The Interaction of Mandarin Fish DDX41 with STING Evokes type I Interferon Responses Inhibiting Ranavirus Replication
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish, Cells, and Virus
2.2. Antibodies and Reagents
2.3. Molecular Cloning of scDDX41 cDNA
2.4. Sequence Analysis
2.5. Tissue Expression Profiles of scDDX41
2.6. Plasmid Construction and Mutations
2.7. Dual-Luciferase Reporter Assays
2.8. RT-qPCR
2.9. Absolute Quantitative Real-time PCR (qPCR) and TaqMan Probe
2.10. Determinations of Virus Titer
2.11. Co-Immunoprecipitation (Co-IP) and Western Blot (WB) Analysis
2.12. Electrophoretic Mobility Shift Assay (EMSA)
2.13. Pull-Down Assay
3. Results
3.1. Molecular Characteristics of scDDX41
3.2. Expressions of scDDX41 in Response to Immune Stimulations
3.3. ScDDX41 and Its Domains Evoked IFN-I and Inflammatory Response
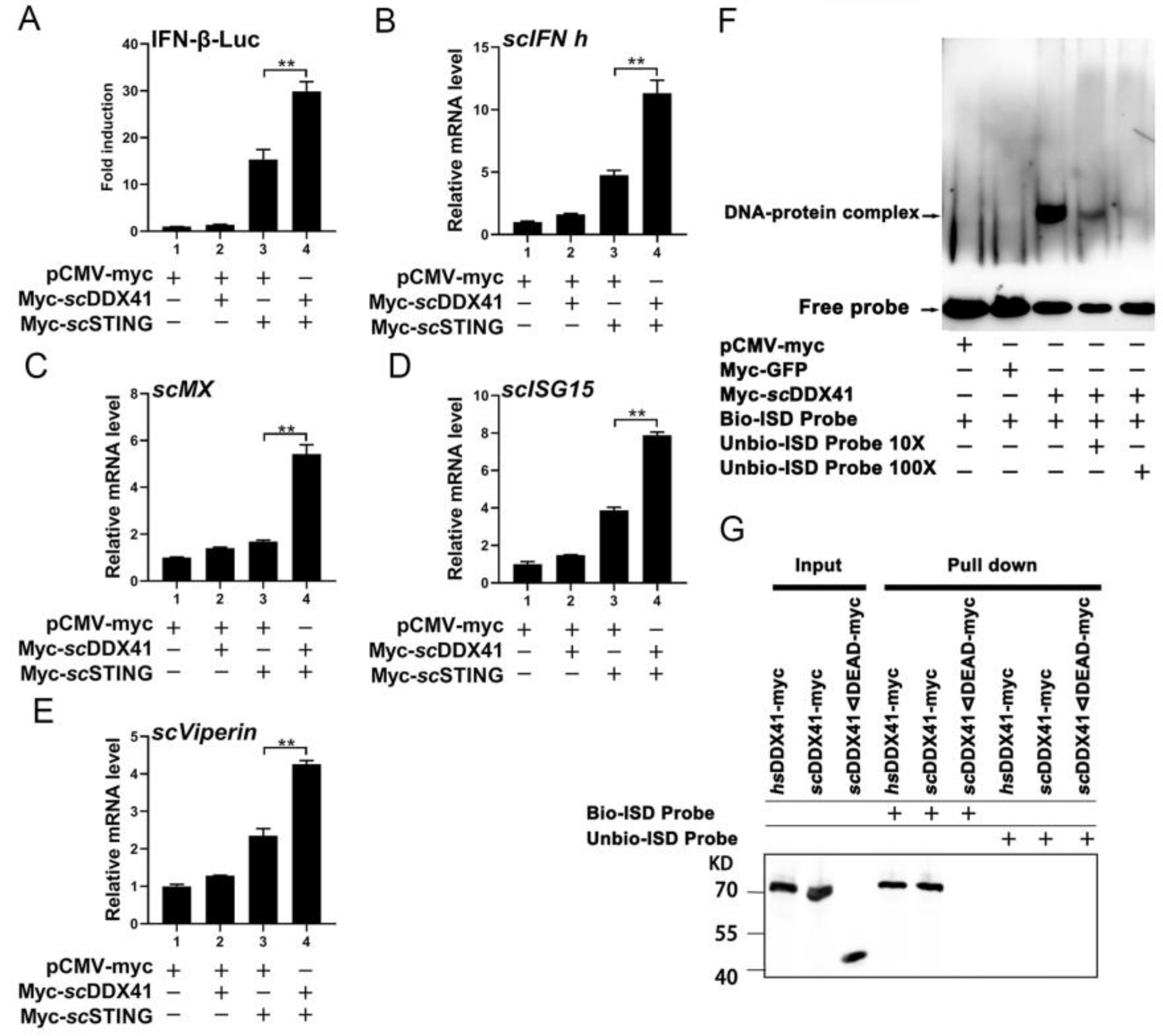
3.4. ScDDX41 Interacted with scSTING
3.5. ScDDX41 Recognizes dsDNA through the DEAD Domain
3.6. ScDDX41 Inhibits the Replication of Ranavirus
4. Discussion
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Linder, P.; Fullerpace, F.V. Looking back on the birth of DEAD-box RNA helicases. Biochim. Biophys. Acta 2013, 1829, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Kadono, M.; Kanai, A.; Nagamachi, A.; Shinriki, S.; Kawata, J.; Iwato, K.; Kyo, T.; Oshima, K.; Yokoyama, A.; Kawamura, T. Biological implications of somatic DDX41 p.R525H mutation in acute myeloid leukemia. Exp. Hematol. 2016, 44, 745–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omura, H.; Oikawa, D.; Nakane, T.; Kato, M.; Ishii, R.; Ishitani, R.; Tokunaga, F.; Nureki, O. Structural and Functional Analysis of DDX41: A bispecific immune receptor for DNA and cyclic dinucleotide. Sci. Rep. 2016, 6, 34756. [Google Scholar] [CrossRef] [PubMed]
- Schmucker, D.; Jackle, H.; Gaul, U. Genetic analysis of the larval optic nerve projection in Drosophila. Development 1997, 124, 937–948. [Google Scholar] [CrossRef]
- Lee, K.G.; Kim, S.S.Y.; Kui, L.; Voon, D.C.C.; Mauduit, M.; Bist, P.; Bi, X.; Pereira, N.A.; Liu, C.; Sukumaran, B. Bruton’s Tyrosine Kinase Phosphorylates DDX41 and Activates Its Binding of dsDNA and STING to Initiate Type 1 Interferon Response. Cell Rep. 2015, 10, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Yuan, B.; Bao, M.; Lu, N.; Kim, T.; Liu, Y.J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011, 12, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvatiyar, K.; Zhang, Z.; Teles, R.M.; Ouyang, S.; Jiang, Y.; Iyer, S.S.; Zaver, S.A.; Schenk, M.; Zeng, S.; Zhong, W.; et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 2012, 13, 1155–1161. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Chen, Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012, 5, a20. [Google Scholar] [CrossRef] [Green Version]
- Soponpong, S.; Amparyup, P.; Tassanakajon, A. A cytosolic sensor, PmDDX41, mediates antiviral immune response in black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 2018, 81, 291–302. [Google Scholar] [CrossRef]
- Ma, J.X.; Li, J.Y.; Fan, D.D.; Feng, W.; Lin, A.F.; Xiang, L.X.; Shao, J.Z. Identification of DEAD-Box RNA Helicase DDX41 as a Trafficking Protein That Involves in Multiple Innate Immune Signaling Pathways in a Zebrafish Model. Front. Immunol. 2018, 9, 1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Huang, Y.; Huang, X.; Li, C.; Ni, S.W.; Yu, Y.; Qin, Q. Grouper DDX41 exerts antiviral activity against fish iridovirus and nodavirus infection. Fish Shellfish Immunol. 2019, 91, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, X.; Wang, S.; Ge, G. Ctenopharyngodon idellus DDX41 initiates IFN I and ISG15 expression in response to GCRV infection. Fish Shellfish Immunol. 2020, 106, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Cheng, J.; Hou, J.; Xia, H.; Chen, W.; Xia, L.; Nie, P.; Lu, Y. Molecular and functional characterization of tilapia DDX41 in IFN regulation. Fish Shellfish Immunol. 2020, 99, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Duan, C.; Zhang, H.; Weng, S.; He, J.; Dong, C. Widespread outbreaks of the emerging mandarinfish ranavirus (MRV) both in natural and ISKNV-FKC vaccinated mandarinfish Siniperca chuatsi in Guangdong, South China. Aquaculture 2017, 520, 734989. [Google Scholar] [CrossRef]
- Guo, C.J.; He, J.; He, J.G. The immune evasion strategies of fish viruses. Fish Shellfish Immunol. 2019, 86, 772–784. [Google Scholar] [CrossRef]
- Qin, X.W.; He, J.; Yu, Y.; Liu, C.; Luo, Z.Y.; Li, Z.M.; Weng, S.P.; Guo, C.J.; He, J.G. The roles of mandarin fish STING in innate immune defense against Infectious spleen and kidney necrosis virus infections. Fish Shellfish Immunol. 2020, 100, 80–89. [Google Scholar] [CrossRef]
- He, J.; Yu, Y.; Qin, X.W.; Zeng, R.Y; Wang, Y.Y.; Li, Z.M.; Mi, S.; Weng, S.P.; Guo, C.J.; He, J.G. Mandarin fish (Siniperca chuatsi) hypoxia-inducible factor-1α involved in the immune response. Fish Shellfish Immunol. 2019, 92, 141–150. [Google Scholar] [CrossRef]
- Dong, C.; Weng, S.; Shi, X.; Xu, X.; Shi, N.; He, J. Development of a mandarin fish Siniperca chuatsi fry cell line suitable for the study of infectious spleen and kidney necrosis virus (ISKNV). Virus Res. 2008, 135, 273–281. [Google Scholar] [CrossRef]
- Zeng, R.Y.; Pan, W.Q.; Lin, Y.F.; He, J.; Guo, C.J. Development of a gene-deleted live attenuated candidate vaccine against fish virus (ISKNV) with low-pathogenicity and high-protection. iScience 2021, 24, 102750. [Google Scholar] [CrossRef]
- Quynh, N.T.; Hikima, J.; Kim, Y.R.; Fagutao, F.F.; Kim, M.S.; Aoki, T.; Jung, T.S. The cytosolic sensor, DDX41, activates antiviral and inflammatory immunity in response to stimulation with double-stranded DNA adherent cells of the olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2015, 44, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhou, X. STING: From Mammals to Insects. Cell Host Microbe 2018, 24, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.L.; Yang, C.R.; Zhang, Y.X.; Peng, L.M.; Chen, X.H.; Rao, Y.L.; Gu, T.L.; Su, J.G. Identification, characterization and immunological response analysis of stimulator of interferon gene (STING) from grass carp Ctenopharyngodon idella. Dev. Comp. Immunol. 2014, 45, 163–176. [Google Scholar] [CrossRef]
- Huang, Y.; Ouyang, Z.; Wang, W.; Yu, Y.; Li, P.; Zhou, S.; Wei, S.; Wei, J.; Huang, X.; Qin, Q. Antiviral role of grouper STING against iridovirus infection. Fish Shellfish Immunol 2015, 47, 157–167. [Google Scholar] [CrossRef] [PubMed]
- De, O.; Orzalli, M.H.; King, D.S.; Kagan, J.C.; Lee, A.; Kranzusch, P.J. Modular Architecture of the STING C-Terminal Tail Allows Interferon and NF-κB Signaling Adaptation. Cell Rep. 2019, 27, 1165–1175. [Google Scholar]





| Names | Sequence (5′–3′) |
|---|---|
| 5′ RACE-F | CTAATAGCACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT |
| 5′ RACE-R | TGAGATGCTGATGCTGGTCAAGGAG |
| 3′ RACE-F | TGATGGATCTTAAAGCCCTGC |
| 3′ RACE-R | ACTCTGCGTTGATACCACTGCTTGCCCTATAGTGAGTGCTATTAG |
| scDDX41-F | CGGAATTCCGGAGACCGACAATCGACCC |
| scDDX41-R | GGGGTACCTTAGAAGTCCATTGAGCTATGAGC |
| scDEAD-F | CGGAATTCCGCCACCAGCAATTCTAAAAGG |
| scDEAD-R | GGGGTACCTTACATCTTGGCCTCCTCTTTG |
| scHELIC-F | CGGAATTCCGCTTTTATTTGCTGAGAAGAAGG |
| scHELIC-R | GGGGTACCTTAATTAATGAACGTAGTGGC |
| scHELIC-YFP-F | CGGAATTCCGATGCTTTTATTTGCTGAGAAGAAG |
| scHELIC-YFP-R | GGGGTACCCCATTAATGAACGTAGTGGC |
| ISD-F | TACAGATCTACTAGTGATCTATGACTGATCTGTACATGATCTACA |
| ISD-R | TGTAGATCATGTACAGATCAGTCATAGATCACTAGTAGATCTGTA |
| Gene Names | Primers | Sequences (5′–3′) | Primer Efficiency |
|---|---|---|---|
| scDDX41 | Forward Reverse | ACGATTATGTTCCGTACATTCCAGTCAA TCCTCATCCCTCTGCTCCTCCC | 0.99 |
| MRV mcp | Forward Reverse | GTCACCCTGCCTTACAACGAAA CACGATGGGCTTGACTTCTCC | 0.96 |
| MRV ICP-18 | Forward Reverse | AGTTTGACGCCAGCTTTCACG TGCCATACCGTCGCACTCG | 0.98 |
| MRV DNA polymerase | Forward Reverse | GGCGTCAAGTGCCATCAG CAGGCGAGACAGTCTTCCAAT | 0.98 |
| IFN-h | Forward Reverse | CGCTCTGCTGTGATTGGC GGGACTCCACCTCTGCCTTT | 0.99 |
| scMX | Forward Reverse | GGATTCTGACATCGGGAGCAA GTGCAGTAGACTCATGCTGT | 0.98 |
| scISG15 | Forward Reverse | CGACGAGACTGTGAGCGACTTC CATTCATCATCTCCCTGCCTTGGT | 0.99 |
| scViperin | Forward Reverse | CCAAGAGGGGCCTCAAACTT CTGACACTTGGGAGCTGGAG | 0.95 |
| scTNF-α | Forward Reverse | AGCCAGGCATCGTTCAGAGTCT CTGTCCTCCTGAGCGGTGTCTT | 0.96 |
| β-actin | Forward Reverse | CCCTCTGAACCCCAAAGCCA CAGCCTGGATGGCAACGTACA | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.-W.; Luo, Z.-Y.; Pan, W.-Q.; He, J.; Li, Z.-M.; Yu, Y.; Liu, C.; Weng, S.-P.; He, J.-G.; Guo, C.-J. The Interaction of Mandarin Fish DDX41 with STING Evokes type I Interferon Responses Inhibiting Ranavirus Replication. Viruses 2023, 15, 58. https://doi.org/10.3390/v15010058
Qin X-W, Luo Z-Y, Pan W-Q, He J, Li Z-M, Yu Y, Liu C, Weng S-P, He J-G, Guo C-J. The Interaction of Mandarin Fish DDX41 with STING Evokes type I Interferon Responses Inhibiting Ranavirus Replication. Viruses. 2023; 15(1):58. https://doi.org/10.3390/v15010058
Chicago/Turabian StyleQin, Xiao-Wei, Zhi-Yong Luo, Wei-Qiang Pan, Jian He, Zhi-Min Li, Yang Yu, Chang Liu, Shao-Ping Weng, Jian-Guo He, and Chang-Jun Guo. 2023. "The Interaction of Mandarin Fish DDX41 with STING Evokes type I Interferon Responses Inhibiting Ranavirus Replication" Viruses 15, no. 1: 58. https://doi.org/10.3390/v15010058






