRef] [PubMed]
- Ooi, K.H.; Liu, M.M.; Tay, J.W.D.; Teo, S.Y.; Kaewsapsak, P.; Jin, S.; Lee, C.K.; Hou, J.; Maurer-Stroh, S.; Lin, W.; et al. An engineered CRISPR-Cas12a variant and DNA-RNA hybrid guides enable robust and rapid COVID-19 testing. Nat. Commun. 2021, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Suo, W.; Goulev, Y.; Sun, L.; Kerr, L.; Paulsson, J.; Zhang, Y.; Lao, T. Handheld microfluidic filtration platform enables rapid, low-cost, and robust self-testing of SARS-CoV-2 virus. Small 2021, 17, e2104009. [Google Scholar] [CrossRef]
- Nouri, R.; Jiang, Y.; Tang, Z.; Lian, X.L.; Guan, W. Detection of SARS-CoV-2 with solid-state CRISPR-Cas12a-assisted nanopores. Nano Lett 2021, 21, 8393–8400. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Valls, M.; Escalona-Noguero, C.; Rodriguez-Diaz, C.; Pardo, D.; Castellanos, M.; Milan-Rois, P.; Martinez-Garay, C.; Coloma, R.; Abreu, M.; Canton, R.; et al. CASCADE: Naked eye-detection of SARS-CoV-2 using Cas13a and gold nanoparticles. Anal. Chim. Acta 2022, 1205, 339749. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.; Hu, Y. Emerging Argonaute-based nucleic acid biosensors. Trends Biotechnol. 2022, 40, 8. [Google Scholar] [CrossRef]
- Xun, G.; Lane, S.T.; Petrov, V.A.; Pepa, B.E.; Zhao, H. A rapid, accurate, scalable, and portable testing system for COVID-19 diagnosis. Nat. Commun. 2021, 12, 2905. [Google Scholar] [CrossRef]
- Koller, G.; Morrell, A.P.; Galao, R.P.; Pickering, S.; MacMahon, E.; Johnson, J.; Ignatyev, K.; Neil, S.J.D.; Elsharkawy, S.; Fleck, R.; et al. More than the eye can see: Shedding new light on SARS-CoV-2 lateral flow device-based immunoassays. ACS Appl. Mater. Interfaces 2021, 13, 25694–25700. [Google Scholar] [CrossRef]
- Wood, B.R.; Kochan, K.; Bedolla, D.E.; Salazar-Quiroz, N.; Grimley, S.L.; Perez-Guaita, D.; Baker, M.J.; Vongsvivut, J.; Tobin, M.J.; Bambery, K.R.; et al. Infrared based saliva screening test for COVID-19. Angew. Chem. 2021, 60, 17102–17107. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Cheng, X.; Liu, L.; Zhang, X.; Yang, X.; Zheng, S.; Rong, Z.; Wang, S. Ultrasensitive and simultaneous detection of two specific SARS-CoV-2 antigens in human specimens using direct/enrichment dual-mode fluorescence lateral flow immunoassay. ACS Appl. Mater. Interfaces 2021, 13, 40342–40353. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Mahmud, A.; Chang, D.; Das, J.; Gomis, S.; Chen, J.B.; Wang, H.; Been, T.; Yip, L.; Coomes, E.; et al. Detection of SARS-CoV-2 viral particles using direct, reagent-free electrochemical sensing. J. Am. Chem. Soc. 2021, 143, 1722–1727. [Google Scholar] [CrossRef] [PubMed]
- Elledge, S.K.; Zhou, X.X.; Byrnes, J.R.; Martinko, A.J.; Lui, I.; Pance, K.; Lim, S.A.; Glasgow, J.E.; Glasgow, A.A.; Turcios, K.; et al. Engineering luminescent biosensors for point-of-care SARS-CoV-2 antibody detection. Nat. Biotechnol. 2021, 39, 928–935. [Google Scholar] [CrossRef]
- Lew, T.T.S.; Aung, K.M.M.; Ow, S.Y.; Amrun, S.N.; Sutarlie, L.; Ng, L.F.P.; Su, X. Epitope-functionalized gold nanoparticles for rapid and selective detection of SARS-CoV-2 IgG antibodies. ACS Nano 2021, 15, 12286–12297. [Google Scholar] [CrossRef]
- Kondo, T.; Iwatani, Y.; Matsuoka, K.; Fujino, T.; Umemoto, S.; Yokomaku, Y.; Ishizaki, K.; Kito, S.; Sezaki, T.; Hayashi, G.; et al. Antibody-like proteins that capture and neutralize SARS-CoV-2. Sci. Adv. 2020, 6, eabd3916. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, J.; Hu, Z.; Chen, Y.; Tao, Y.; Wang, L.; Li, L.; Wang, P.; Li, H.Y.; Zhang, J.; et al. All-solid-state SARS-CoV-2 protein biosensor employing colloidal quantum dots-modified electrode. Biosens. Bioelectron. 2022, 202, 113974. [Google Scholar] [CrossRef]
- Han, S.; Ko, O.; Lee, G.; Jeong, S.W.; Choi, Y.J.; Lee, J.B. Rapid diagnosis of coronavirus by RNA-directed RNA transcription using an engineered RNA-based platform. Nano Lett. 2021, 21, 462–468. [Google Scholar] [CrossRef]
- Abrego-Martinez, J.C.; Jafari, M.; Chergui, S.; Pavel, C.; Che, D.; Siaj, M. Aptamer-based electrochemical biosensor for rapid detection of SARS-CoV-2: Nanoscale electrode-aptamer-SARS-CoV-2 imaging by photo-induced force microscopy. Biosens. Bioelectron. 2022, 195, 113595. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, D.K.; Nandwana, V.; Henrich, S.E.; Josyula, V.; Thaxton, C.S.; Qi, C.; Simons, L.M.; Hultquist, J.F.; Ozer, E.A.; Shekhawat, G.S.; et al. Highly sensitive and ultra-rapid antigen-based detection of SARS-CoV-2 using nanomechanical sensor platform. Biosens. Bioelectron. 2022, 195, 113647. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef]
- Liang, Y.; Lin, H.; Zou, L.; Deng, X.; Tang, S. Rapid detection and tracking of Omicron variant of SARS-CoV-2 using CRISPR-Cas12a-based assay. Biosens. Bioelectron. 2022, 205, 114098. [Google Scholar] [CrossRef]
- Zhong, Z.; Wang, J.; He, S.; Su, X.; Huang, W.; Chen, M.; Zhuo, Z.; Zhu, X.; Fang, M.; Li, T.; et al. An encodable multiplex microsphere-phase amplification sensing platform detects SARS-CoV-2 mutations. Biosens. Bioelectron. 2022, 203, 114032. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Conley, B.M.; Shin, M.; Choi, J.H.; Bektas, C.K.; Choi, J.W.; Lee, K.B. Ultrasensitive electrochemical detection of mutated viral RNAs with single-nucleotide resolution using a nanoporous electrode array (NPEA). ACS Nano 2022, 16, 5764–5777. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Chen, J.; Wang, M.; Zhang, T.; Luo, W.; Li, Y.; Wu, Y.; Zeng, B.; Zhang, K.; et al. Detection of SARS-CoV-2 and its mutated variants via CRISPR-Cas13-based transcription amplification. Anal. Chem. 2021, 93, 3393–3402. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Kim, H.; Joung, Y.; Kang, H.; Moon, J.; Jang, H.; Park, S.; Kwon, H.J.; Lee, I.C.; Kim, S.; et al. Surface-enhanced Raman scattering-based immunoassay for severe acute respiratory syndrome coronavirus 2. Biosens. Bioelectron. 2022, 202, 114008. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.E.; Lee, P.W.; Trick, A.Y.; Park, J.S.; Chen, L.; Shah, K.; Mostafa, H.; Carroll, K.C.; Hsieh, K.; Wang, T.H. Point-of-care CRISPR-Cas-assisted SARS-CoV-2 detection in an automated and portable droplet magnetofluidic device. Biosens. Bioelectron. 2021, 190, 113390. [Google Scholar] [CrossRef]
- Dai, Z.; Xu, X.; Wang, Y.; Li, M.; Zhou, K.; Zhang, L.; Tan, Y. Surface plasmon resonance biosensor with laser heterodyne feedback for highly-sensitive and rapid detection of COVID-19 spike antigen. Biosens. Bioelectron. 2022, 206, 114163. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Y.; Chen, Y.; Ho, N.R.Y.; Sundah, N.R.; Natalia, A.; Liu, Y.; Miow, Q.H.; Wang, Y.; Tambyah, P.A.; et al. Accessible detection of SARS-CoV-2 through molecular nanostructures and automated microfluidics. Biosens. Bioelectron. 2021, 194, 113629. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Pan, J.; Zhang, Y.; Zhang, L.; Wang, C.; Yan, X.; Liu, X.; Lu, G. Ultrasensitive detection of SARS-CoV-2 spike protein in untreated saliva using SERS-based biosensor. Biosens. Bioelectron. 2021, 190, 113421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Miao, X.; Song, C.; Chen, N.; Xiong, J.; Gan, H.; Ni, J.; Zhu, Y.; Cheng, K.; Wang, L. Non-enzymatic signal amplification-powered point-of-care SERS sensor for rapid and ultra-sensitive assay of SARS-CoV-2 RNA. Biosens. Bioelectron. 2022, 212, 114379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, Y.; Liu, Y.; Fang, H.; Huang, X.; Leng, Y.; Liu, Z.; Hou, L.; Zhang, W.; Lai, W.; et al. Controlled copper in situ growth-amplified lateral flow sensors for sensitive, reliable, and field-deployable infectious disease diagnostics. Biosens. Bioelectron. 2021, 171, 112753. [Google Scholar] [CrossRef]
- Xu, X.; Sun, J.; Nie, S.; Li, H.; Kong, Y.; Liang, M.; Hou, J.; Huang, X.; Li, D.; Ma, T.; et al. Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat. Med. 2020, 26, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Xiong, J.; Nyaruaba, R.; Li, J.; Muturi, E.; Liu, H.; Yu, J.; Yang, H.; Wei, H. Rapid determination of infectious SARS-CoV-2 in PCR-positive samples by SDS-PMA assisted RT-qPCR. Sci. Total Environ. 2021, 797, 149085. [Google Scholar] [CrossRef]
- Qian, J.; Yu, Q.; Jiang, L.; Yang, H.; Wu, W. Food cold chain management improvement: A conjoint analysis on COVID-19 and food cold chain systems. Food Control 2022, 137, 108940. [Google Scholar] [CrossRef]
- Chi, Y.; Wang, Q.; Chen, G.; Zheng, S. The long-term presence of SARS-CoV-2 on cold-chain food packaging surfaces indicates a new COVID-19 winter outbreak: A mini review. Front. Public Health 2021, 9, 650493. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Han, L.; Chen, T.; Wang, L.; Li, H.; Li, S.; He, L.; Fu, X.; Chen, S.; et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens. Bioelectron. 2020, 166, 112437. [Google Scholar] [CrossRef]
- Iftekhar, A.; Cui, X. Blockchain-based traceability system that ensures food safety measures to protect consumer safety and COVID-19 free supply chains. Foods 2021, 10, 1289. [Google Scholar] [CrossRef]
- Xu, J.; Ma, R.; Stankovski, S.; Liu, X.; Zhang, X. Intelligent dynamic quality prediction of chilled chicken with integrated IoT flexible sensing and knowledge rules extraction. Foods 2022, 11, 836. [Google Scholar] [CrossRef]
- Skawinska, E.; Zalewski, R.I. Economic impact of temperature control during food transportation-A COVID-19 perspective. Foods 2022, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Urbano, O.; Perles, A.; Pedraza, C.; Rubio-Arraez, S.; Castello, M.L.; Ortola, M.D.; Mercado, R. Cost-effective implementation of a temperature traceability system based on smart RFID tags and IoT services. Sensors 2020, 20, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, A.K.; Dhau, J.S.; Gohel, H.; Mishra, Y.K.; Kateb, B.; Kim, N.Y.; Goswami, D.Y. Electrochemical SARS-CoV-2 sensing at point-of-care and artificial intelligence for intelligent COVID-19 management. ACS Appl. Bio Mater. 2020, 3, 7306–7325. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Tian, F.; Liu, C.; Liu, Y.; Zhao, S.; Fu, T.; Sun, J.; Tan, W. Rapid one-step detection of viral particles using an aptamer-based thermophoretic assay. J. Am. Chem. Soc. 2021, 143, 7261–7266. [Google Scholar] [CrossRef]
- Zhou, L.; Chandrasekaran, A.R.; Punnoose, J.A.; Bonenfant, G.; Charles, S.; Levchenko, O.; Badu, P.; Cavaliere, C.; Pager, C.T.; Halvorsen, K. Programmable low-cost DNA-based platform for viral RNA detection. Sci. Adv. 2020, 6, eabc6246. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, P.; Islam, M.S.; Duarte, P.M.; Tazerji, S.S.; Sobur, M.A.; El Zowalaty, M.E.; Ashour, H.M.; Rahman, M.T. Impact of the COVID-19 pandemic on food production and animal health. Trends Food Sci. Technol. 2022, 121, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Dai, B.; Wang, B.; Zha, Y.; Song, Q. Traceability in food processing: Problems, methods, and performance evaluations-a review. Crit. Rev. Food Sci. Nutr. 2022, 62, 679–692. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Masotti, F.; Cattaneo, S.; Stuknyte, M.; Pica, V.; De Noni, I. Transmission routes, preventive measures and control strategies of SARS-CoV-2 in the food factory. Crit. Rev. Food Sci. Nutr. 2022, 62, 4821–4831. [Google Scholar] [CrossRef]
- Abdeldayem, O.M.; Dabbish, A.M.; Habashy, M.M.; Mostafa, M.K.; Elhefnawy, M.; Amin, L.; Al-Sakkari, E.G.; Ragab, A.; Rene, E.R. Viral outbreaks detection and surveillance using wastewater-based epidemiology, viral air sampling, and machine learning techniques: A comprehensive review and outlook. Sci. Total Environ. 2022, 803, 149834. [Google Scholar] [CrossRef] [PubMed]
- Nzediegwu, C.; Chang, S.X. Improper solid waste management increases potential for COVID-19 spread in developing countries. Resour. Conserv. Recycl. 2020, 161, 104947. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qiao, J.; Han, X.; Zhao, Z.; Kou, J.; Zhang, W.; Man, S.; Ma, L. Needs, Challenges and Countermeasures of SARS-CoV-2 Surveillance in Cold-Chain Foods and Packaging to Prevent Possible COVID-19 Resurgence: A Perspective from Advanced Detections. Viruses 2023, 15, 120. https://doi.org/10.3390/v15010120
Li Y, Qiao J, Han X, Zhao Z, Kou J, Zhang W, Man S, Ma L. Needs, Challenges and Countermeasures of SARS-CoV-2 Surveillance in Cold-Chain Foods and Packaging to Prevent Possible COVID-19 Resurgence: A Perspective from Advanced Detections. Viruses. 2023; 15(1):120. https://doi.org/10.3390/v15010120
Chicago/Turabian StyleLi, Yaru, Jiali Qiao, Xiao Han, Zhiying Zhao, Jun Kou, Wenlu Zhang, Shuli Man, and Long Ma. 2023. "Needs, Challenges and Countermeasures of SARS-CoV-2 Surveillance in Cold-Chain Foods and Packaging to Prevent Possible COVID-19 Resurgence: A Perspective from Advanced Detections" Viruses 15, no. 1: 120. https://doi.org/10.3390/v15010120






