Long-Term Follow-Up of COVID-19 Convalescents—Immune Response Associated with Reinfection Rate and Symptoms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Convalescents and Blood Samples
2.2. Follow-Up Assessment of Convalescents
2.3. T-Cell and Antibody Responses
2.4. Software and Statistical Analysis
3. Results
3.1. Clinical Characteristics of COVID-19 Convalescents
3.2. Prevalence of Long-Term Symptoms and Severity
3.3. Vaccinations
3.4. Reinfections
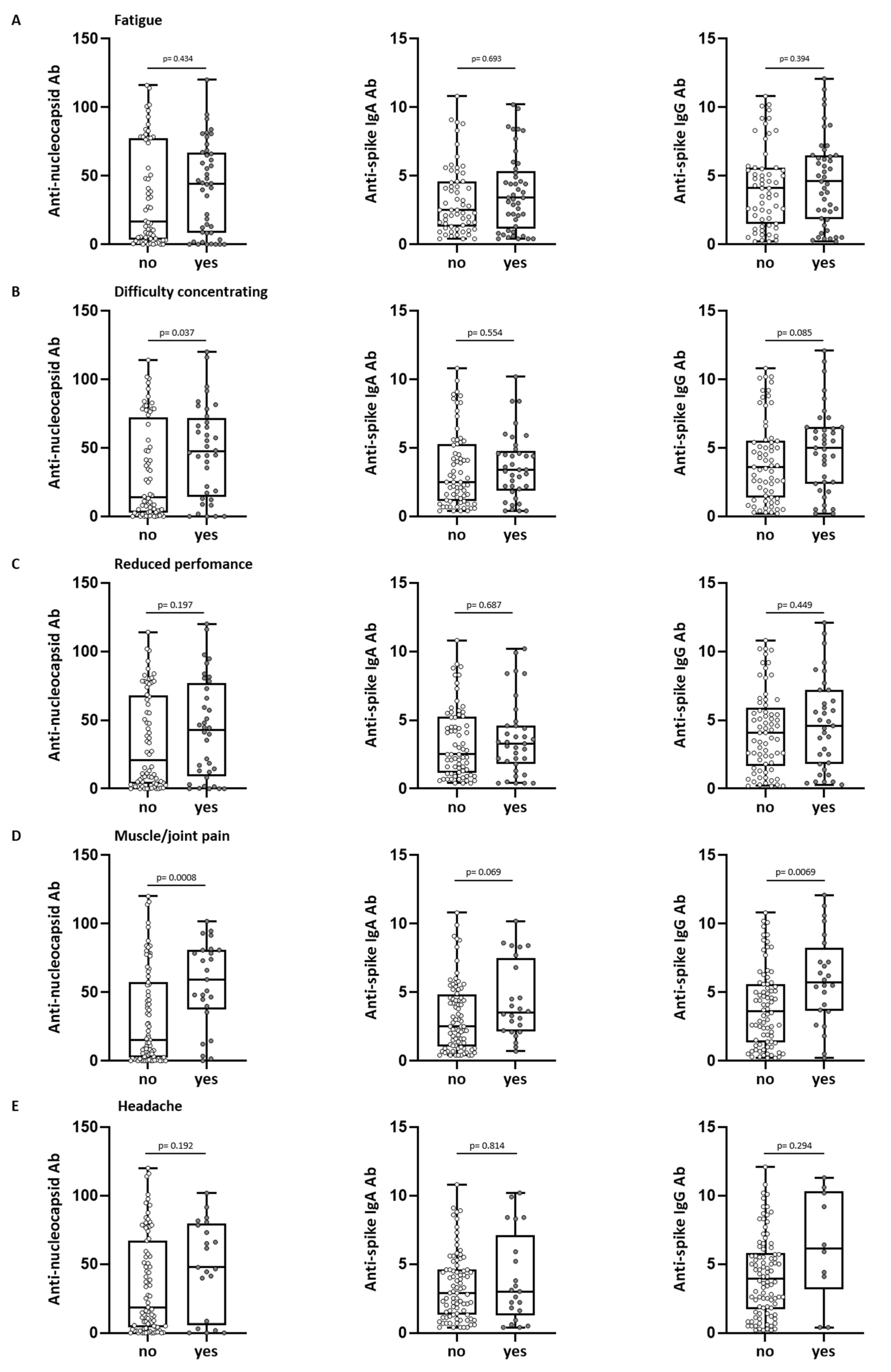
3.5. Antibody Levels and Long-Term COVID-19 Symptoms
3.6. Antibody Levels and Reinfections
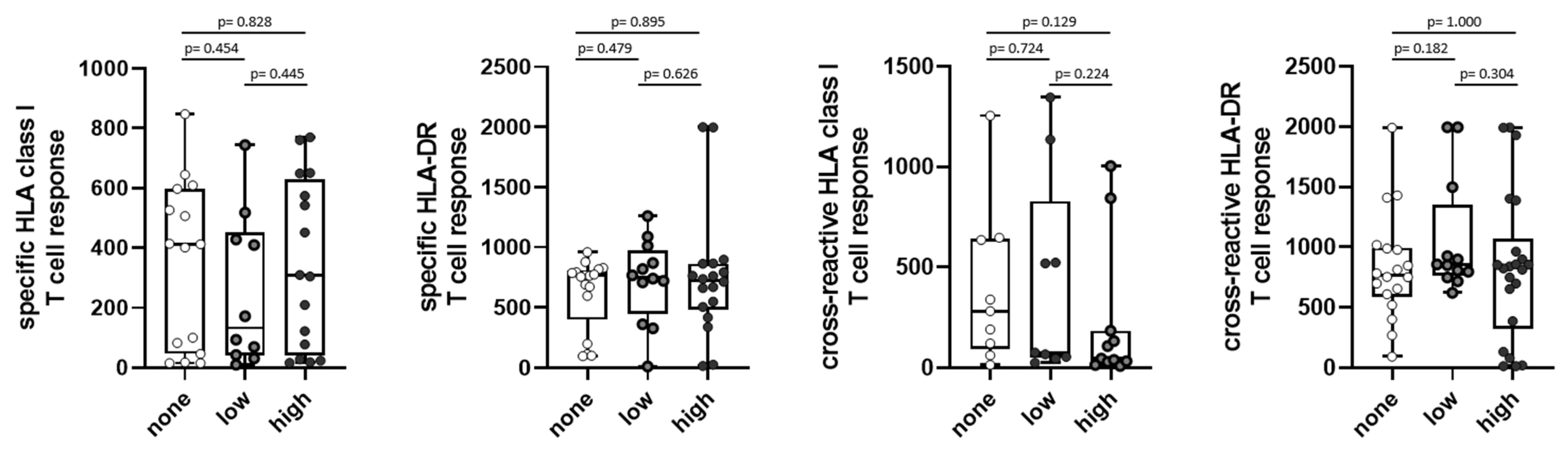
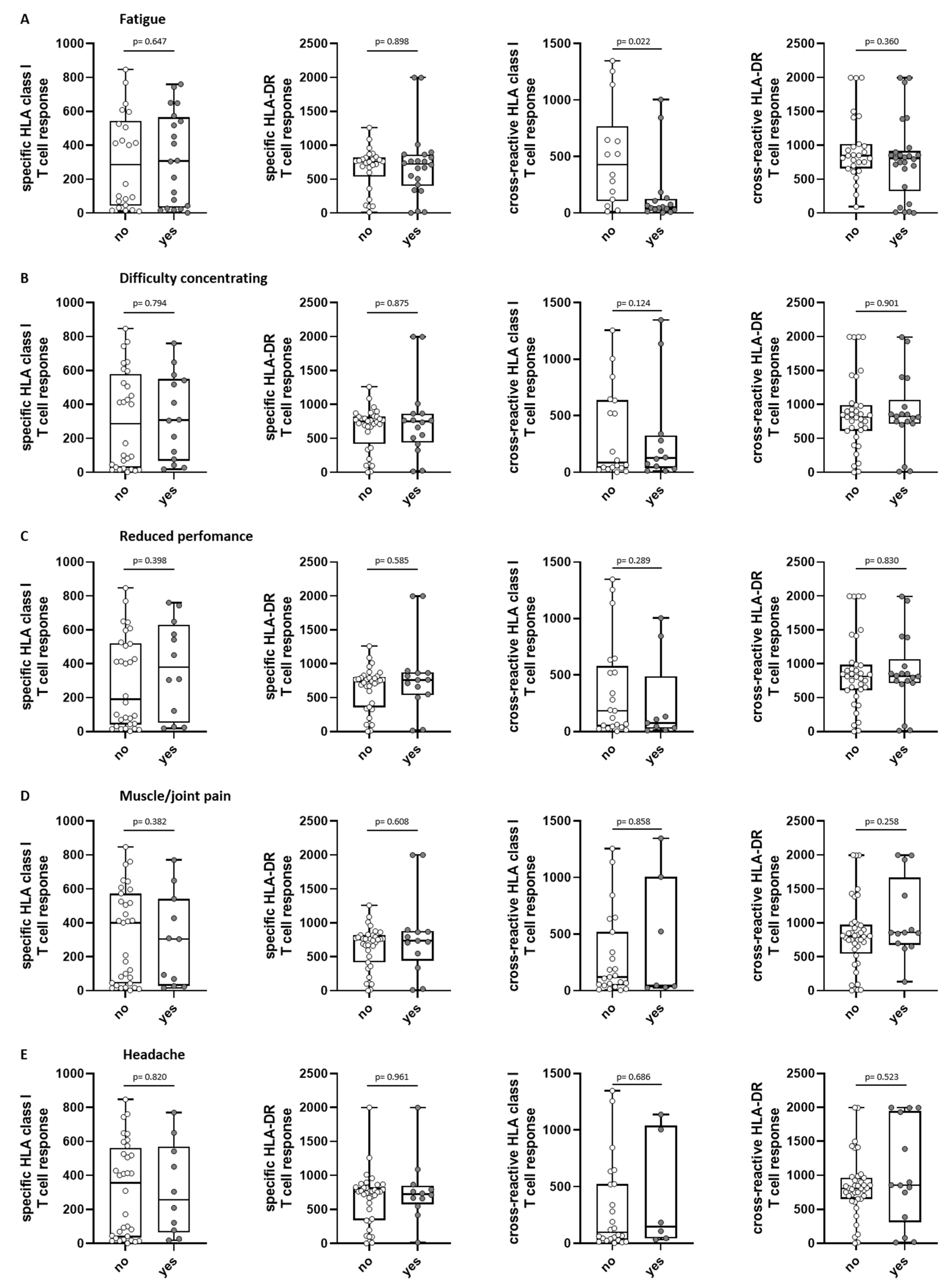
3.7. T-Cell Immunity in Association to Long-Term COVID-19 Symptoms and Further Infections
3.8. Post-COVID Syndrome after 5–6 Months and Long-Term Symptoms at 2.5 Years after First Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 7 March 2023).
- Briggs, A.; Vassall, A. Count the cost of disability caused by COVID-19. Nature 2021, 593, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Huang, T.; Wang, Y.Q.; Wang, Z.P.; Liang, Y.; Huang, T.B.; Zhang, H.Y.; Sun, W.; Wang, Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020, 92, 577–583. [Google Scholar] [CrossRef]
- Arnold, D.T.; Hamilton, F.W.; Milne, A.; Morley, A.J.; Viner, J.; Attwood, M.; Noel, A.; Gunning, S.; Hatrick, J.; Hamilton, S.; et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: Results from a prospective UK cohort. Thorax 2021, 76, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Dennis, A.; Wamil, M.; Alberts, J.; Oben, J.; Cuthbertson, D.J.; Wootton, D.; Crooks, M.; Gabbay, M.; Brady, M.; Hishmeh, L.; et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open 2021, 11, e048391. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Colombo, M.G.; Wetzel, A.J.; Martus, P.; Joos, S. Persisting Symptoms After COVID-19—Prevalence and Risk Factors in a Population-Based Cohort. Dtsch. Ärzteblatt Int. 2022, 119, 167–174. [Google Scholar] [CrossRef]
- Lund, L.C.; Hallas, J.; Nielsen, H.; Koch, A.; Mogensen, S.H.; Brun, N.C.; Christiansen, C.F.; Thomsen, R.W.; Pottegård, A. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: A Danish population-based cohort study. Lancet Infect. Dis. 2021, 21, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Seeßle, J.; Waterboer, T.; Hippchen, T.; Simon, J.; Kirchner, M.; Lim, A.; Müller, B.; Merle, U. Persistent Symptoms in Adult Patients 1 Year After Coronavirus Disease 2019 (COVID-19): A Prospective Cohort Study. Clin. Infect. Dis. 2022, 74, 1191–1198. [Google Scholar] [CrossRef]
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: A population-based cohort study. Thorax 2021, 76, 405–407. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. Jama 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Nakamichi, K.; Shen, J.Z.; Lee, C.S.; Lee, A.; Roberts, E.A.; Simonson, P.D.; Roychoudhury, P.; Andriesen, J.; Randhawa, A.K.; Mathias, P.C.; et al. Hospitalization and mortality associated with SARS-CoV-2 viral clades in COVID-19. Sci. Rep. 2021, 11, 4802. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Liu, F.; Xu, X.; Ling, Y.; Huang, W.; Zhu, Z.; Guo, M.; Lin, Y.; Fu, Z.; Liang, D.; et al. Durability of neutralizing antibodies and T-cell response post SARS-CoV-2 infection. Front. Med. 2020, 14, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Ramonell, R.P.; Nguyen, D.C.; Cashman, K.S.; Saini, A.S.; Haddad, N.S.; Ley, A.M.; Kyu, S.; Howell, J.C.; Ozturk, T.; et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat. Immunol. 2020, 21, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Hackenbruch, C.; Maringer, Y.; Tegeler, C.M.; Walz, J.S.; Nelde, A.; Heitmann, J.S. Elevated SARS-CoV-2-Specific Antibody Levels in Patients with Post-COVID Syndrome. Viruses 2023, 15, 701. [Google Scholar] [CrossRef] [PubMed]
- Tegeler, C.M.; Bilich, T.; Maringer, Y.; Salih, H.R.; Walz, J.S.; Nelde, A.; Heitmann, J.S. Prevalence of COVID-19-associated symptoms during acute infection in relation to SARS-CoV-2-directed humoral and cellular immune responses in a mild-diseased convalescent cohort. Int. J. Infect. Dis. 2022, 120, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lübke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-derived peptides define heterologous and COVID-19-induced T cell recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Son, K.B.; Lee, T.J.; Hwang, S.S. Disease severity classification and COVID-19 outcomes, Republic of Korea. Bull. World Health Organ. 2021, 99, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Bilich, T.; Nelde, A.; Heitmann, J.S.; Maringer, Y.; Roerden, M.; Bauer, J.; Rieth, J.; Wacker, M.; Peter, A.; Hörber, S.; et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci. Transl. Med. 2021, 13, eabf7517. [Google Scholar] [CrossRef]
- Beavis, K.G.; Matushek, S.M.; Abeleda, A.P.F.; Bethel, C.; Hunt, C.; Gillen, S.; Moran, A.; Tesic, V. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. J. Clin. Virol. 2020, 129, 104468. [Google Scholar] [CrossRef]
- Muench, P.; Jochum, S.; Wenderoth, V.; Ofenloch-Haehnle, B.; Hombach, M.; Strobl, M.; Sadlowski, H.; Sachse, C.; Torriani, G.; Eckerle, I.; et al. Development and Validation of the Elecsys Anti-SARS-CoV-2 Immunoassay as a Highly Specific Tool for Determining Past Exposure to SARS-CoV-2. J. Clin. Microbiol. 2020, 58, 10-1128. [Google Scholar] [CrossRef]
- Robert-Koch-Institut. Wöchentlicher Lagebericht des RKI zur Coronavirus-Krankheit-2019 (COVID-19) 27.01.2022. Available online: https://web.archive.org/web/20220127185752/https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Wochenbericht/Wochenbericht_2022-01-27.pdf?__blob=publicationFile (accessed on 4 May 2023).
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y.; Xie, C.; Ma, K.; Shang, K.; Wang, W.; et al. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Ji, P.; Pang, J.; Zhong, Z.; Li, H.; He, C.; Zhang, J.; Zhao, C. Clinical characteristics of 3062 COVID-19 patients: A meta-analysis. J. Med. Virol. 2020, 92, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Horton, D.B.; Barrett, E.S.; Roy, J.; Gennaro, M.L.; Andrews, T.; Greenberg, P.; Bruiners, N.; Datta, P.; Ukey, R.; Velusamy, S.K.; et al. Determinants and dynamics of SARS-CoV-2 infection in a diverse population: 6-month evaluation of a prospective cohort study. J. Infect. Dis. 2021, 224, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- García-Abellán, J.; Padilla, S.; Fernández-González, M.; García, J.A.; Agulló, V.; Andreo, M.; Ruiz, S.; Galiana, A.; Gutiérrez, F.; Masiá, M. Antibody Response to SARS-CoV-2 is Associated with Long-term Clinical Outcome in Patients with COVID-19: A Longitudinal Study. J. Clin. Immunol. 2021, 41, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Marklund, E.; Leach, S.; Axelsson, H.; Nyström, K.; Norder, H.; Bemark, M.; Angeletti, D.; Lundgren, A.; Nilsson, S.; Andersson, L.M.; et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS ONE 2020, 15, e0241104. [Google Scholar] [CrossRef]
- Van Elslande, J.; Oyaert, M.; Ailliet, S.; Van Ranst, M.; Lorent, N.; Vande Weygaerde, Y.; André, E.; Lagrou, K.; Vandendriessche, S.; Vermeersch, P. Longitudinal follow-up of IgG anti-nucleocapsid antibodies in SARS-CoV-2 infected patients up to eight months after infection. J. Clin. Virol. 2021, 136, 104765. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Martín-Guerrero, J.D.; Pellicer-Valero, Ó.J.; Navarro-Pardo, E.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Arias-Navalón, J.A.; Cigarán-Méndez, M.; Hernández-Barrera, V.; Arendt-Nielsen, L. Female Sex Is a Risk Factor Associated with Long-Term Post-COVID Related-Symptoms but Not with COVID-19 Symptoms: The LONG-COVID-EXP-CM Multicenter Study. J. Clin. Med. 2022, 11, 413. [Google Scholar] [CrossRef]
- Mariani, C.; Borgonovo, F.; Capetti, A.F.; Oreni, L.; Cossu, M.V.; Pellicciotta, M.; Armiento, L.; Bocchio, S.; Dedivitiis, G.; Lupo, A.; et al. Persistence of Long-COVID symptoms in a heterogenous prospective cohort. J. Infect. 2022, 84, 722–746. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef] [PubMed]
- Frithiof, R.; Rostami, E.; Kumlien, E.; Virhammar, J.; Fällmar, D.; Hultström, M.; Lipcsey, M.; Ashton, N.; Blennow, K.; Zetterberg, H.; et al. Critical illness polyneuropathy, myopathy and neuronal biomarkers in COVID-19 patients: A prospective study. Clin. Neurophysiol. 2021, 132, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Barkhuizen, A.; Rosen, H.R.; Wolf, S.; Flora, K.; Benner, K.; Bennett, R.M. Musculoskeletal pain and fatigue are associated with chronic hepatitis C: A report of 239 hepatology clinic patients. Am. J. Gastroenterol. 1999, 94, 1355–1360. [Google Scholar] [CrossRef]
- Javelle, E.; Ribera, A.; Degasne, I.; Gaüzère, B.A.; Marimoutou, C.; Simon, F. Specific management of post-chikungunya rheumatic disorders: A retrospective study of 159 cases in Reunion Island from 2006–2012. PLoS Negl. Trop. Dis. 2015, 9, e0003603. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Vandy, A.O.; Stretch, R.; Otieno, D.; Prajapati, M.; Calderon, M.; Vandi, M. Sequelae and Other Conditions in Ebola Virus Disease Survivors, Sierra Leone, 2015. Emerg. Infect. Dis. 2017, 23, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, J.S.; Bae, S.; Cha, H.H.; Lim, J.S.; Kim, M.C.; Chung, J.W.; Park, S.Y.; Lee, M.J.; Kim, B.N.; et al. SARS-CoV-2-Specific Antibody and T Cell Response Kinetics According to Symptom Severity. Am. J. Trop. Med. Hyg. 2021, 105, 395–400. [Google Scholar] [CrossRef]
- Toor, S.M.; Saleh, R.; Sasidharan Nair, V.; Taha, R.Z.; Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 2021, 162, 30–43. [Google Scholar] [CrossRef]
- Cohen, C.; Kleynhans, J.; von Gottberg, A.; McMorrow, M.L.; Wolter, N.; Bhiman, J.N.; Moyes, J.; du Plessis, M.; Carrim, M.; Buys, A.; et al. SARS-CoV-2 incidence, transmission and reinfection in a rural and an urban setting: Results of the PHIRST-C cohort study, South Africa, 2020–2021. medRxiv 2021. [Google Scholar] [CrossRef]
- Guedes, A.R.; Oliveira, M.S.; Tavares, B.M.; Luna-Muschi, A.; Lazari, C.d.S.; Montal, A.C.; de Faria, E.; Maia, F.L.; Barboza, A.d.S.; Leme, M.D.; et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci. Rep. 2023, 13, 712. [Google Scholar] [CrossRef]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M.; Mølbak, K.; Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Cao, S.; Lee, A.S.; Manohar, M.; Sindher, S.B.; Ahuja, N.; Artandi, M.; Blish, C.A.; Blomkalns, A.L.; Chang, I.; et al. Anti-nucleocapsid antibody levels and pulmonary comorbid conditions are linked to post-COVID-19 syndrome. JCI Insight 2022, 7, e156713. [Google Scholar] [CrossRef] [PubMed]
- Atti, A.; Insalata, F.; Carr, E.J.; Otter, A.D.; Castillo-Olivares, J.; Wu, M.; Harvey, R.; Howell, M.; Chan, A.; Lyall, J.; et al. Antibody correlates of protection from SARS-CoV-2 reinfection prior to vaccination: A nested case-control within the SIREN study. J. Infect. 2022, 85, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A., 3rd; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test With Risk of Future Infection. JAMA Intern. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Islamoglu, M.S.; Cengiz, M.; Uysal, B.B.; Ikitimur, H.; Ozdemir, Z.; Karamehmetoglu, A.; Akbulut, A.E.; Bakdur, A.N.; Ozdemir, A.; Kayıkcıoglu, H.; et al. Relationship between Antibody Levels and SARS-CoV-2 Reinfection. Ann. Clin. Lab. Sci. 2021, 51, 750–755. [Google Scholar]
- Team, C.-F. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef]
- Mentzer, D.; Keller-stanislawski, B. Verdachtsfälle von Nebenwirkungen oder Impfkomplikationen nach Impfung mit den Omikron-Adaptierten Bivalenten COVID-19-Impfstoffen Comirnaty Original/Omicron BA.1, Comirnaty Original/Omicron BA.4-5, Spikevax Bivalent/Omicron BA.1 (bis 31.10.2022 in Deutschland Gemeldet), in PEI Sicherheitsbericht; Paul Ehrlich Institut, Bundesinstitut für Arzneimittel und Medizinprodukte: Langen, Germany, 2022. [Google Scholar]
- Hosseini, R.; Askari, N. A review of neurological side effects of COVID-19 vaccination. Eur. J. Med. Res. 2023, 28, 102. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef]
- Finsterer, J. A Case Report: Long Post-COVID Vaccination Syndrome During the Eleven Months After the Third Moderna Dose. Cureus 2022, 14, e32433. [Google Scholar] [CrossRef]
- Maddox, G.L.; Douglass, E.B. Self-assessment of health: A longitudinal study of elderly subjects. J. Health Soc. Behav. 1973, 14, 87–93. [Google Scholar] [CrossRef]
- Ward, M.; Gruppen, L.; Regehr, G. Measuring self-assessment: Current state of the art. Adv. Health Sci. Educ. Theory Pract. 2002, 7, 63–80. [Google Scholar] [CrossRef]


| Presence of symptoms 2.5 years after first infection [n (%)] | |||||
| 0 symptoms regardless of the severity | 38 (35) | ||||
| ≥1 symptom regardless of the severity | 72 (65) | ||||
| Maximum severity achieved in patients for any of assessed symptoms [n (%)] | |||||
| None | 38 (35) | ||||
| Mild | 28 (25) | ||||
| Noderate | 27 (25) | ||||
| Severe | 17 (15) | ||||
| Symptom | n | Patients with symptoms [n (%)] | Severity [n (%)] | ||
| Mild | Moderate | Severe | |||
| Breathlessness | 110 | 33 (30) | 17 (15) | 16 (15) | 0 (0) |
| Fatigue | 110 | 46 (42) | 27 (25) | 9 (8) | 10 (9) |
| Difficulty concentrating | 110 | 37 (34) | 19 (17) | 13 (12) | 5 (5) |
| Reduced performance | 110 | 37 (34) | 18 (16) | 16 (15) | 3 (3) |
| Sleep disorders | 110 | 31 (28) | 19 (17) | 7 (6) | 5 (5) |
| Depression | 110 | 24 (22) | 13 (12) | 10 (9) | 1 (1) |
| Loss of taste/smell | 110 | 14 (13) | 9 (8) | 2 (2) | 3 (3) |
| Muscle/joint pain | 110 | 25 (23) | 14 (13) | 6 (5) | 5 (5) |
| Hearing loss/disorder | 110 | 12 (11) | 9 (8) | 1 (1) | 2 (2) |
| Headache | 110 | 22 (20) | 14 (13) | 7 (6) | 1 (1) |
| Menstruation disorders | 55 | 1 (2) | 0 (0) | 0 (0) | 1 (2) |
| Number of vaccinations after first infection (n [%]) | ||||||
| 0 vaccinations | 4 (4) | |||||
| 1 vaccination | 7 (6) | |||||
| 2 vaccinations | 47 (43) | |||||
| 3 vaccinations | 45 (41) | |||||
| 4 vaccinations | 7 (6) | |||||
| Long-term symptoms after vaccination (n [%]) | ||||||
| Self-assessed presence of symptoms | 3 (3) | |||||
| Distribution of used vaccines (n [%]) | ||||||
| Tozinameran [n (%)] | Elasomeran [n (%)] | AZD1222 [n (%)] | Ad26.COV2.S [n (%)] | Other [n (%)] | n | |
| First vaccine | 65 (61) | 16 (15) | 20 (19) | 4 (4) | 1 (1) | 106 |
| Second vaccine | 73 (74) | 22 (22) | 3 (3) | 0 (0) | 1 (1) | 99 |
| Third vaccine | 35 (67) | 15 (29) | 1 (1) | 0 (0) | 1 (1) | 52 |
| Fourth vaccine | 5 (71) | 2 (29) | 0 (0) | 0 (0) | 0 (0) | 7 |
| Sum of used vaccines | 178 (67) | 55 (21) | 24 (9) | 4 (2) | 3 (1) | ntotal = 264 |
| Reinfection Rate after First Infection [n (%)] | |
| No reinfection | 59 (53) |
| ≥2 infections | 51 (47) |
| 3 infections | 4 (4) |
| Score for Long-Term Symptoms at 2.5 Years | No Post-COVID Syndrome at 5–6 Months | Post-COVID Syndrome at 5–6 Months | p-Value |
| None (0 symptoms) [n (%)] | 6 (75) | 2 (25) | 0.7189 |
| Low (1–2 symptoms) [n (%)] | 9 (82) | 2 (18) | |
| None (0 symptoms) [n (%)] | 6 (75) | 2 (25) | 0.0487 |
| High (3–10 symptoms) [n (%)] | 4 (31) | 9 (69) | |
| Low (1–2 symptoms) [n (%)] | 9 (82) | 2 (18) | 0.0124 |
| High (3–10 symptoms) [n (%)] | 4 (31) | 9 (69) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seller, A.; Hackenbruch, C.; Walz, J.S.; Nelde, A.; Heitmann, J.S. Long-Term Follow-Up of COVID-19 Convalescents—Immune Response Associated with Reinfection Rate and Symptoms. Viruses 2023, 15, 2100. https://doi.org/10.3390/v15102100
Seller A, Hackenbruch C, Walz JS, Nelde A, Heitmann JS. Long-Term Follow-Up of COVID-19 Convalescents—Immune Response Associated with Reinfection Rate and Symptoms. Viruses. 2023; 15(10):2100. https://doi.org/10.3390/v15102100
Chicago/Turabian StyleSeller, Anna, Christopher Hackenbruch, Juliane S. Walz, Annika Nelde, and Jonas S. Heitmann. 2023. "Long-Term Follow-Up of COVID-19 Convalescents—Immune Response Associated with Reinfection Rate and Symptoms" Viruses 15, no. 10: 2100. https://doi.org/10.3390/v15102100





