The Epidemiology of Chickenpox in England, 2016–2022: An Observational Study Using General Practitioner Consultations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Case Definition
2.3. Data Sources
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bernal, J.L.; Hobbelen, P.; Amirthalingam, G. Burden of varicella complications in secondary care, England, 2004 to 2017. Eurosurveillance 2019, 24, 1900233. [Google Scholar] [CrossRef] [PubMed]
- WHO. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly. Epidemiol. Rec. 2014, 89, 265–287. [Google Scholar]
- Hobbelen, P.H.; Stowe, J.; Amirthalingam, G.; Miller, L.; van Hoek, A.J. The burden of hospitalisation for varicella and herpes zoster in England from 2004 to 2013. J. Infect. 2016, 73, 241–253. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. Varicella: The Green Book, Chapter 34. Available online: https://www.gov.uk/government/publications/varicella-the-green-book-chapter-34 (accessed on 13 July 2023).
- Lee, Y.H.; Choe, Y.J.; Lee, J.; Kim, E.; Lee, J.Y.; Hong, K.; Yoon, Y.; Kim, Y.K. Global varicella vaccination programs. Clin. Exp. Pediatr. 2022, 65, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, G.; Ramsay, M. Should the UK introduce a universal childhood varicella vaccination programme? Arch. Dis. Child. 2016, 101, 2–3. [Google Scholar] [CrossRef]
- Akpo, E.I.H.; Cristeau, O.; Hunjan, M.; Casabona, G. Epidemiological impact and cost-effectiveness of varicella vaccination strategies in the United Kingdom. Clin. Infect. Dis. 2021, 73, e3617–e3626. [Google Scholar] [CrossRef]
- UK Health Security Agency. Chickenpox: Public Health Management and Guidance—The Diagnosis, Management and Epidemiology of Chickenpox (Varicella). Available online: https://www.gov.uk/government/collections/chickenpox-public-health-management-and-guidance (accessed on 13 July 2023).
- Bardsley, M.; Morbey, R.A.; Hughes, H.E.; Beck, C.R.; Watson, C.H.; Zhao, H.; Ellis, J.; Smith, G.E.; Elliot, A.J. Epidemiology of respiratory syncytial virus in children younger than 5 years in England during the COVID-19 pandemic, measured by laboratory, clinical, and syndromic surveillance: A retrospective observational study. Lancet Infect. Dis. 2023, 23, 56–66. [Google Scholar] [CrossRef]
- Nash, K.; Lai, J.; Sandhu, K.; Chandan, J.S.; Shantikumar, S.; Ogunlayi, F.; Coleman, P.C. Impact of national COVID-19 restrictions on incidence of notifiable communicable diseases in England: An interrupted time series analysis. BMC Public Health 2022, 22, 2318. [Google Scholar] [CrossRef]
- O’Dowd, A. New agency will focus on preparing and protecting UK from future pandemics. BMJ 2021, 372, n819. [Google Scholar] [CrossRef]
- UK Health Security Agency. Available online: https://www.gov.uk/government/organisations/uk-health-security-agency (accessed on 18 October 2023).
- Triple, S. Assessment of syndromic surveillance in Europe. Lancet 2011, 378, 1833–1834. [Google Scholar] [CrossRef]
- UK Health Security Agency. Syndromic Surveillance: Systems and Analyses. GP in Hours Syndromic Surveillance System. Available online: https://www.gov.uk/government/collections/syndromic-surveillance-systems-and-analyses#gp-in-hours-syndromic-surveillance-system (accessed on 13 July 2023).
- Office for National Statistics (ONS). Population Estimates for the UK, England, Wales, Scotland and Northern Ireland: Mid-2021. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2021 (accessed on 23 October 2023).
- UK Parliament. Coronavirus: A History of ‘Lockdown Laws’ in England. UK Parliament House of Commons Library. 2021. Available online: https://commonslibrary.parliament.uk/research-briefings/cbp-9068/ (accessed on 13 July 2023).
- Fleming, D.M.; Elliot, A.J. Changing disease incidence: The consulting room perspective. Br. J. Gen. Pract. 2006, 56, 820–824. [Google Scholar] [PubMed]
- Walker, J.L.; Andrews, N.J.; Mathur, R.; Smeeth, L.; Thomas, S.L. Trends in the burden of varicella in UK general practice. Epidemiol. Infect. 2017, 145, 2678–2682. [Google Scholar] [CrossRef] [PubMed]
- Widgren, K.; Giesecke, J.; Lindquist, L.; Tegnell, A. The burden of chickenpox disease in Sweden. BMC Infect. Dis. 2016, 16, 666. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Sinnathamby, M.A.; Elgohari, S.; Zhao, H.; Obi, C.; Coughlan, L.; Lampos, V.; Simmons, R.; Tessier, E.; Campbell, H.; et al. The impact of social and physical distancing measures on COVID-19 activity in England: Findings from a multi-tiered surveillance system. Eurosurveillance 2021, 26, 2001062. [Google Scholar] [CrossRef]
- Ross, A.M.; Fleming, D.M. Chickenpox increasingly affects preschool children. Commun. Dis. Public Health 2000, 3, 213–215. [Google Scholar]
- Liaw, S.T.; Kuziemsky, C.; Schreiber, R.; Jonnagaddala, J.; Liyanage, H.; Chittalia, A.; Bahniwal, R.; He, J.W.; Ryan, B.L.; Lizotte, D.J.; et al. Primary care informatics response to COVID-19 pandemic: Adaptation, progress, and lessons from four countries with high ICT development. Yearb. Med. Inform. 2021, 30, 44–55. [Google Scholar] [CrossRef]
- Murphy, M.; Scott, L.J.; Salisbury, C.; Turner, A.; Scott, A.; Denholm, R.; Lewis, R.; Iyer, G.; Macleod, J.; Horwood, J. Implementation of remote consulting in UK primary care following the COVID-19 pandemic: A mixed-methods longitudinal study. Br. J. Gen. Pract. 2021, 71, e166–e177. [Google Scholar] [CrossRef]
- Hughes, H.E.; Hughes, T.C.; Morbey, R.; Challen, K.; Oliver, I.; Smith, G.E.; Elliot, A.J. Emergency department use during COVID-19 as described by syndromic surveillance. Emerg. Med. J. 2020, 37, 600–604. [Google Scholar] [CrossRef]
- Ferraro, C.F.; Findlater, L.; Morbey, R.; Hughes, H.E.; Harcourt, S.; Hughes, T.C.; Elliot, A.J.; Oliver, I.; Smith, G.E. Describing the indirect impact of COVID-19 on healthcare utilisation using syndromic surveillance systems. BMC Public Health 2021, 21, 2019. [Google Scholar] [CrossRef]
- Yeoh, D.K.; Foley, D.A.; Minney-Smith, C.A.; Martin, A.C.; Mace, A.O.; Sikazwe, C.T.; Le, H.; Levy, A.; Blyth, C.C.; Moore, H.C. Impact of coronavirus disease 2019 public health measures on detections of influenza and respiratory syncytial virus in children during the 2020 Australian winter. Clin. Infect. Dis. 2021, 72, 2199–2202. [Google Scholar] [CrossRef]
- Leston, M.; Elson, W.H.; Watson, C.; Lakhani, A.; Aspden, C.; Bankhead, C.R.; Borrow, R.; Button, E.; Byford, R.; Elliot, A.J.; et al. Representativeness, vaccination uptake, and COVID-19 clinical outcomes 2020–2021 in the UK Oxford-Royal College of General Practitioners Research and Surveillance Network: Cohort profile summary. JMIR Public Health Surveill. 2022, 8, e39141. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Social Care. Minute of the JCVI Shingles/Varicella Sub-Committee Meeting Monday 26 September 2022 13:00–16:00. Available online: https://app.box.com/s/vdlafy8wm4t5asq2qyfpc4dw6fzeyypf/file/1079273167905 (accessed on 13 July 2023).

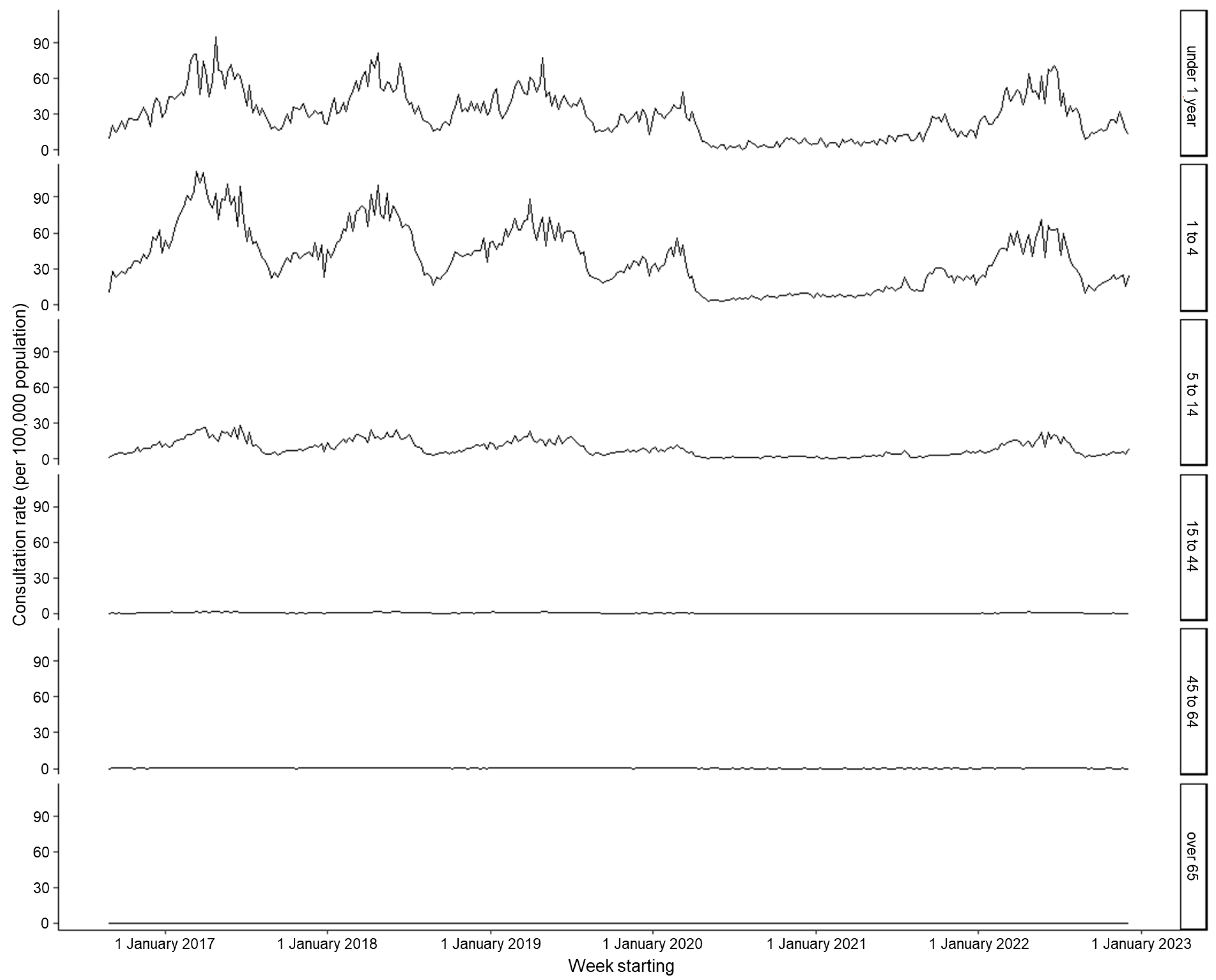
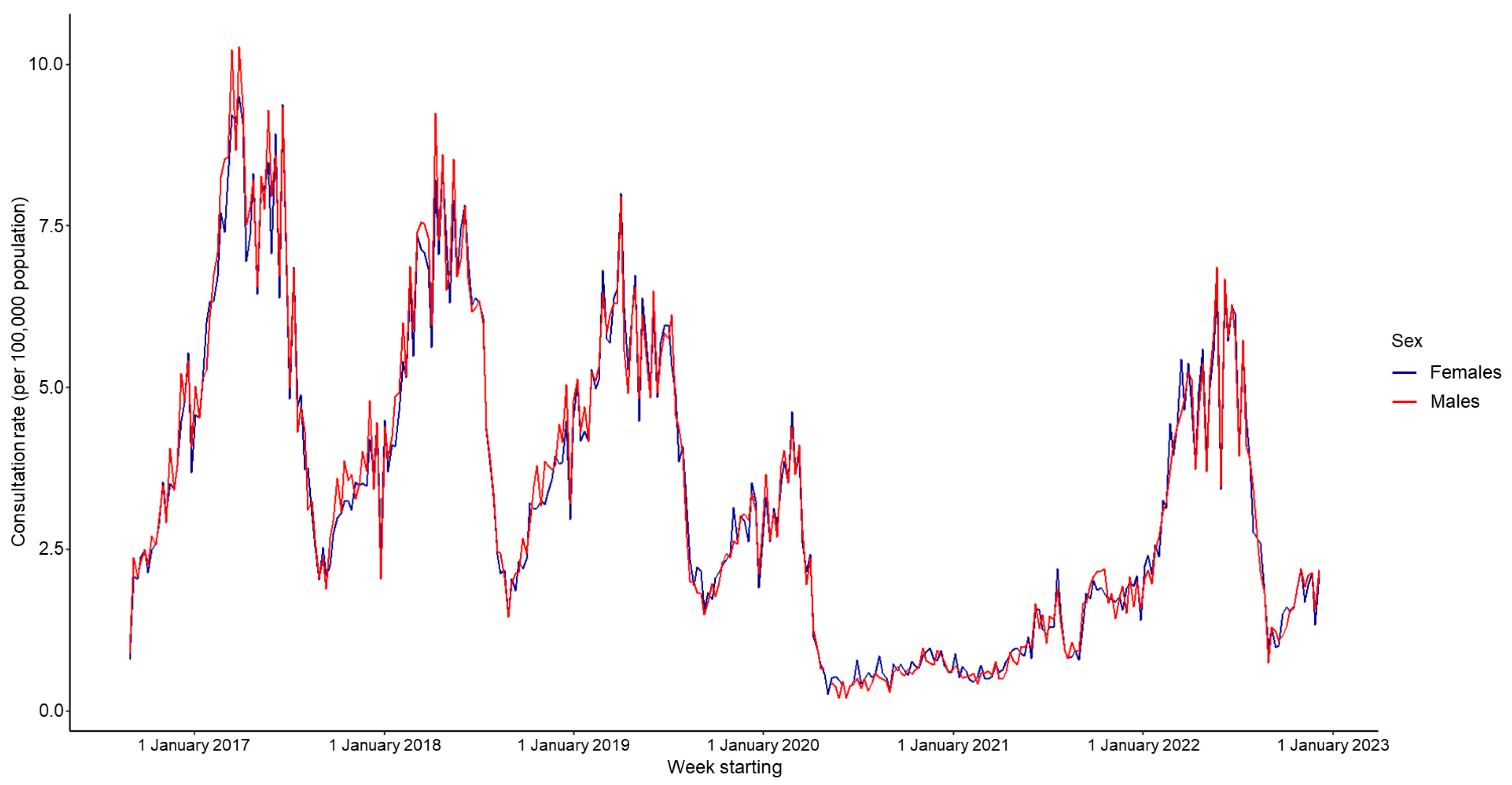

| Year | Mean Weekly Rate 1 | Lower 95% CI | Upper 95% CI | Activity Range (Rate) | Peak Week Number | |
|---|---|---|---|---|---|---|
| Minimum | Maximum (Peak Week) | |||||
| 2017 | 5.52 | 4.86 | 6.18 | 1.97 | 9.87 | 13 |
| 2018 | 4.88 | 4.33 | 5.42 | 1.46 | 8.73 | 15 |
| 2019 | 4.18 | 3.70 | 4.66 | 1.53 | 7.93 | 14 |
| 2020 | 1.28 | 0.93 | 1.62 | 0.23 | 4.49 | - |
| 2021 | 1.20 | 1.05 | 1.35 | 0.48 | 2.06 | - |
| 2022 2 | 3.36 | 2.88 | 3.84 | 0.84 | 6.69 | 21 |
| Whole Study Period (1 September 2016 to 9 December 2022) | Pre COVID-19 (1 September 2016 to 8 March 2020) | Post COVID-19 (9 March 2020 to 9 December 2022) | ||||
|---|---|---|---|---|---|---|
| Characteristic | Rate per 100,000 | RR 1 | Rate per 100,000 | RR | Rate per 100,000 | RR |
| Age | ||||||
| <1 | 29.53 | - | 31.49 | - | 24.41 | - |
| 1 to 4 | 38.01 | 1.29 | 41.90 | 1.33 | 27.86 | 1.14 |
| 5 to 14 | 8.96 | 0.30 | 9.73 | 0.31 | 6.97 | 0.29 |
| 15 to 44 | 0.67 | 0.02 | 0.73 | 0.02 | 0.50 | 0.02 |
| 45 to 64 | 0.19 | 0.01 | 0.21 | 0.01 | 0.13 | 0.01 |
| Over 65 | 0.09 | 0.00 | 0.10 | 0.00 | 0.06 | 0.00 |
| Sex | ||||||
| Male | 3.43 | - | 3.81 | - | 2.44 | - |
| Female | 3.38 | 0.99 | 3.74 | 1.02 | 2.45 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bardsley, M.; Loveridge, P.; Bednarska, N.G.; Smith, S.; Morbey, R.A.; Amirthalingam, G.; Elson, W.H.; Bates, C.; de Lusignan, S.; Todkill, D.; et al. The Epidemiology of Chickenpox in England, 2016–2022: An Observational Study Using General Practitioner Consultations. Viruses 2023, 15, 2163. https://doi.org/10.3390/v15112163
Bardsley M, Loveridge P, Bednarska NG, Smith S, Morbey RA, Amirthalingam G, Elson WH, Bates C, de Lusignan S, Todkill D, et al. The Epidemiology of Chickenpox in England, 2016–2022: An Observational Study Using General Practitioner Consultations. Viruses. 2023; 15(11):2163. https://doi.org/10.3390/v15112163
Chicago/Turabian StyleBardsley, Megan, Paul Loveridge, Natalia G. Bednarska, Sue Smith, Roger A. Morbey, Gayatri Amirthalingam, William H. Elson, Chris Bates, Simon de Lusignan, Daniel Todkill, and et al. 2023. "The Epidemiology of Chickenpox in England, 2016–2022: An Observational Study Using General Practitioner Consultations" Viruses 15, no. 11: 2163. https://doi.org/10.3390/v15112163






