Serological and Molecular Epidemiology of Chikungunya Virus Infection in Vietnam, 2017–2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Population and Study Methodology
2.2. Viruses and Cell Lines
2.3. ELISA Identification of Anti-CHIKV IgM Antibodies
2.4. ELISA Identification of Anti-CHIKV IgG Antibodies
2.5. Detection of Neutralization Antibody against CHIKV by FRNT50
2.6. CHIKV Genome Detection Using Real-Time Quantitative PCR (RT–qPCR)
2.7. Genomic Characterization of CHIKV
2.8. Data Analysis
3. Results
3.1. Demographic Characteristics
3.2. Prevalence of Anti–IgM and Anti–IgG CHIKV Antibodies in the Study Population
3.3. Prevalence of Anti-CHIKV Neutralizing Antibodies (NAbs) in This Study
3.4. Correlation between CHIKV IgG and IgM Antibodies with Neutralizing Antibody (NAbs)
3.5. CHIKV Genome Detection and Correlation between the CHIKV Genome and Antibodies
3.6. CHIKV Sequence Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- David, M.; Knine, P.M.H. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; ISBN 9781119650836. [Google Scholar]
- Caglioti, C.; Lalle, E.; Castilletti, C.; Carletti, F.; Capobianchi, M.R.; Bordi, L. Chikungunya Virus Infection: An Overview. New Microbiol. 2013, 36, 211–227. [Google Scholar]
- Suhrbier, A. Rheumatic Manifestations of Chikungunya: Emerging Concepts and Interventions. Nat. Rev. Rheumatol. 2019, 15, 597–611. [Google Scholar] [CrossRef] [PubMed]
- Seyler, T.; Hutin, Y.; Ramanchandran, V.; Ramakrishnan, R.; Manickam, P.; Murhekar, M. Estimating the Burden of Disease and the Economic Cost Attributable to Chikungunya, Andhra Pradesh, India, 2005–2006. Trans. R. Soc. Trop. Med. Hyg. 2010, 104, 133–138. [Google Scholar] [CrossRef]
- WHO. Guidelines on Clinical Management of Chikungunya Fever; WHO: New Delhi, India, 2008. [Google Scholar]
- WHO; Regional Office for South-East Asia. Guidelines for Prevention and Control of Chikungunya Fever 2009; WHO—Regional Office for South-East Asia: New Delhi, India, 2009; ISBN 9789290223375. [Google Scholar]
- Reddy, V.; Ravi, V.; Desai, A.; Parida, M.; Powers, A.M.; Johnson, B.W. Utility of IgM ELISA, TaqMan Real-Time PCR, Reverse Transcription PCR, and RT-LAMP Assay for the Diagnosis of Chikungunya Fever. J. Med. Virol. 2012, 84, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.W.; Russell, B.J.; Goodman, C.H. Laboratory Diagnosis of Chikungunya Virus Infections and Commercial Sources for Diagnostic Assays. J. Infect. Dis. 2016, 214, S471–S474. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.L.A.; Da Fonseca, B.A.L. Sensitivity and Detection of Chikungunya Viral Genetic Material Using Several PCR-Based Approaches. Rev. Soc. Bras. Med. Trop. 2017, 50, 465–469. [Google Scholar] [CrossRef]
- Khan, A.H.; Morita, K.; Del Carmen, M.; Hasebe, F.; Mathenge, E.G.M.; Igarashi, A. Printed in Great Britain Complete Nucleotide Sequence of Chikungunya Virus and Evidence for an Internal Polyadenylation Site. J. Gen. Virol. 2002, 83, 3075–3084. [Google Scholar] [CrossRef]
- Kendall, C.; Khalid, H.; Müller, M.; Banda, D.H.; Kohl, A.; Merits, A.; Stonehouse, N.J.; Tuplin, A. Structural and Phenotypic Analysis of Chikungunya Virus RNA Replication Elements. Nucleic Acids Res. 2019, 47, 9296–9312. [Google Scholar] [CrossRef]
- Ross, R.W. A Laboratory Technique for Studying the Insect Transmission of Animal Viruses, Employing a Bat-Wing Membrane, Demonstrated with Two African Viruses; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Hayes, C.G.; O’Rourke, T.; Sarr, A. Chikungunya Fever among U.S. Peace Corps Volunteers—Republic of the Philippines. MMWR Morb. Mortal Wkly. Rep. 2006, 35, 573–574. [Google Scholar]
- Porter, K.R.; Tan, R.; Istary, Y.; Suharyono, W.; Sutaryo; Widjaja, S.; Ma, C.; Listiyaningsih, E.; Kosasih, H.; Hueston, L.; et al. A Serological Study of Chikungunya Virus Transmission in Yogyakarta, Indonesia: Evidence for the First Outbreak since 1982. Southeast Asian J. Trop. Med. Public Health 2004, 35, 408–415. Available online: https://pubmed.ncbi.nlm.nih.gov/15691147/ (accessed on 23 August 2023).
- Deller, J.J., Jr.; Russell, P.K. An Analysis of Fevers of Unknown Origin in American Soldiers in Vietnam. J. Occup. Environ. Med. 1967, 66, 1129–1143. Available online: https://journals.lww.com/joem/citation/1968/02000/an_analysis_of_fevers_of_unknown_origin_in.30.aspx (accessed on 23 August 2023). [CrossRef]
- Hammon, W.M.D.; Rudnick, A.; Sather, G.E. Viruses Associated with Epidemic Hemorrhagic Fevers of the Philippines and Thailand. Science 1960, 131, 1102–1103. [Google Scholar] [CrossRef]
- Organ mond Sante, B.; Khai Ming, C.; Thein, S.; Thaung, U.; Saw Myint, K.; Swe, T.; Halstead, S.B.; Diwan, A.R. Clinical and Laboratory Studies on Haemorrhagic Fever in Burma, 1970–1972*. Bull. World Health Organ. 1974, 51, 227–235. [Google Scholar]
- Zeller, H.; Van Bortel, W.; Sudre, B. Chikungunya: Its History in Africa and Asia and Its Spread to New Regions in 2013–2014. J. Infect. Dis. 2016, 214, S436–S440. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Angheben, A.; Degani, M.; Bisoffi, Z. Emergence and Surveillance of Chikungunya. Curr. Trop. Med. Rep. 2015, 2, 4–12. [Google Scholar] [CrossRef]
- Duong, V.; Andries, A.C.; Ngan, C.; Sok, T.; Richner, B.; Asgari-Jirhandeh, N.; Bjorge, S.; Huy, R.; Ly, S.; Laurent, D.; et al. Reemergence of Chikungunya Virus in Cambodia. Emerg. Infect. Dis. 2012, 18, 2066. [Google Scholar] [CrossRef] [PubMed]
- Soulaphy, C.; Souliphone, P.; Phanthavong, K.; Phonekeo, D.; Phimmasine, S.; Khamphaphongphane, B.; Kitthiphong, V.; Lewis, H.C. Emergence of Chikungunya in Moonlapamok and Khong Districts, Champassak Province, the Lao People’s Democratic Republic, May to September 2012. West. Pacific Surveill. Response J. WPSAR 2013, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.C.; Tan, L.K.; Tan, C.H.; Tan, S.S.Y.; Hapuarachchi, H.C.; Pok, K.Y.; Lai, Y.L.; Lam-Phua, S.G.; Bucht, G.; Lin, R.T.P.; et al. Entomologic and Virologic Investigation of Chikungunya, Singapore. Emerg. Infect. Dis. 2009, 15, 1243. [Google Scholar] [CrossRef]
- Haque, F.; Rahman, M.; Banu, N.N.; Sharif, A.R.; Jubayer, S.; Shamsuzzaman, A.; Alamgir, A.; Erasmus, J.H.; Guzman, H.; Forrester, N.; et al. An Epidemic of Chikungunya in Northwestern Bangladesh in 2011. PLoS ONE 2019, 14, e0212218. [Google Scholar] [CrossRef]
- Hassan, R.; Rahman, M.M.; Moniruzzaman, M.; Rahim, A.; Barua, S.; Biswas, R.; Biswas, P.; Mowla, S.G.M.; Chowdhury, M.A.J. Chikungunya—An Emerging Infection in Bangladesh: A Case Series. J. Med. Case Rep. 2014, 8, 67. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, H.C.; Bandara, K.B.A.T.; Sumanadasa, S.D.M.; Hapugoda, M.D.; Lai, Y.L.; Lee, K.S.; Tan, L.K.; Lin, R.T.P.; Ng, L.F.P.; Bucht, G.; et al. Re-Emergence of Chikungunya Virus in South-East Asia: Virological Evidence from Sri Lanka and Singapore. J. Gen. Virol. 2010, 91, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Yergolkar, P.N.; Tandale, B.V.; Arankalle, V.A.; Sathe, P.S.; Sudeep, A.B.; Gandhe, S.S.; Gokhle, M.D.; Jacob, G.P.; Hundekar, S.L.; Mishra, A.C. Chikungunya Outbreaks Caused by African Genotype, India. Emerg. Infect. Dis. 2006, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Khongwichit, S.; Chansaenroj, J.; Thongmee, T.; Benjamanukul, S.; Wanlapakorn, N.; Chirathaworn, C.; Poovorawan, Y. Large-Scale Outbreak of Chikungunya Virus Infection in Thailand, 2018–2019. PLoS ONE 2021, 16, e0247314. [Google Scholar] [CrossRef]
- Naveca, F.G.; Claro, I.; Giovanetti, M.; de Jesus, J.G.; Xavier, J.; de Melo Iani, F.C.; do Nascimento, V.A.; de Souza, V.C.; Silveira, P.P.; Lourenço, J.; et al. Genomic, Epidemiological and Digital Surveillance of Chikungunya Virus in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2019, 13, e0007065. [Google Scholar] [CrossRef]
- WHO PAHO/WHO Data—Weekly Report. Available online: https://www3.paho.org/data/index.php/en/mnu-topics/chikv-en/550-chikv-weekly-en.html (accessed on 23 August 2023).
- Vu-Qui-Dai; Nguyen-Thi Kim-Thoa; Ly-Quoc-Bang [Study of Anti-Chikungunya Antibodies in Vietnamese Children in Saigon]. Bull. Soc. Pathol. Exot. Fil. 1967, 60, 353–359.
- Quyen, N.T.H.; Kien, D.T.H.; Rabaa, M.; Tuan, N.M.; Vi, T.T.; Van Tan, L.; Hung, N.T.; Tuan, H.M.; Tram, T.V.; Da Ha, N.L.; et al. Chikungunya and Zika Virus Cases Detected against a Backdrop of Endemic Dengue Transmission in Vietnam. Am. J. Trop. Med. Hyg. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Pham Thi, K.L.; Briant, L.; Gavotte, L.; Labbe, P.; Perriat-Sanguinet, M.; Cornillot, E.; Vu, T.D.; Nguyen, T.Y.; Tran, V.P.; Nguyen, V.S.; et al. Incidence of Dengue and Chikungunya Viruses in Mosquitoes and Human Patients in Border Provinces of Vietnam. Parasites Vectors 2017, 10, 556. [Google Scholar] [CrossRef]
- Kim Lien, P.T.; Briant, L.; Tang, T.B.; Trang, B.M.; Gavotte, L.; Cornillot, E.; Duoc, V.T.; Duong, T.N.; Frutos, R.; Nga, P.T. Surveillance of Dengue and Chikungunya Infection in Dong Thap, Vietnam: A 13-Month Study. Asian Pac. J. Trop. Med. 2016, 9, 39–43. [Google Scholar] [CrossRef]
- Neupane, B.; Pandey, K.; Pandey, B.D.; Morita, K.; Tun, M.M.N. Detection of Chikungunya Virus in Nepal. Am. J. Trop. Med. Hyg. 2015, 93, 697–700. [Google Scholar] [CrossRef]
- Ngwe Tun, M.M.; Kyaw, A.K.; Nabeshima, T.; Dumre, S.P.; Soe, A.M.; Nwe, K.M.; Myaing, S.S.; Lwin, E.P.; Win, Y.T.; Inoue, S.; et al. Coinfection and Circulation of Chikungunya Virus and Dengue Virus in Pediatric Patients in Myanmar, 2019. Microbes Infect. 2023, 25, 105129. [Google Scholar] [CrossRef] [PubMed]
- Tun, M.M.N.; Inoue, S.; Thant, K.Z.; Talemaitoga, N.; Aryati, A.; Dimaano, E.M.; Matias, R.R.; Buerano, C.C.; Natividad, F.F.; Abeyewickreme, W.; et al. Retrospective Seroepidemiological Study of Chikungunya Infection in South Asia, Southeast Asia and the Pacific Region. Epidemiol. Infect. 2016, 144, 2268–2275. [Google Scholar] [CrossRef]
- Luvai, E.A.C.; Kyaw, A.K.; Sabin, N.S.; Yu, F.; Hmone, S.W.; Thant, K.Z.; Inoue, S.; Morita, K.; Tun, M.M.N. Evidence of Chikungunya Virus Seroprevalence in Myanmar among Denguesuspected Patients and Healthy Volunteers in 2013, 2015, and 2018. PLoS Negl. Trop. Dis. 2021, 15, e0009961. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Pandey, B.D.; Chaurasiya, R.R.; Thakur, M.; Neupane, B.; Shah, Y.; Tun, M.M.N.; Morita, K. Evidence of Chikungunya Virus Circulation in the Terai Region of Nepal in 2014 and 2015. Trans. R. Soc. Trop. Med. Hyg. 2017, 111, 294–299. [Google Scholar] [CrossRef]
- Kyaw, A.K.; Ngwe Tun, M.M.; Nabeshima, T.; Soe, A.M.; Mon, K.M.; Thida; Aung, T. H.; Htwe, T.T.; Myaing, S.S.; Mar, T.T.; et al. Chikungunya Virus Infection in Blood Donors and Patients during Outbreak, Mandalay, Myanmar, 2019. Emerg. Infect. Dis. 2020, 26, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Ngwe Tun, M.M.; Thant, K.Z.; Inoue, S.; Nabeshima, T.; Aoki, K.; Kyaw, A.K.; Myint, T.; Tar, T.; Maung, K.T.T.; Hayasaka, D.; et al. Detection of East/Central/South African Genotype of Chikungunya Virus in Myanmar, 2010. CDC. Emerg. Infect. Dis. 2014, 20, 1378–1381. [Google Scholar] [CrossRef]
- Hasebe, F.; Parquet, M.C.; Pandey, B.D.; Mathenge, E.G.M.; Morita, K.; Balasubramaniam, V.; Saat, Z.; Yusop, A.; Sinniah, M.; Natkunam, S.; et al. Combined Detection and Genotyping of Chikungunya Virus by a Specific Reverse Transcription-Polymerase Chain Reaction. J. Med. Virol. 2002, 67, 370–374. [Google Scholar] [CrossRef]
- Imad, H.A.; Phadungsombat, J.; Nakayama, E.E.; Kludkleeb, S.; Matsee, W.; Ponam, T.; Suzuki, K.; Leaungwutiwong, P.; Piyaphanee, W.; Phumratanaprapin, W.; et al. Chikungunya Manifestations and Viremia in Patients Who Presented to the Fever Clinic at Bangkok Hospital for Tropical Diseases during the 2019 Outbreak in Thailand. Trop. Med. Infect. Dis. 2021, 6, 12. [Google Scholar] [CrossRef]
- Ho, P.S.; Ng, M.M.L.; Chu, J.J.H. Establishment of One-Step SYBR Green-Based Real Time-PCR Assay for Rapid Detection and Quantification of Chikungunya Virus Infection. Virol. J. 2010, 7, 13. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Pasteur Institute in Ho Chi Minh City Vietnam Dengue National Program, Annual Data Report; Pasteur Institute in Ho Chi Minh city: Ho Chi Minh city, Vietnam, unpublished.
- WHO World Mosquito Program. Available online: https://www.worldmosquitoprogram.org/en/global-progress/vietnam (accessed on 17 August 2023).
- WHO Dengue in Viet Nam. Available online: https://www.who.int/vietnam/health-topics/dengue (accessed on 17 August 2023).
- Taurel, A.F.; Luong, C.Q.; Nguyen, T.T.T.; Do, K.Q.; Diep, T.H.; Nguyen, T.V.; Cao, M.T.; Hoang, T.N.D.; Huynh, P.T.; Huynh, T.K.L.; et al. Age Distribution of Dengue Cases in Southern Vietnam from 2000 to 2015. PLoS Negl. Trop. Dis. 2023, 17, e0011137. [Google Scholar] [CrossRef]
- National Center for Vector Borne Diseases Control (NCVBDC). CHIKUNGUNYA SITUATION IN INDIA. Available online: https://ncvbdc.mohfw.gov.in/index4.php?lang=1&level=0&linkid=486&lid=3765 (accessed on 12 August 2023).
- Kabir, I.; Dhimal, M.; Müller, R.; Banik, S.; Haque, U. The 2017 Dhaka Chikungunya Outbreak. Lancet Infect. Dis. 2017, 17, 1118. [Google Scholar] [CrossRef] [PubMed]
- Capeding, M.R.; Chua, M.N.; Hadinegoro, S.R.; Hussain, I.I.H.M.; Nallusamy, R.; Pitisuttithum, P.; Rusmil, K.; Thisyakorn, U.; Thomas, S.J.; Huu Tran, N.; et al. Dengue and Other Common Causes of Acute Febrile Illness in Asia: An Active Surveillance Study in Children. PLoS Negl. Trop. Dis. 2013, 7, e2331. [Google Scholar] [CrossRef]
- Foeller, M.E.; Nosrat, C.; Krystosik, A.; Noel, T.; Gérardin, P.; Cudjoe, N.; Mapp-Alexander, V.; Mitchell, G.; Macpherson, C.; Waechter, R.; et al. Chikungunya Infection in Pregnancy—Reassuring Maternal and Perinatal Outcomes: A Retrospective Observational Study. BJOG 2021, 128, 1077. [Google Scholar] [CrossRef]
- Watanaveeradej, V.; Endy, T.P.; Simasathien, S.; Kerdpanich, A.; Polprasert, N.; Aree, C.; Vaughn, D.W.; Nisalak, A. Transplacental Chikungunya Virus Antibody Kinetics, Thailand. Emerg. Infect. Dis. 2006, 12, 1770. [Google Scholar] [CrossRef]
- Weger-Lucarelli, J.; Carrau, L.; Levi, L.I.; Rezelj, V.; Vallet, T.; Blanc, H.; Boussier, J.; Megrian, D.; Coutermarsh-Ott, S.; LeRoith, T.; et al. Host Nutritional Status Affects Alphavirus Virulence, Transmission, and Evolution. PLoS Pathog. 2019, 15, e1008089. [Google Scholar] [CrossRef] [PubMed]
- Jean-Baptiste, E.; Von Oettingen, J.; Larco, P.; Raphaël, F.; Larco, N.C.; Cauvin, M.M.; Charles, R. Chikungunya Virus Infection and Diabetes Mellitus: A Double Negative Impact. Am. J. Trop. Med. Hyg. 2016, 95, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Roques, P.; Thiberville, S.D.; Dupuis-Maguiraga, L.; Lum, F.M.; Labadie, K.; Martinon, F.; Gras, G.; Lebon, P.; Ng, L.F.P.; de Lamballerie, X.; et al. Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection. Viruses 2018, 10, 268. [Google Scholar] [CrossRef]
- Rueda, J.C.; Arcos-Burgos, M.; Santos, A.M.; Martin-Arsanios, D.; Villota-Erazo, C.; Reyes, V.; Bernal-Macías, S.; Peláez-Ballestas, I.; Cardiel, M.H.; Londono, J. Human Genetic Host Factors and Its Role in the Pathogenesis of Chikungunya Virus Infection. Front. Med. 2022, 9, 654395. [Google Scholar] [CrossRef]
- Srivastava, P.; Kumar, A.; Hasan, A.; Mehta, D.; Kumar, R.; Sharma, C.; Sunil, S. Disease Resolution in Chikungunya—What Decides the Outcome? Front. Immunol. 2020, 11, 488753. [Google Scholar] [CrossRef]
- Do Nascimento Costa, D.M.; Coêlho, M.R.C.D.; Da Cruz Gouveia, P.A.; Bezerra, L.A.; Marques, C.D.L.; Duarte, A.L.B.P.; Valente, L.M.; Da Silveira, V.M. Long-Term Persistence of Serum-Specific Anti-Chikungunya IgM Antibody—A Case Series of Brazilian Patients. Rev. Soc. Bras. Med. Trop. 2021, 54, e0855. [Google Scholar] [CrossRef]
- Chelluboina, S.; Robin, S.; Aswathyraj, S.; Arunkumar, G. Persistence of Antibody Response in Chikungunya. VirusDisease 2019, 30, 469. [Google Scholar] [CrossRef]
- Oliver, M.; Grandadam, M.; Marimoutou, C.; Rogier, C.; Botelho-Nevers, E.; Tolou, H.; Moalic, J.L.; Kraemer, P.; Morillons, M.; Morand, J.J.; et al. Persisting Mixed Cryoglobulinemia in Chikungunya Infection. PLoS Negl. Trop. Dis. 2009, 3, e374. [Google Scholar] [CrossRef] [PubMed]
- Borgherini, G.; Poubeau, P.; Jossaume, A.; Gouix, A.; Cotte, L.; Michault, A.; Arvin-Berod, C.; Paganin, F. Persistent Arthralgia Associated with Chikungunya Virus: A Study of 88 Adult Patients on Reunion Island. Clin. Infect. Dis. 2008, 47, 469–475. [Google Scholar] [CrossRef]
- Chua, C.L.; Sam, I.C.; Chiam, C.W.; Chan, Y.F. The Neutralizing Role of IgM during Early Chikungunya Virus Infection. PLoS ONE 2017, 12, e0171989. [Google Scholar] [CrossRef]
- Fortuna, C.; Marsili, G.; Nowotny, N.; Myat Ngwe Tun, M.; Kyaw Kyaw, A.; Mya Nwe, K.; Su Myaing, S.; Thu Win, Y.; Inoue, S.; Takamatsu, Y.; et al. Burden of Chikungunya Virus Infection during an Outbreak in Myanmar. Viruses 2023, 15, 1734. [Google Scholar] [CrossRef]
- Barr, K.L.; Khan, E.; Farooqi, J.Q.; Imtiaz, K.; Prakoso, D.; Malik, F.; Lednicky, J.A.; Long, M.T. Evidence of Chikungunya Virus Disease in Pakistan since 2015 with Patients Demonstrating Involvement of the Central Nervous System. Front. Public Health 2018, 6, e130509. [Google Scholar] [CrossRef]
- Nayak, K.; Jain, V.; Kaur, M.; Khan, N.; Gottimukkala, K.; Aggarwal, C.; Sagar, R.; Gupta, S.; Rai, R.C.; Dixit, K.; et al. Antibody Response Patterns in Chikungunya Febrile Phase Predict Protection versus Progression to Chronic Arthritis. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Win, M.K.; Chow, A.; Dimatatac, F.; Go, C.J.; Leo, Y.S. Chikungunya Fever in Singapore: Acute Clinical and Laboratory Features, and Factors Associated with Persistent Arthralgia. J. Clin. Virol. 2010, 49, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Laurent, P.; Le Roux, K.; Grivard, P.; Bertil, G.; Naze, F.; Picard, M.; Staikowsky, F.; Barau, G.; Schuffenecker, I.; Michault, A. Development of a Sensitive Real-Time Reverse Transcriptase PCR Assay with an Internal Control to Detect and Quantify Chikungunya Virus. Clin. Chem. 2007, 53, 1408–1414. [Google Scholar] [CrossRef]
- Appassakij, H.; Khuntikij, P.; Kemapunmanus, M.; Wutthanarungsan, R.; Silpapojakul, K. Viremic Profiles in Asymptomatic and Symptomatic Chikungunya Fever: A Blood Transfusion Threat? Transfusion 2013, 53, 2567–2574. [Google Scholar] [CrossRef] [PubMed]
- Riswari, S.F.; Ma’roef, C.N.; Djauhari, H.; Kosasih, H.; Perkasa, A.; Yudhaputri, F.A.; Artika, I.M.; Williams, M.; van der Ven, A.; Myint, K.S.; et al. Study of Viremic Profile in Febrile Specimens of Chikungunya in Bandung, Indonesia. J. Clin. Virol. 2016, 74, 61–65. [Google Scholar] [CrossRef]
- Prince, H.E.; Seaton, B.L.; Matud, J.L.; Batterman, H.J. Chikungunya Virus RNA and Antibody Testing at a National Reference Laboratory since the Emergence of Chikungunya Virus in the Americas. Clin. Vaccine Immunol. 2015, 22, 291–297. [Google Scholar] [CrossRef] [PubMed]
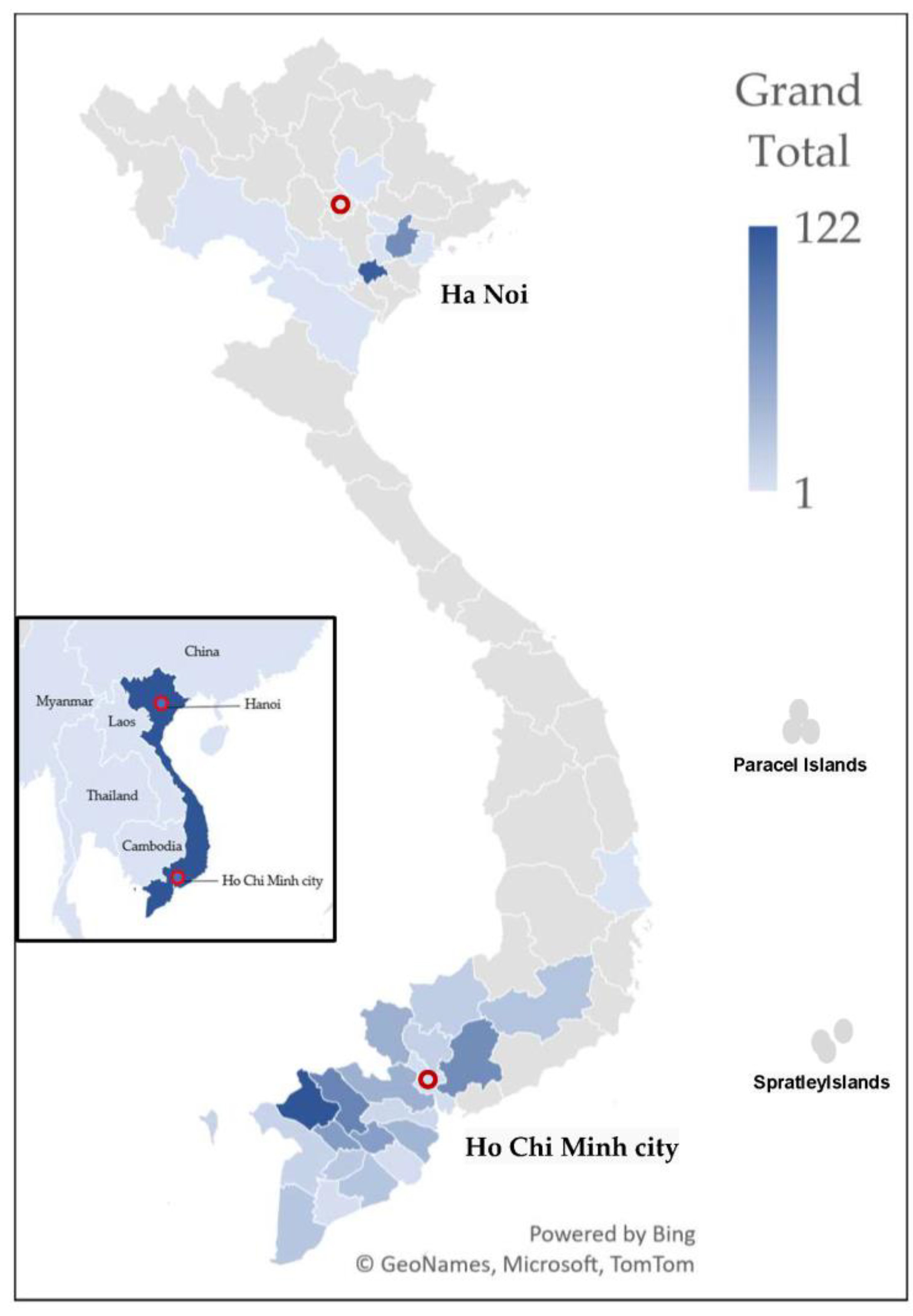







| Variables | Overall (n, % +) | Female (n, % ++) | Male (n, % ++) |
|---|---|---|---|
| Year of illness | |||
| 2017 | 368 (34.6) | 157 (42.7) | 211 (57.3) |
| 2018 | 326 (30.7) | 143 (43.9) | 183 (56.1) |
| 2019 | 369 (34.7) | 170 (46.1) | 199 (53.9) |
| Regions | |||
| North | 283 (26.6) | 117 (41.3) | 166 (58.7) |
| South | 780 (73.4) | 353 (45.3) | 427 (54.7) |
| Age groups * | |||
| ≤5 | 125 (11.8) | 46 (36.8) | 79 (63.2) |
| 6–15 | 392 (36.9) | 171 (43.6) | 221 (56.4) |
| 16–45 | 370 (34.8) | 168 (45.4) | 202 (54.6) |
| ≥46 | 176 (16.6) | 85 (48.3) | 91 (51.7) |
| Clinical diagnoses ** | |||
| Non-specific febrile illness | 361 (34) | 147 (40.7) | 214 (59.3) |
| Suspected Dengue | 58 (5.5) | 23 (39.7) | 35 (60.3) |
| Dengue without warning sign | 604 (56.8) | 280 (46.4) | 324 (53.6) |
| Dengue with warning sign | 26 (2.4) | 10 (38.5) | 16 (61.5) |
| Severe Dengue | 14 (1.3) | 10 (71.4) | 4 (28.6) |
| Overall | 1063 | 470 (44.2) | 593 (55.8) |
| Variable | n = 1063 (%) + | IgM (+) n, (%) ++, (1) | IgG (+) n, (%) ++, (2) | IgM (+) and IgG (+) n, (%) ++, (3) | IgM (+) and/or IgG (+) n, (%) ++, (4) | p-Value (χ² or Fisher Test) |
|---|---|---|---|---|---|---|
| Year of illness | ||||||
| 2017 | 368 (34.6) | 44 (12) | 87 (23.6) | 12 (3.3) | 119 (32.3) | (1) 0.006 (2) 0.022 (3) 0.742 (4) 0.994 |
| 2018 | 326 (30.7) | 68 (20.9) | 50 (15.3) | 13 (4) | 105 (32.2) | |
| 2019 | 369 (34.7) | 57 (15.4) | 77 (20.9) | 16 (4.3) | 118 (32) | |
| Regions | ||||||
| North | 283 (26.6) | 29 (10.2) | 41 (14.5) | 8 (2.8) | 62 (21.9) | (1) 0.002 (2) 0.006 (3) 0.293 (4) <0.0001 |
| South | 780 (73.4) | 140 (17.9) | 173 (22.2) | 33 (4.2) | 280 (35.9) | |
| Genders | ||||||
| Female | 470 (44.2) | 66 (14) | 104 (22.1) | 21 (4.5) | 149 (31.7) | (1) 0.141 (2) 0.149 (3) 0.357 (4) 0.770 |
| Male | 593 (55.8) | 103 (17.4) | 110 (18.5) | 20 (3.4) | 193 (32.5) | |
| Age groups * | ||||||
| ≤5 | 136 (12.8) | 24 (17.6) | 62 (45.6) | 12 (8.8) | 74 (54.4) | (1) 0.012 (2) <0.0001 (3) 0.009 (4) <0.0001 |
| 6–15 | 423 (39.8) | 83 (19.6) | 60 (14.2) | 12 (2.8) | 131 (31) | |
| 16–45 | 382 (35.9) | 51 (13.4) | 48 (12.6) | 11 (2.9) | 88 (23) | |
| ≥46 | 122 (11.5) | 11 (9) | 44 (36.1) | 6 (4.9) | 49 (40.2) | |
| Clinical diagnoses ** | ||||||
| Non-specific febrile illness | 361 (34) | 49 (13.6) | 70 (19.4) | 14 (3.9) | 105 (29.1) | (1) 0.076 (2) 0.664 (3) 0.563 (4) 0.196 |
| Suspected Dengue | 58 (5.5) | 9 (15.5) | 12 (20.7) | 4 (6.9) | 17 (29.3) | |
| Dengue without warning sign | 604 (56.8) | 108 (17.9) | 121 (20) | 22 (3.6) | 207 (34.3) | |
| Dengue with warning sign | 26 (2.4) | (0) | 6 (23.1) | (0) | 6 (23.1) | |
| Severe Dengue | 14 (1.3) | 3 (21.4) | 5 (35.7) | 1 (7.1) | 7 (50) | |
| Overall | 1063 (100) | 169 (15.9) | 214 (20.1) | 41 (3.9) | 342 (32.2) | |
| Variable | Patients n (%) + | FRNT50 n (%) ++ | p Value (χ² or Fisher Test) |
|---|---|---|---|
| Gender | |||
| Female | 470 (44.2) | 54 (11.5) | 0.32 |
| Male | 593 (55.8) | 57 (9.6) | |
| Age groups | |||
| ≤5 | 136 (12.8) | 19 (14) | <0.0001 |
| 6–15 | 423 (39.8) | 34 (8) | |
| 16–45 | 382 (35.9) | 21 (5.5) | |
| ≥46 | 122 (11.5) | 37 (30.3) | |
| Regions | |||
| North | 283 (26.6) | 21 (7.4) | 0.052 |
| South | 780 (73.4) | 90 (11.5) | |
| Year of illness * | |||
| 2017 | 368 (34.6) | 39 (10.6) | 0.991 |
| 2018 | 326 (30.7) | 34 (10.4) | |
| 2019 | 369 (34.7) | 38 (10.3) | |
| Clinical diagnoses ** | |||
| Non-specific febrile illness | 361 (34) | 36 (10) | 0.14 |
| Suspected Dengue | 58 (5.5) | 8 (13.8) | |
| Dengue without warning sign | 604 (56.8) | 62 (10.3) | |
| Dengue with warning sign | 26 (2.4) | 1 (3.8) | |
| Severe Dengue | 14 (1.3) | 4 (28.6) | |
| Overall | 1063 (100) | 111 (10.4) | |
| Variable | Patients n (%) + | RNA Detection Rate n (%) ++ | p Value (χ² or Fisher Test) |
|---|---|---|---|
| Genders | |||
| Female | 209 (44.5) | 52 (24.9) | 0.218 |
| Male | 260 (55.4) | 78 (30.0) | |
| Age groups | |||
| ≤5 | 90 (19.2) | 20 (22.2) | 0.434 |
| 6–15 | 187 (39.9) | 55 (29.4) | |
| 16–45 | 133 (28.4) | 41 (30.8) | |
| ≥46 | 59 (12.6) | 14 (23.7) | |
| Regions | |||
| North | 66 (14.1) | 13 (19.7) | 0.116 |
| South | 403 (85.9) | 117 (29) | |
| Year of illness * | |||
| 2017 | 149 (31.8) | 42 (28.2) | 0.116 |
| 2018 | 179 (38.2) | 41 (22.9) | |
| 2019 | 141 (44.6) | 47 (33.3) | |
| Clinical diagnoses ** | |||
| Non-specific febrile illness | 131 (27.9) | 33 (25.2) | 0.905 |
| Suspected Dengue | 27 (5.8) | 9 (33.3) | |
| Dengue without warning sign | 292 (62.3) | 82 (28.1) | |
| Dengue with warning sign | 9 (1.9) | 3 (33.3) | |
| Severe Dengue | 10 (2.1) | 3 (30) | |
| Overall | 469 (100) | 130 (27.7) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.V.; Ngwe Tun, M.M.; Cao, M.T.; Dao, H.M.; Luong, C.Q.; Huynh, T.K.L.; Nguyen, T.T.T.; Hoang, T.N.D.; Morita, K.; Le, T.Q.M.; et al. Serological and Molecular Epidemiology of Chikungunya Virus Infection in Vietnam, 2017–2019. Viruses 2023, 15, 2065. https://doi.org/10.3390/v15102065
Nguyen TV, Ngwe Tun MM, Cao MT, Dao HM, Luong CQ, Huynh TKL, Nguyen TTT, Hoang TND, Morita K, Le TQM, et al. Serological and Molecular Epidemiology of Chikungunya Virus Infection in Vietnam, 2017–2019. Viruses. 2023; 15(10):2065. https://doi.org/10.3390/v15102065
Chicago/Turabian StyleNguyen, Thanh Vu, Mya Myat Ngwe Tun, Minh Thang Cao, Huy Manh Dao, Chan Quang Luong, Thi Kim Loan Huynh, Thi Thanh Thuong Nguyen, Thi Nhu Dao Hoang, Kouichi Morita, Thi Quynh Mai Le, and et al. 2023. "Serological and Molecular Epidemiology of Chikungunya Virus Infection in Vietnam, 2017–2019" Viruses 15, no. 10: 2065. https://doi.org/10.3390/v15102065





