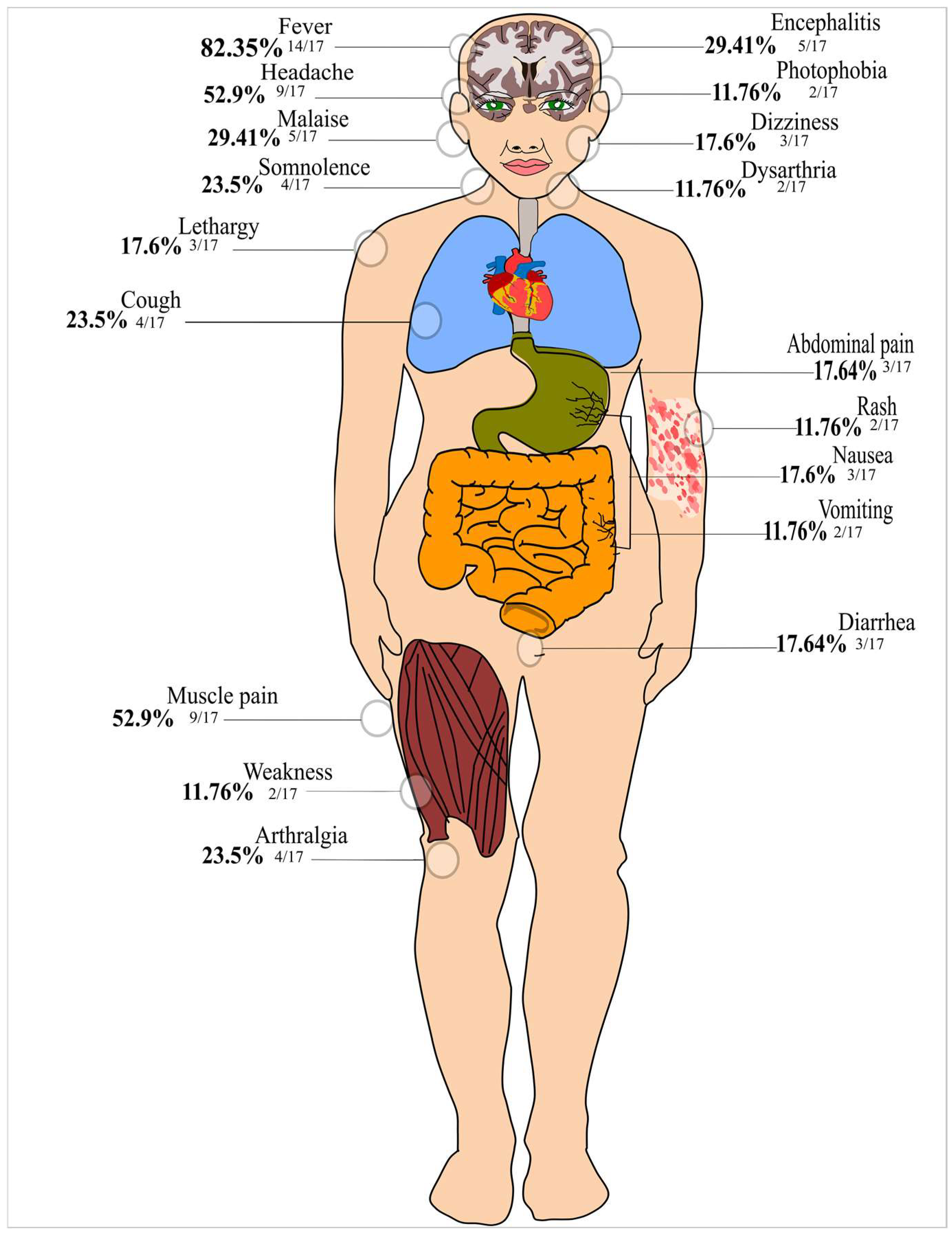
Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Systematic Review and Meta-Analysis
2.1.1. Search Strategy
2.1.2. Data Analysis
2.1.3. Statistical Analyses
3. Results
3.1. Characteristics of the Included Studies and Quality Assessment
3.2. Sensitivity Analysis and Publication Bias in the Meta-Analysis
3.3. A Meta-Analysis to Estimate the Pooled Frequency of ILHV Infection
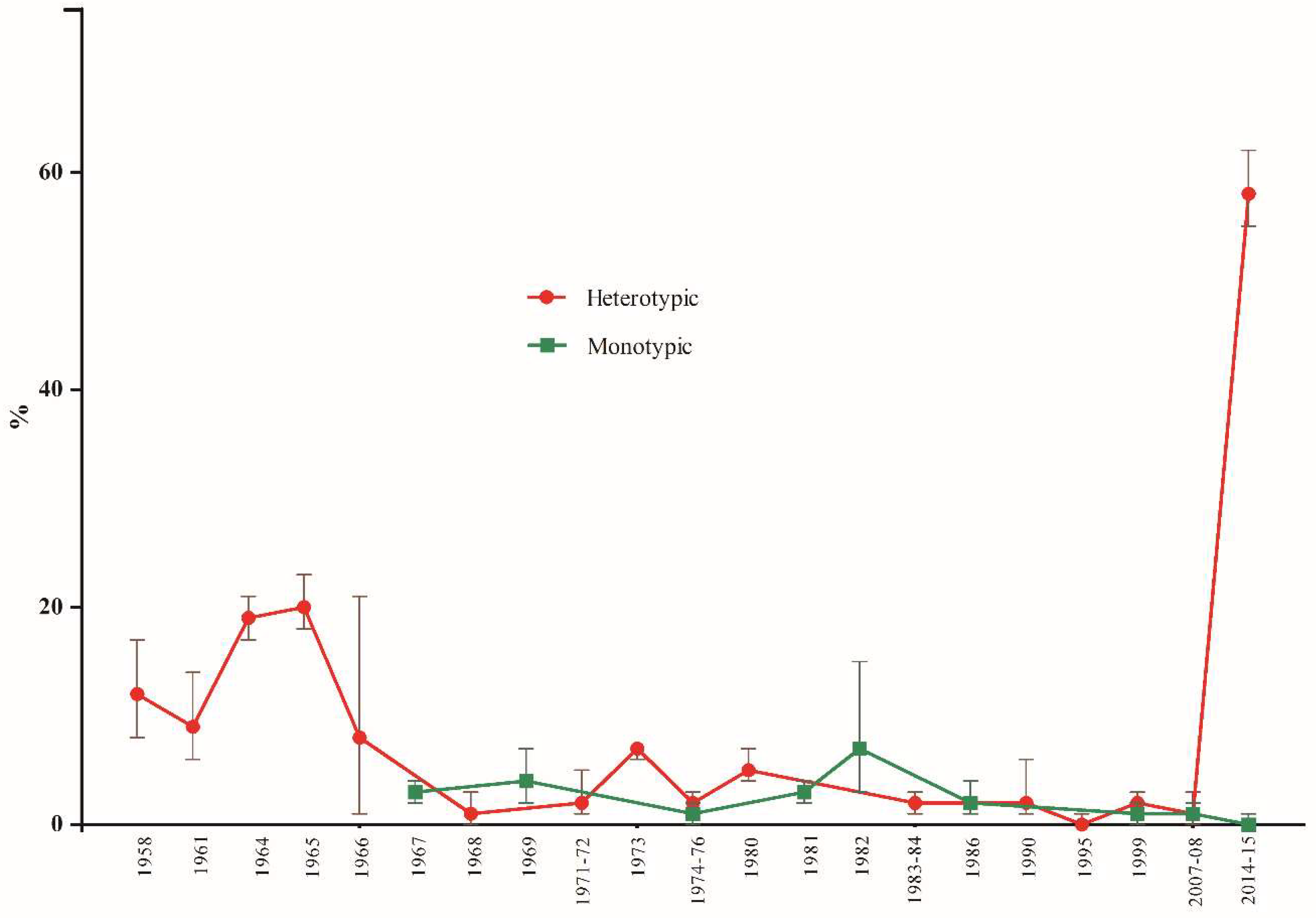
3.3.1. Subgroup Analysis by Origin and Year of Publication of the Studies
3.3.2. Subgroup Analysis by Sex and Age of Participants, and Serological Assays Used
3.3.3. A Meta-Analysis to Evaluate Factors Associated with ILHV Positivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laemmert, J.R.; Hugo, W.; Hughes; Thomas, P. The virus of Ilheus encephalitis: Isolation, serological specificity and trans-mission. J. Immunol. 1947, 55, 61–67. [Google Scholar] [PubMed]
- Cruz, A.C.; da Rosa, A.P.; Ferreira, I.I.; Albuquerque, M.M.; Galler, R. Ilheus virus (Flaviviridae, Flavivirus) is closely related to Japanese encephalitis virus complex. Intervirology 1997, 40, 220–225. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, K.; Quek, J.P.; Luo, D. Molecular Insights into the Flavivirus Replication Complex. Viruses 2021, 13, 956. [Google Scholar] [CrossRef] [PubMed]
- Saivish, M.V.; Menezes, G.L.; Costa, V.G.D.; Silva, G.C.D.D.; Marques, R.E.; Nogueira, M.L.; Silva, R.A.D. Predict-ing Antigenic Peptides from Rocio Virus NS1 Protein for Immunodiagnostic Testing Using Immunoinformatics and Molecular Dynamics Simulation. Int. J. Mol. Sci. 2022, 23, 7681. [Google Scholar] [CrossRef] [PubMed]
- Causey, O.R.; Causey, C.E.; Maroja, O.M.; Macedo, D.G. The isolation of arthropod-borne viruses, including members of two hitherto undescribed serological groups, in the Amazon region of Brazil. Am. J. Trop. Med. Hyg. 1961, 10, 227–249. [Google Scholar] [CrossRef]
- Southam, C.M.; Moore, A.E. West Nile, Ilheus, and Bunyamwera Virus Infections in Man 1,2,3. Am. J. Trop. Med. Hyg. 1951, 31, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Spence, L.; Anderson, C.R.; Downs, W.G. Isolation of Ilhéus virus from human beings in Trinidad, West Indies. Trans. R. Soc. Trop. Med. Hyg. 1962, 56, 504–509. [Google Scholar] [CrossRef]
- Srihongse, S.; Johnson, C.M. The Isolation of Ilhéus Virus from Man in Panamá. Am. J. Trop. Med. Hyg. 1967, 16, 516–518. [Google Scholar] [CrossRef]
- Nassar, E.; Coimbra, T.; Rocco, I.; Pereira, L.; Ferreira, I.; De Souza, L.; De Souza, D.; Ueda-Ito, M.; Moura, J.; Bergo, R. Human Disease Caused by an Arbovirus Closely Related to Ilheus Virus: Report of five cases. Intervirology 1997, 40, 247–252. [Google Scholar] [CrossRef]
- Venegas, E.A.; Aguilar, P.V.; Cruz, C.; Guevara, C.; Kochel, T.J.; Vargas, J.; Halsey, E.S. Ilheus Virus Infection in Human, Bolivia. Emerg. Infect. Dis. 2012, 18, 516–518. [Google Scholar] [CrossRef]
- Milhim, B.H.G.A.; Estofolete, C.F.; da Rocha, L.C.; Liso, E.; Brienze, V.M.S.; Vasilakis, N.; Terzian, A.C.B.; Nogueira, M.L. Fatal Outcome of Ilheus Virus in the Cerebrospinal Fluid of a Patient Diagnosed with Encephalitis. Viruses 2020, 12, 957. [Google Scholar] [CrossRef] [PubMed]
- Prías-Landínez, E.; Bernal-Cubides, C.; Morales-Alarcón, A. Isolation of Ilhéus Virus from Man in Colombia*. Am. J. Trop. Med. Hyg. 1968, 17, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Panon, G.; Fauran, P.; Digoutte, J.P. Isolation of Ilheus virus in french Guyana. Bull. Soc. Pathol. Exot. Fil. 1979, 72, 315–318. [Google Scholar]
- Johnson, B.W.; Cruz, C.; Felices, V.; Espinoza, W.R.; Manock, S.R.; Guevara, C.; Olson, J.G.; Kochel, T.J. Ilheus Virus Isolate from a Human, Ecuador. Emerg. Infect. Dis. 2007, 13, 956–958. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.B.; Pereira, L.E.; Rocco, I.M.; Marti, A.T.; de Souza, L.T.; Iversson, L.B. Surveillance of arbovirus infections in the Atlantic forest region, state of São Paulo, Brazil. Rev. Inst. Med. Trop. São Paulo 1994, 36, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.E.; Suzuki, A.; Coimbra, T.L.; de Souza, R.P.; Chamelet, E.L. Arbovírus Ilheus em aves silvestres (Sporophila caerulescens e Molothrus bonariensis) [Ilheus arbovirus in wild birds (Sporophila caerulescens and Molothrus bonariensis)]. Rev. Saúde Pública 2001, 35, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Casseb, A.R.; Cruz, A.V.; Jesus, I.S.; Chiang, J.O.; Martins, L.C.; Silva, S.P.; Henriques, D.F.; Casseb, L.M.; Vasconcelos, P.F. Seroprevalence of flaviviruses antibodies in water buffaloes (Bubalus bubalis) in Brazilian Amazon. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 9. [Google Scholar] [CrossRef] [Green Version]
- Iversson, L.B.; Silva, R.A.; da Rosa, A.P.; Barros, V.L. Circulation of eastern equine encephalitis, western equine encephalitis, Ilhéus, Maguari and Tacaiuma viruses in equines of the Brazilian Pantanal, South America. Rev. Inst. Med. Trop. Sao Paulo 1993, 35, 355–359. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Campos, Z.; Juliano, R.; Velez, J.; Nogueira, R.M.; Komar, N. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS. Negl. Trop. Dis. 2014, 8, e2706. [Google Scholar] [CrossRef] [Green Version]
- Medlin, S.; Deardorff, E.R.; Hanley, C.S.; Vergneau-Grosset, C.; Siudak-Campfield, A.; Dallwig, R.; da Rosa, A.T.; Tesh, R.B.; Martin, M.P.; Weaver, S.C.; et al. Serosurvey of selected arboviral pathogens in free-ranging, two-toed sloths (Choloepus hoffmanni) and three-toed sloths (Bradypus variegatus) in Costa Rica, 2005–2007. J. Wildl. Dis. 2016, 52, 883–892. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.A.; Fabbri, C.M.; Zunino, G.E.; Kowalewski, M.M.; Luppo, V.C.; Enría, D.A.; Levis, S.C.; Calderón, G.E. Detection of the mosquito-borne flaviviruses, West Nile, Dengue, Saint Louis Encephalitis, Ilheus, Bussuquara, and Yellow Fever in free-ranging black howlers (Alouatta caraya) of Northeastern Argentina. PLoS. Negl. Trop. Dis. 2017, 10, e0005351. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.S.; Ferreira, M.; Martins, L.C.; De Vleeschouwer, K.M.; Cassano, C.R.; Oliveira, L.C.; Canale, G.; Deem, S.L.; Tello, J.S.; Parker, P.; et al. Surveillance of Arboviruses in Primates and Sloths in the Atlantic Forest, Bahia, Brazil. EcoHealth 2018, 15, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.N.; Auguste, A.J.; Coombs, D.; Blitvich, B.J.; Carrington, C.V.; da Rosa, A.P.; Wang, E.; Chadee, D.D.; Drebot, M.A.; Tesh, R.B.; et al. Serological evidence of flaviviruses and alphaviruses in livestock and wildlife in Trinidad. Vector Borne Zoonotic Dis. 2012, 12, 969–978. [Google Scholar] [CrossRef] [Green Version]
- Vieira, C.J.D.S.P.; Andrade, C.D.; Kubiszeski, J.R.; Silva, D.J.F.D.; Barreto, E.S.; Massey, A.L.; Canale, G.R.; Bernardo, C.S.S.; Levi, T.; Peres, C.A.; et al. Detection of Ilheus virus in mosquitoes from southeast Amazon, Brazil. Trans. R. Soc. Trop. Med. Hyg. 2019, 113, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.A.B.; Santos, E.D.; Cardoso, J.D.C.; Noll, C.A.; Lima, M.M.; Silva, F.A.E.; Ferreira, M.S.; Martins, L.C.; Vasconcelos, P.F.D.C.; Bicca-Marques, J.C. Detection of antibodies against Icoaraci, Ilhéus, and Saint Louis Encephalitis arboviruses during yellow fever monitoring surveillance in non-human primates (Alouatta caraya) in southern Brazil. J. Med. Primatol. 2019, 48, 211–217. [Google Scholar] [CrossRef]
- Pauvolid-Corrêa, A.; Kenney, J.L.; Couto-Lima, D.; Campos, Z.M.; Schatzmayr, H.G.; Nogueira, R.M.; Brault, A.C.; Komar, N. Ilheus virus isolation in the Pantanal, west-central Brazil. PLoS Negl. Trop. Dis. 2013, 18, e2318. [Google Scholar] [CrossRef] [Green Version]
- da Silva Ferreira, R.; de Toni Aquino da Cruz, L.C.; de Souza, V.J.; da Silva Neves, N.A.; de Souza, V.C.; Filho, L.C.F.; da Silva Lemos, P.; de Lima, C.P.S.; Naveca, F.G.; Atanaka, M.; et al. Insect-specific viruses and arboviruses in adult male culicids from Midwestern Brazil. Infect. Genet. Evol. 2020, 85, 104561. [Google Scholar] [CrossRef]
- Cunha, M.S.; Luchs, A.; Dos Santos, F.C.P.; Caleiro, G.S.; Nogueira, M.L.; Maiorka, P.C. Applying a pan-flavivirus RT-qPCR assay in Brazilian public health surveillance. Arch. Virol. 2020, 165, 1863–1868. [Google Scholar] [CrossRef]
- Araújo, P.A.; Freitas, M.O.; Chiang, J.O.; Silva, F.A.; Chagas, L.L.; Casseb, S.M.; Silva, S.P.; Nunes-Neto, J.P.; Rosa-Júnior, J.W.; Nascimento, B.S.; et al. Investigation about the Occurrence of Transmission Cycles of Arbovirus in the Tropical Forest, Amazon Region. Viruses 2019, 11, 774. [Google Scholar] [CrossRef] [Green Version]
- Cunha, M.S.; Luchs, A.; da Costa, A.C.; Ribeiro, G.O.; Dos Santos, F.C.P.; Nogueira, J.S.; Komninakis, S.V.; Marinho, R.D.S.S.; Witkin, S.S.; Villanova, F.; et al. Detection and characterization of Ilheus and Iguape virus genomes in historical mosquito samples from Southern Brazil. Acta Trop. 2020, 205, 105401. [Google Scholar] [CrossRef]
- De Rodaniche, E.; Galindo, P. Isolation of Ilhéus virus from Sabethes chloropterus captured in Guatemala in 1956. Am. J. Trop. Med. Hyg. 1957, 6, 686–687. [Google Scholar] [CrossRef] [PubMed]
- De Rodaniche, E.; Galindo, P. Isolation of the virus of IIheus encephalitis from mosquitoes captured in Panama. Am. J. Trop. Med. Hyg. 1961, 10, 393–394. [Google Scholar] [CrossRef] [PubMed]
- De Rodaniche, E. Isolation of the virus of IIhéus encephalitis from mosquitoes of the genus Psorophora captured in Honduras. Am. J. Trop. Med. Hyg. 1956, 5, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Aitken, T.H.; Anderson, C.R.; Downs, W.G. The isolation of Ilhéus virus from wild caught forest mosquitoes in Trinidad. Am. J. Trop. Med. Hyg. 1956, 5, 621–625. [Google Scholar] [CrossRef]
- Galindo, P.; de Rodaniche, E. Birds as hosts of Ilheus encephalitis virus in Panama. Am. J. Trop. Med. Hyg. 1961, 10, 395–396. [Google Scholar] [CrossRef]
- de Rodaniche, E.; Galindo, P. Ecological Observations on Ilhéus Virus in the Vicinity of Almirante, Republic of Panama*. Am. J. Trop. Med. Hyg. 1963, 12, 924–928. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses; Ottawa Health Research Institute: Ottawa, ON, USA, 2021; Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 26 July 2022).
- Freeman, M.F.; Tukey, J.W. Transformations related to the angular and the square root. Ann. Math. Stat. 1950, 21, 607–611. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-analysis of prevalence. J. Epidemiol. Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Groot, H.; Riberiro, R.B. Neutralizing and haemagglutination-inhibiting antibodies to yellow fever 17 years after vaccination with 17D vaccine. Bull. World Health Organ. 1962, 27, 699–707. [Google Scholar] [PubMed]
- Ehrenkranz, N.J.; Pond, W.L.; Pennington, R.M.; Carter, M.J. Arthropod-borne virus disease in Florida: Report of a 1958 outbreak in Miami and a serologic survey of Miami residents. Am. J. Med. 1963, 35, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Macias, M.J. Estudios Epidemiológicos Sobre Virus Arbor en el Sureste de México; Salud Pública de México: Cuernavaca, Mexico, 1963; Volume 5, pp. 523–527. Available online: File:///F:/Perfil%20Vivaldo/Downloads/3881-Texto%20del%20art%C3%ADculo-3828-1-10-20141111.pdf (accessed on 25 July 2022).
- Príans-landínez, E.; Bernal-Cúbides, C.; Torres, S.V.; Romero-León, M. Encuesta Serologica de Virus Transmitidos Por Artropodos; Boletín de la Oficina Sanitaria Panamericana: Bogotá, Colombia, 1966; Available online: https://iris.paho.org/bitstream/handle/10665.2/14510/v68n2p134.pdf?sequence=1&isAllowed=y (accessed on 22 July 2022).
- Neel, J.V.; Andrade, A.H.P.; Stollerman, G.H.; Weinstein, E.D.; Wheeler, A.H.; Brown, G.E.; Goobar, J.; Eveland, W.E.; Sodeman, W.A. Further Studies of the Xavante Indians. Am. J. Trop. Med. Hyg. 1968, 17, 486–498. [Google Scholar] [CrossRef]
- Niederman, J.C.; Henderson, J.R.; Opton, E.M.; Black, F.L.; Skvrnova, K. A nationwide serum survey of brazilian military recruits, 1964: Ii. antibody patterns with arboviruses, polioviruses, measles and mumps. Am. J. Epidemiol. 1967, 86, 319–329. [Google Scholar] [CrossRef]
- Evans, A.S.; Casals, J.; Opton, E.M.; Borman, E.K.; Levine, L.; Cuadrado, R.R. A nationwide serum survey of colombian military recruits, 1966. Am. J. Trop. Med. Hyg. 1969, 90, 292–303. [Google Scholar] [CrossRef]
- Black, F.L.; Woodall, J.P.; Evans, A.S.; Liebhaber, H.; Henle, G. Prevalence of antibody against viruses in the Tiriyo, an isolated amazon tribe. Am. J. Epidemiol. 1970, 91, 430–438. [Google Scholar] [CrossRef]
- Buckley, S.M.; Davis, J.L.; Madalengoitia, J.; Flores, W.; Casals, J. Arbovirus neutralization tests with Peruvian sera in Vero cell cultures. Bull. World Health Organ. 1972, 46, 451–455. [Google Scholar] [PubMed]
- Madalengoitia, J.; Flores, W.; Casals, J. Arbovirus Antibody Survey of Sera from Residents of Eastern Peru; English Edition; Boletín de la Oficina Sanitaria Panamericana (OSP): Lima, Peru, 1973; p. 7. Available online: https://iris.paho.org/handle/10665.2/11677 (accessed on 14 July 2022).
- Pinheiro, F.P.; Bensabath, G.; Andrade, A.H.; Lins, Z.C.; Fraiha, H.; Tang, A.T.; Lainson, R.; Shaw, J.J.; Azevedo, M.C. Infectious Diseases along Brazil’s Trans-Amazon Highway: Surveillance and Research; Pan American Health Organization (PAHO): Washington, DC, USA, 1974; Volume 8, Available online: https://iris.paho.org/handle/10665.2/27094 (accessed on 8 July 2022).
- Pinheiro, F.P.; Schatzmayr, H.; Rosa, A.P.A.T.; Homma, A.; Bensabath, G. Arbovirus Antibodies in Children of Rural Guanabara, Brazil. Intervirology 1975, 5, 93–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiland, H.T.; Williams, M.C.; Hull, B. Serologic survey of dengue and other arboviruses in Curaçao and Aruba, 1973. Bull. Pan Am. Health Organ. 1978, 12, 134–142. [Google Scholar] [PubMed]
- Dixon, K.E.; Llewellyn, C.H.; da Rosa, A.P.T.; da Rosa, J.F.T. A multidisciplinary program of infectious disease surveillance along the Transamazon highway in Brazil: Epidemiology of arbovirus infections. Bull. Pan Am. Health Organ. 1981, 15, 11–25. [Google Scholar] [PubMed]
- Iversson, L.B.; Rosa; Amélia, P.A.T.; Rosa, J.T. Estudos sorológicos para pesquisa de anticorpos de arbovírus em população humana da região do Vale do Ribeira: Ii-inquérito em pacientes do hospital regional de pariquera-açú, 1980. Rev. Saúde Pública 1981, 15, 587–602. [Google Scholar] [CrossRef] [Green Version]
- Iversson, L.B.; da Rosa, A.P.A.T.; da Rosa, J.T.; Costa, C.S. Estudos sorológicos para pesquisa de anticorpos de arbovírus em população humana da região do Vale do Ribeira: III-inquérito em coabitantes com casos de encefalite por Flavivirus Rocio. Rev. Saúde Pública 1982, 16, 160–170. [Google Scholar] [CrossRef]
- Figueiredo, L.T.M.; de Rosa, A.P.A.T.; Fiorillo, A.M. Níveis de anticorpos para arbovírus em indivíduos da região de Ribeirão Preto, SP (Brasil). Rev. Saúde Pública 1986, 20, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Tavares-Neto, J.; Rosa, A.P.A.T.d.; Vasconcelos, P.F.C.; Costa, J.M.L.; de Rosa, J.F.S.T.; Marsden, P.D. Pesquisa de anticorpos para arbovírus no soro de residentes no povoado de Corte de Pedra, Valença, Bahia. Mem. Inst. Oswaldo Cruz 1986, 81, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Degallier, N.; da Rosa, A.M.P.T.; Vasconcelos, P.F.C.; Hervé, J.P.; Filho, G.C.S.; da Rosa, J.F.S.T.; da Rosa, E.S.T.; Rodrigues, S.G. Modifications of arbovirus transmission in relation to construction of dams in Brazilian Amazonia. Sci. Cult 1992, 44, 124–135. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_6/b_fdi_33-34/38274.pdf (accessed on 5 July 2022).
- Straatmann, A.; Santos-Torres, S.; Vasconcelos, P.F.; Da Rosa, A.P.T.; Rodrigues, S.G.; Tavares-Neto, J. Evidências sorológicas da circulação do arbovírus Rocio (Flaviviridae) na Bahia. Rev. Soc. Bras. Med. Trop. 1997, 30, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Romano-Lieber, N.S.; Iversson, L.B. Inquérito soroepidemiológico para pesquisa de infecções por arbovírus em moradores de reserva ecológica. Rev Saúde Pública 2000, 34, 236–242. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, T.P.; Rodrigues, S.G.; Costa, M.I.W.D.A.; Vasconcelos, P.F.D.C.; Da Rosa, A.P.T. Diagnóstico sorológico de infecções por dengue e febre amarela em casos suspeitos no Estado do Pará, Brasil, 1999. Rev. Soc. Bras. Med. Trop. 2002, 35, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Tavares-Neto, J.; Freitas-Carvalho, J.; Nunes, M.R.T.; Rocha, G.; Rodrigues, S.G.; Damasceno, E.; Darub, R.; Viana, S.; Vasconcelos, P.F.C. Pesquisa de anticorpos contra arbovírus e o vírus vacinal da febre amarela em uma amostra da população de Rio Branco, antes e três meses após a vacina 17D. Rev. Soc. Bras. Med. Trop. 2004, 37, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.C.; Prazeres, A.S.C.; Gama, E.C.; Lima, M.F.; Azevedo, R.S.S.; Casseb, L.M.N.; Neto, J.P.N.; Martins, L.C.; Chiang, J.O.; Rodrigues, S.G.; et al. Serological survey for arboviruses in Juruti, para state, brazil. Cad Saude Publica 2009, 25, 2517–2523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, B.B.; de Jesus, M.F.C.; Pereira, R.L.; Chiang, J.O.; de Oliveira, F.M.N.; Ferreira, M.S.; Martins, L.C.; da Costa Vasconcelos, P.F.; Ganoza, C.; Lalwani, P. Prevalence of arbovirus antibodies in young healthy adult population in Brazil. Parasites Vectors 2021, 14, 403. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, L.S.; Ferreira, M.S.; Fernandes, D.; Padda, H.; Travassos-da-Rosa, E.S.; Deem, S.L.; Vasconcelos, P.F.C.; Martins, L.C. Individual, household and environmental factors associated with arboviruses in rural human populations, Brazil. Zoonoses Public Health 2021, 68, 203–212. [Google Scholar] [CrossRef]
- Pinheiro, F.P.; Travassos da Rosa, A.P.A.; Freitas, R.B.; Travasos da Rosa, J.F.S.; Vasconcelos, P.F.C. Aspectos Clínicos-Epidemiológicos: 50 anos do Instituto Evandro Chagas. Belem. Fundação Servidos Saude Publica 1986, 1, 375–407. [Google Scholar]
- Chastel, C. Asymptomatic infections in man: A Trojan horse for the introduction and spread of mosquito-borne arboviruses in non-endemic areas? Bull. Soc. Pathol. Exot. 2011, 104, 213–219. [Google Scholar] [CrossRef] [Green Version]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic arboviral diseases: Priorities for research and public health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.; Chen, H.; Gao, N.; An, J. Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases. Viruses 2022, 14, 1213. [Google Scholar] [CrossRef]
- Qian, X.; Qi, Z. Mosquito-Borne Flaviviruses and Current Therapeutic Advances. Viruses 2022, 14, 1226. [Google Scholar] [CrossRef] [PubMed]
- Rufalco-Moutinho, P.; de Noronha, L.A.G.; de Souza Cardoso Quintão, T.; Nobre, T.F.; Cardoso, A.P.S.; Cilião-Alves, D.C.; Bellocchio Júnior, M.A.; von Glehn, M.P.; Haddad, R.; Romero, G.A.S.; et al. Evidence of co-circulation of multiple arboviruses transmitted by Aedes species based on laboratory syndromic surveillance at a health unit in a slum of the Federal District, Brazil. Parasites Vectors 2021, 19, 610. [Google Scholar] [CrossRef] [PubMed]
- Srihongse, S.; Johnson, C.M. The first isolation of Bussuquara virus from man. Trans. R. Soc. Trop. Med. Hyg. 1971, 65, 541–542. [Google Scholar] [CrossRef] [PubMed]
- Batista, W.C.; Tavares, G.D.S.B.; Vieira, D.S.; Honda, E.R.; Pereira, S.S.; Tada, M.S. Notification of the first isolation of Cacipacore virus in a human in the State of Rondônia, Brazil. Rev. Soc. Bras. Med. Trop. 2011, 44, 528–530. [Google Scholar] [CrossRef] [Green Version]
- Shope, R.E. Epidemiology of Other Arthropod-borne Flaviviruses infecting humans. Adv. Virus Res. 2003, 61, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.P.S.; John, A.L.S. Cross-Reactive Immunity Among Flaviviruses. Front. Immunol. 2020, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- John, A.L.S.; Rathore, A.P.S. Adaptive immune responses to primary and secondary dengue virus infections. Nat. Rev. Immunol. 2019, 19, 218–230. [Google Scholar] [CrossRef]
- Amarilla, A.A.; Fumagalli, M.J.; Figueiredo, M.L.; Lima-Junior, D.S.; Santos-Junior, N.N.; Alfonso, H.L.; Lippi, V.; Trabuco, A.C.; Lauretti, F.; Muller, V.D.; et al. Ilheus and Saint Louis encephalitis viruses elicit cross-protection against a lethal Rocio virus challenge in mice. PLoS ONE 2018, 13, e0199071. [Google Scholar] [CrossRef]
- Fumagalli, M.J.; de Souza, W.M.; de Castro-Jorge, L.A.; de Carvalho, R.V.H.; Castro, Í.A.; de Almeida, L.G.N.; Consonni, S.R.; Zamboni, D.S.; Figueiredo, L.T.M. Chikungunya Virus Exposure Partially Cross-Protects against Mayaro Virus Infection in Mice. J. Virol. 2021, 95, e0112221. [Google Scholar] [CrossRef]
- Sather, G.E.; Hammon, W.M. Protection against St. Louis encephalitis and West Nile arboviruses by previous dengue virus (types 1–4) infection. Proc. Soc. Exp. Biol. Med. 1970, 135, 573–578. [Google Scholar] [CrossRef]
- Hirst, G.K. The quantitative determination of influenza virus and antibodies by means of red cell agglutination. J. Exp. Med. 1942, 75, 49. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Maeda, J. Review of diagnostic plaque reduction neutralization tests for flavivirus infection. Vet. J. 2013, 195, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T.; Hombach, J.; Barrett, A.D.T. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008, 21, 123–132. [Google Scholar] [CrossRef]
- Musso, D.; Despres, P. Serological Diagnosis of Flavivirus-Associated Human Infections. Diagnostics 2020, 10, 302. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa, V.G.; Saivish, M.V.; Lino, N.A.B.; Bittar, C.; de Freitas Calmon, M.; Nogueira, M.L.; Rahal, P. Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis. Viruses 2023, 15, 92. https://doi.org/10.3390/v15010092
da Costa VG, Saivish MV, Lino NAB, Bittar C, de Freitas Calmon M, Nogueira ML, Rahal P. Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis. Viruses. 2023; 15(1):92. https://doi.org/10.3390/v15010092
Chicago/Turabian Styleda Costa, Vivaldo Gomes, Marielena Vogel Saivish, Nikolas Alexander Borsato Lino, Cíntia Bittar, Marília de Freitas Calmon, Maurício Lacerda Nogueira, and Paula Rahal. 2023. "Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis" Viruses 15, no. 1: 92. https://doi.org/10.3390/v15010092
APA Styleda Costa, V. G., Saivish, M. V., Lino, N. A. B., Bittar, C., de Freitas Calmon, M., Nogueira, M. L., & Rahal, P. (2023). Clinical Landscape and Rate of Exposure to Ilheus Virus: Insights from Systematic Review and Meta-Analysis. Viruses, 15(1), 92. https://doi.org/10.3390/v15010092