Identification of 2,4-Diaminoquinazoline Derivative as a Potential Small-Molecule Inhibitor against Chikungunya and Ross River Viruses
Abstract
:1. Introduction
2. Material and Methods
2.1. Cells and Viruses
2.2. DRDE Chemical Repository (DCR) Library
2.3. Preparation of Compound Stock Solution
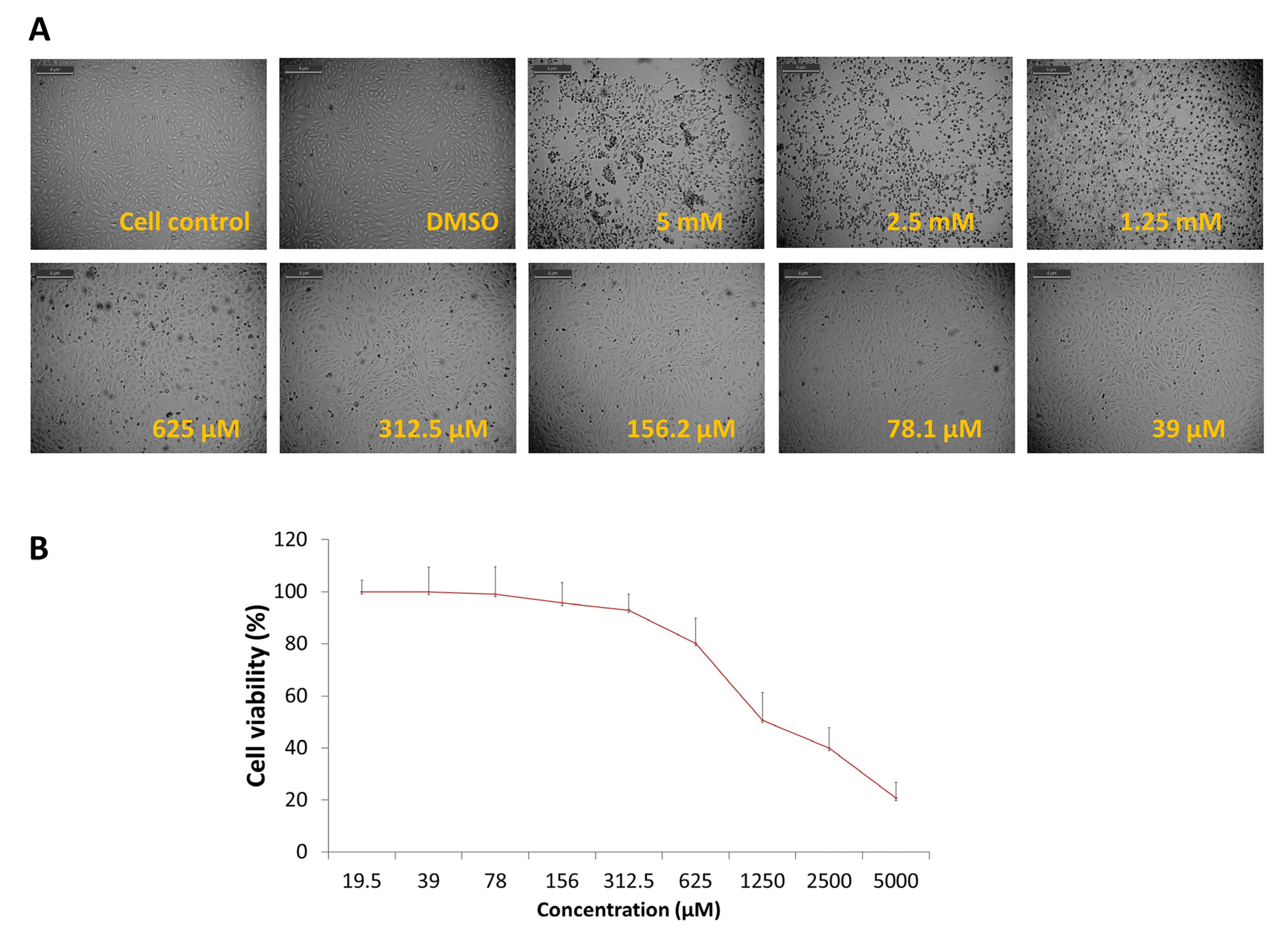
2.4. Cytotoxicity Assay for Screening Small Molecules
2.5. In Vitro Screening of Compounds against CHIKV
2.6. Evaluation of Antiviral Activity of DCR-137 against CHIKV
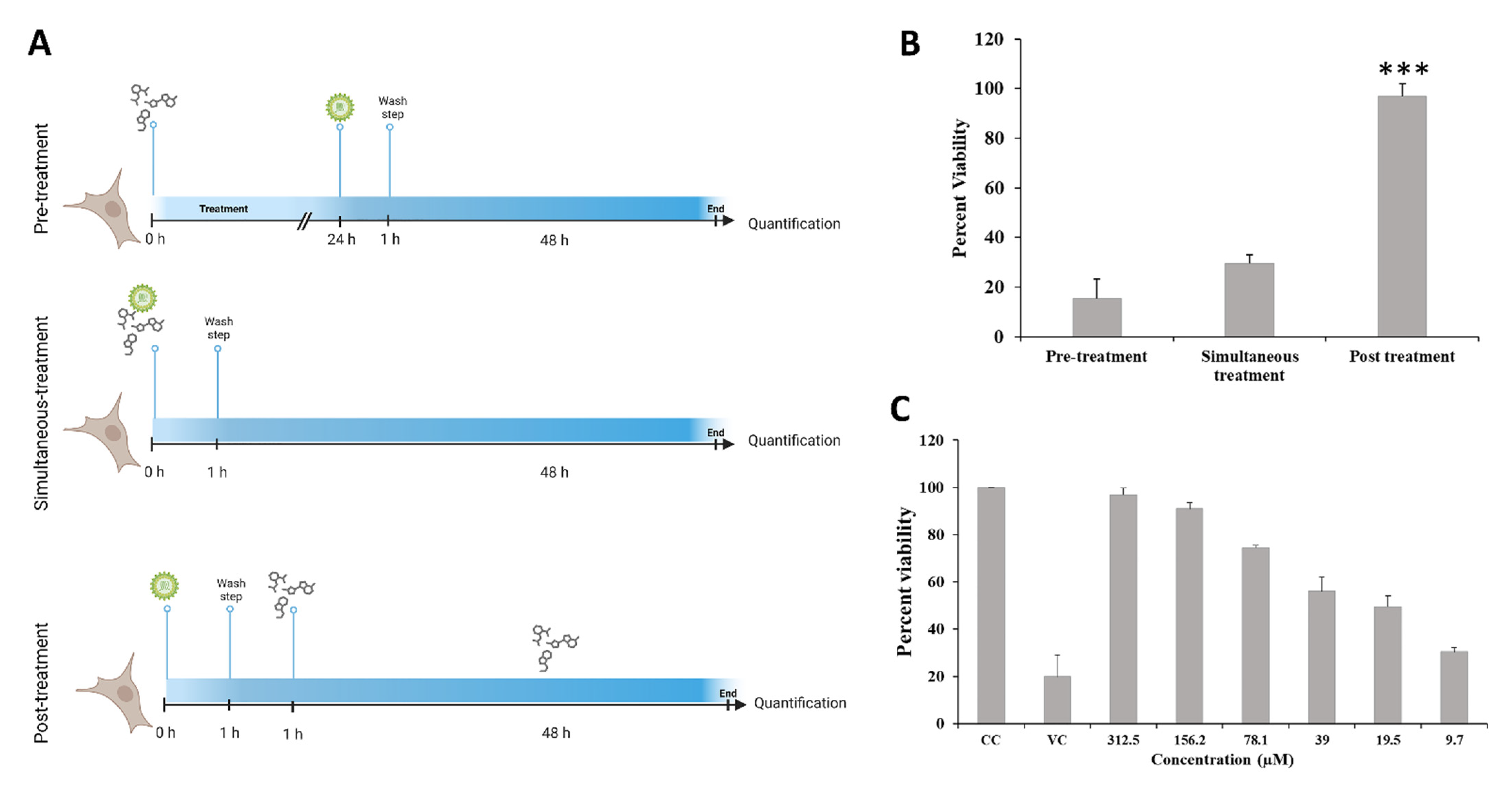
2.6.1. Mode of Inhibition
2.6.2. Cytopathic Effect Inhibition and Cell Viability Assay
2.6.3. Plaque Assay
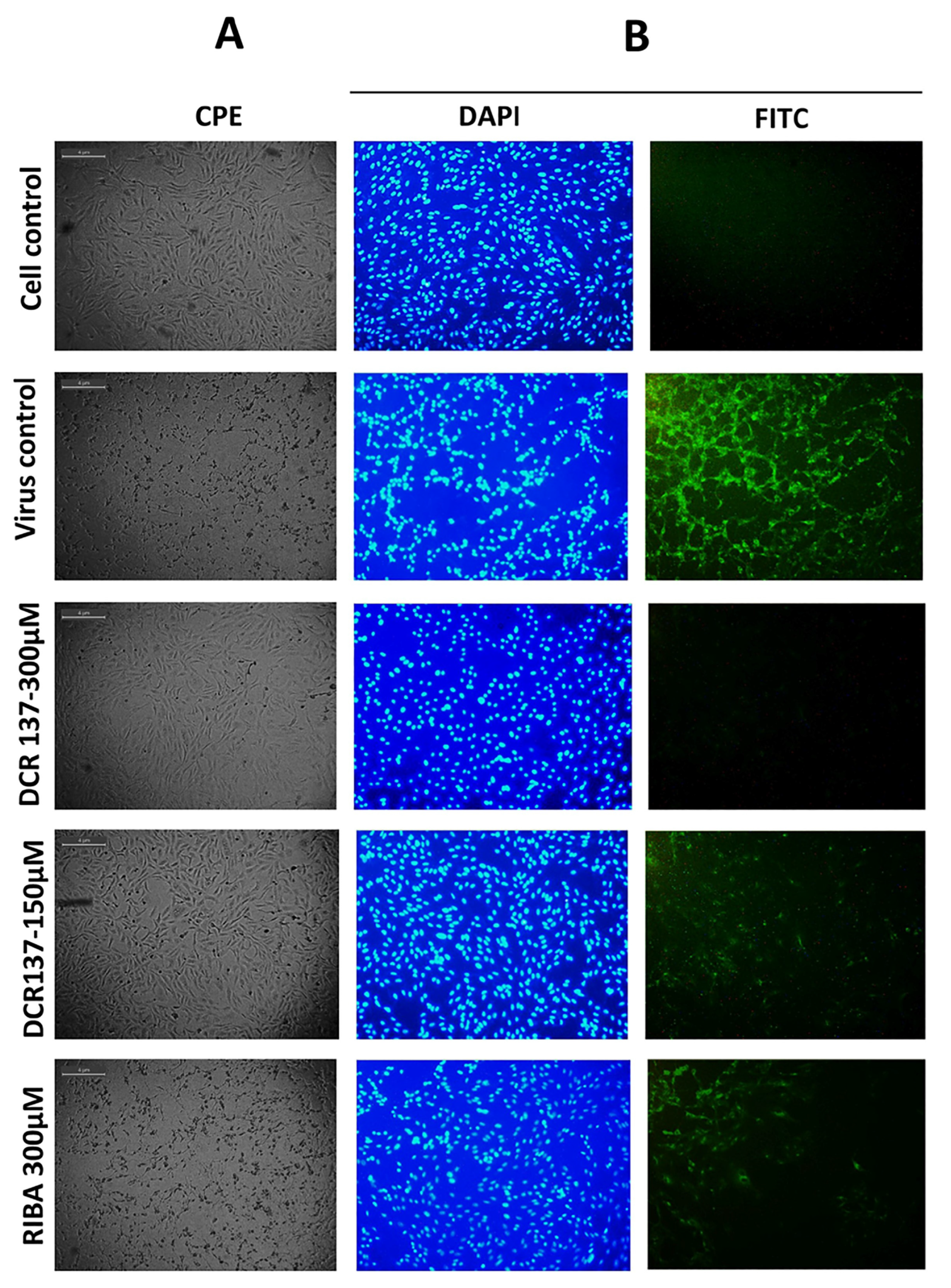
2.6.4. Immunofluorescence Assay
2.6.5. Flow Cytometric Analysis
2.6.6. Western Blot
2.7. Evaluation of Antiviral Activity of DCR-137 against RRV
2.8. Statistical Analyses
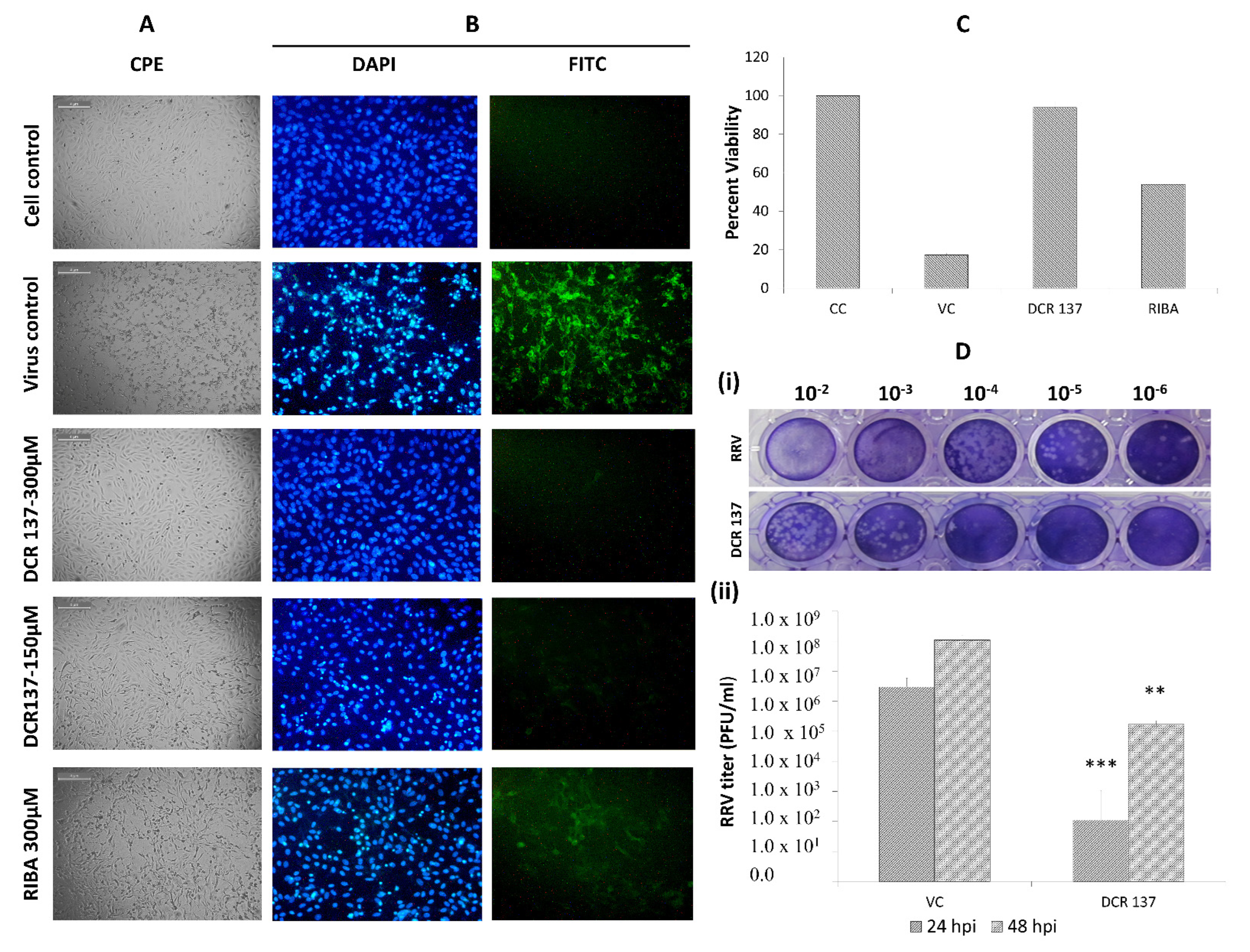
3. Results
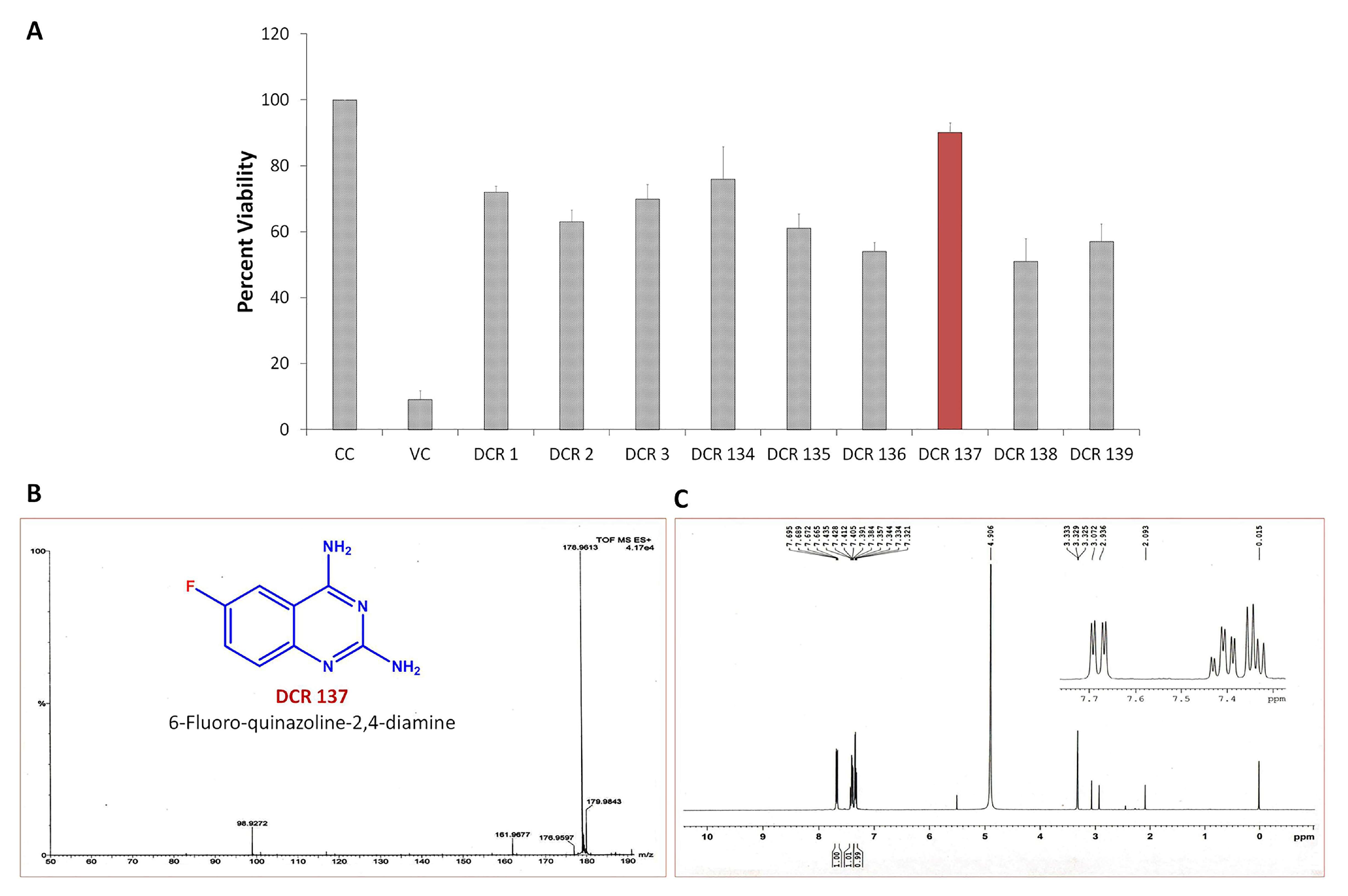
3.1. In Vitro Antiviral Screening of Small Molecules
3.2. Evaluation of Antiviral Activity of DCR-137 against CHIKV
3.3. Evaluation of Antiviral Activity of DCR-137 against RRV
4. Discussion
5. Limitations of the Study and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microbiol. Rev. 1994, 58, 491–562. [Google Scholar] [CrossRef]
- Powers, A.M.; Brault, A.C.; Shirako, Y.; Strauss, E.G.; Kang, W.; Strauss, J.H.; Weaver, S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001, 75, 10118–10131. [Google Scholar] [CrossRef] [PubMed]
- Weaver, S.C.; Winegar, R.; Manger, I.D.; Forrester, N.L. Alphaviruses: Population genetics and determinants of emergence. Antivir. Res. 2012, 94, 242–257. [Google Scholar] [CrossRef]
- Weaver, S.C. Arrival of chikungunya virus in the new world: Prospects for spread and impact on public health. PLoS Negl. Trop. Dis. 2014, 8, e2921. [Google Scholar] [CrossRef] [PubMed]
- Solignat, M.; Gay, B.; Higgs, S.; Briant, L.; Devaux, C. Replication cycle of chikungunya: A re-emerging arbovirus. Virology 2009, 393, 183–197. [Google Scholar] [CrossRef]
- Powers, A.M. Risks to the Americas associated with the continued expansion of chikungunya virus. J. Gen. Virol. 2015, 96 Pt 1, 1. [Google Scholar] [CrossRef]
- Weaver, S.C.; Lecuit, M. Chikungunya virus and the global spread of a mosquito-borne disease. N. Engl. J. Med. 2015, 372, 1231–1239. [Google Scholar] [CrossRef]
- Presti, A.L.; Cella, E.; Angeletti, S.; Ciccozzi, M. Molecular epidemiology, evolution and phylogeny of Chikungunya virus: An updating review. Infect. Genet. Evol. 2016, 41, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Briolant, S.; Garin, D.; Scaramozzino, N.; Jouan, A.; Crance, J.M. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: Synergistic effect of interferon-α and ribavirin combination. Antivir. Res. 2004, 61, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Saluja, M.; Venugopalan, A. Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis Rheumatol. 2014, 66, 319–326. [Google Scholar] [CrossRef]
- Delogu, I.; de Lamballerie, X. Chikungunya disease and chloroquine treatment. J. Med. Virol. 2011, 83, 1058. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Manian, M. Ribavirin therapy for Chikungunya arthritis. J. Infect. Dev. Ctries. 2008, 2, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Puhl, A.C.; Lane, T.R.; Urbina, F.; Ekins, S. The need for speed and efficiency: A brief review of small molecule antivirals for COVID-19. Front. Drug Discov. 2022, 2, 837587. [Google Scholar] [CrossRef]
- Apaydın, Ç.B.; Çınar, G.; Cihan-Üstündağ, G. Small-molecule antiviral agents in ongoing clinical trials for COVID-19. Curr. Drug Targets 2021, 22, 1986–2005. [Google Scholar] [CrossRef] [PubMed]
- Sargsyan, K.; Mazmanian, K.; Lim, C. A strategy for evaluating potential antiviral resistance to small molecule drugs and application to SARS-CoV-2. Sci. Rep. 2023, 13, 502. [Google Scholar] [CrossRef]
- Xiang, R.; Yu, Z.; Wang, Y.; Wang, L.; Huo, S.; Li, Y.; Liang, R.; Hao, Q.; Ying, T.; Gao, Y.; et al. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharm. Sin. B 2022, 12, 1591–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhao, Z.; Peng, X.; Zou, J.; Yang, S. Recent advances in small-molecular therapeutics for COVID-19. Precis. Clin. Med. 2022, 5, pbac024. [Google Scholar] [CrossRef]
- Lei, S.; Chen, X.; Wu, J.; Duan, X.; Men, K. Small molecules in the treatment of COVID-19. Signal Transduct. Target. Ther. 2022, 7, 387. [Google Scholar] [CrossRef]
- Chen, Z.; Cui, Q.; Caffrey, M.; Rong, L.; Du, R. Small molecule inhibitors of influenza virus entry. Pharmaceuticals 2021, 14, 587. [Google Scholar] [CrossRef]
- Han, J.; Perez, J.; Schafer, A.; Cheng, H.; Peet, N.; Rong, L.; Manicassamy, B. Influenza virus: Small molecule therapeutics and mechanisms of antiviral resistance. Curr. Med. Chem. 2018, 25, 5115–5127. [Google Scholar] [CrossRef]
- Li, Y.; Huo, S.; Yin, Z.; Tian, Z.; Huang, F.; Liu, P.; Liu, Y.; Yu, F. The current state of research on influenza antiviral drug development: Drugs in clinical trial and licensed drugs. Mbio 2023, e01273-23. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, X.; Sun, Q.; Zhang, C.; Yang, S.; Li, L.; Jia, Z. Progress of small molecular inhibitors in the development of anti-influenza virus agents. Theranostics 2017, 7, 826. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Bhagyawant, S.S.; Parida, M.; Dash, P.K. Vector-delivered artificial miRNA effectively inhibited replication of Chikungunya virus. Antivir. Res. 2016, 134, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.K.; Agarwal, A.; Sharma, S.; Saha, A.; Joshi, G.; Gopalan, N.; Sukumaran, D.; Parida, M.M. Development of a SYBR green I-based quantitative RT-PCR for Ross River virus: Application in vector competence studies and antiviral drug evaluation. J. Virol. Methods 2016, 234, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Shelke, N.B.; Ghorpade, R.; Pratap, A.; Tak, V.; Acharya, B.N. SN Ar reaction in aqueous medium in the presence of mixed organic and inorganic bases. RSC Adv. 2015, 5, 31226–31230. [Google Scholar] [CrossRef]
- Kumar, J.S.; Khan, M.; Gupta, G.; Bhoopati, M.; Lakshmana Rao, P.V.; Parida, M. Production, characterization, and application of monoclonal antibodies specific to recombinant (E2) structural protein in antigen-capture ELISA for clinical diagnosis of Chikungunya virus. Viral Immunol. 2012, 25, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Acharya, B.N.; Priya, R.; Tripathi, N.K.; Shrivastava, A.; Rao, M.K.; Kesari, P.; Narwal, M.; Tomar, S.; Bhagyawant, S.S.; et al. Development of nsP2 protease based cell free high throughput screening assay for evaluation of inhibitors against emerging Chikungunya virus. Sci. Rep. 2018, 8, 10831. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.N.; Thavaselvam, D.; Kaushik, M.P. Synthesis and antimalarial evaluation of novel pyridine quinoline hybrids. Med. Chem. Res. 2008, 17, 487–494. [Google Scholar] [CrossRef]
- Strauss, E.G.; Strauss, J.H. Structure and replication of the alphavirus genome. In The Togaviridae and Flaviviridae; Springer: Boston, MA, USA, 1986; pp. 35–90. [Google Scholar]
- Strauss, J.H.; Strauss, E.G. Evolution of RNA viruses. Annu. Rev. Microbiol. 1988, 42, 657–683. [Google Scholar] [CrossRef]
- Bartholomeeusen, K.; Daniel, M.; LaBeaud, D.A.; Gasque, P.; Peeling, R.W.; Stephenson, K.E.; Ng, L.F.; Ariën, K.K. Chikungunya fever. Nat. Rev. Dis. Primers. 2023, 9, 17. [Google Scholar] [CrossRef]
- Yuen, K.Y.; Bielefeldt-Ohmann, H. Ross River virus infection: A cross-disciplinary review with a veterinary perspective. Pathogens 2021, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.P.; Sasse, F.; Brönstrup, M.; Diez, J.; Meyerhans, A. Antiviral drug discovery: Broad-spectrum drugs from nature. Nat. Prod. Rep. 2015, 32, 29–48. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.E.; Whitmore, A.C.; Shabman, R.S.; Lidbury, B.A.; Mahalingam, S.; Heise, M.T. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J. Virol. 2006, 80, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.E. Alphaviruses. In Fields Virology, 4th ed.; Fields, B.N., Knipe, D.M., Howley, P.M., Griffin, D.E., Eds.; Lippincott, Williams, & Wilkins: Philadelphia, PA, USA, 2001; pp. 917–962. [Google Scholar]
- Zhang, R.; Kim, A.S.; Fox, J.M.; Nair, S.; Basore, K.; Klimstra, W.B.; Rimkunas, R.; Fong, R.H.; Lin, H.; Poddar, S.; et al. Mxra8 is a receptor for multiple arthritogenic alphaviruses. Nature 2018, 557, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives. Int. J. Med. Chem. 2014, 2014, 395637. [Google Scholar] [CrossRef] [PubMed]
- Jafari, E.; Khajouei, M.R.; Hassanzadeh, F.; Hakimelahi, G.H.; Khodarahmi, G.A. Quinazolinone and quinazoline derivatives: Recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci. 2016, 11, 1–14. [Google Scholar] [PubMed]
- Wynne, G.M.; De Moor, O.; Johnson, P.D.; Vickers, R.J. Use of Compounds for Preparing Anti-Tuberculosis Agents. United States Patent Application US 12/666,769, 16 December 2010. [Google Scholar]
- Dhuguru, J.; Ghoneim, O.A. Quinazoline based HDAC dual inhibitors as potential anti-cancer agents. Molecules 2022, 27, 2294. [Google Scholar] [CrossRef]
- Wdowiak, P.; Matysiak, J.; Kuszta, P.; Czarnek, K.; Niezabitowska, E.; Baj, T. Quinazoline derivatives as potential therapeutic agents in urinary bladder cancer therapy. Front. Chem. 2021, 9, 765552. [Google Scholar] [CrossRef]
- Jamroskovic, J.; Doimo, M.; Chand, K.; Obi, I.; Kumar, R.; Brännström, K.; Hedenström, M.; Nath Das, R.; Akhunzianov, A.; Deiana, M.; et al. Quinazoline ligands induce cancer cell death through selective STAT3 inhibition and G-quadruplex stabilization. J. Am. Chem. Soc. 2020, 142, 2876–2888. [Google Scholar] [CrossRef]
- Partin, J.V.; Anglin, I.E.; Kyprianou, N. Quinazoline-based α1-adrenoceptor antagonists induce prostate cancer cell apoptosis via TGF-β signalling and IκBα induction. Br. J. Cancer 2003, 88, 1615–1621. [Google Scholar] [CrossRef]
- Chao, B.; Tong, X.K.; Tang, W.; Li, D.W.; He, P.L.; Garcia, J.M.; Zeng, L.M.; Gao, A.H.; Yang, L.; Li, J.; et al. Discovery and optimization of 2,4-diaminoquinazoline derivatives as a new class of potent dengue virus inhibitors. J. Med. Chem. 2012, 55, 3135–3143. [Google Scholar] [CrossRef]

| S. No. | DCR Code | Series Type |
|---|---|---|
| 1. | DCR 1-DCR 3 | Pyridine-quinolone hybrids |
| 2. | DCR 4-DCR 53 | Hydrazones of nicotic acid and isonicotinic acid |
| 3. | DCR 54-DCR 63 | Triaryl pyrazoline |
| 4. | DCR 64-DCR 71 | Oxazin-3-one |
| 5. | DCR 72-DCR 85 | Oxadiazoles |
| 6. | DCR 86-DCR 112 | Dihydropyrimidine dione |
| 7. | DCR 113-DCR 133 | Xanthenes |
| 8. | DCR 134-DCR 139 | Quinazolines |
| 9. | DCR 140-DCR 150 | Substituted benzonitriles |
| DCR Code | Compound | Systematic Name | Structure | Group | Mol.Wt. (Da) | MNTD | CC50, EC50, SI | Cell Viability ** |
|---|---|---|---|---|---|---|---|---|
| DCR 1 | C14H7BrN2O2 | 5-bromo-7,12-dioxa-1,11-diaza-benzo[a]anthracene | 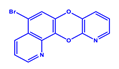 | Pyridine-quinolone hybrids | 315.12 | 50 µM | n.d. * | 72% |
| DCR 2 | C18H8Br2N2O2 | 5,12-dibromo-[1,4]dioxino[2,3-h:5,6-h’]diquinoline | 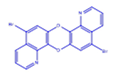 | Pyridine-quinolone hybrids | 444.07 | 50 µM | n.d. | 63% |
| DCR 3 | C19H10ClN3O3 | 7-[(2-chloropridin-3-yl)oxy]-5,12-dioxa-1,11-diazatetraphene |  | Pyridine-quinolone hybrids | 363.754 | 50 µM | n.d. | 69.9% |
| DCR 134 | C8H8N4 | Quinazoline-2,4-diamine | 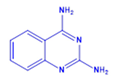 | Quinazoline derivative | 160.18 | 150 µM | n.d. | 75.9% |
| DCR 135 | C8H7FN4 | 5-fluoro-quinazoline-2,4-diamine | 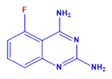 | Quinazoline derivative | 178.17 | 250 µM | n.d. | 61% |
| DCR 136 | C8H7FN4 | 7-fluoro-quinazoline-2,4-diamine |  | Quinazoline derivative | 178.17 | 250 µM | n.d. | 54% |
| DCR 137 | C8H7FN4 | 6-fluoro-quinazoline-2,4-diamine | 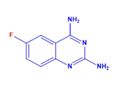 | Quinazoline derivative | 178.17 | 300 µM | CC50 > 1100 µM, EC50 37 µM, SI 31 | 90.3% |
| DCR 138 | C8H6F2N4 | 6,7-difluoro-quinazoline-2,4-diamine | 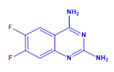 | Quinazoline derivative | 196.16 | 250 µM | n.d. | 51% |
| DCR 139 | C9H10N4O | 5-methoxy-quinazoline-2,4-diamine | 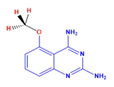 | Quinazoline derivative | 186.25 | 250 µM | n.d. | 57% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, A.; Acharya, B.N.; Parida, M.; Saxena, N.; Rajaiya, J.; Dash, P.K. Identification of 2,4-Diaminoquinazoline Derivative as a Potential Small-Molecule Inhibitor against Chikungunya and Ross River Viruses. Viruses 2023, 15, 2194. https://doi.org/10.3390/v15112194
Saha A, Acharya BN, Parida M, Saxena N, Rajaiya J, Dash PK. Identification of 2,4-Diaminoquinazoline Derivative as a Potential Small-Molecule Inhibitor against Chikungunya and Ross River Viruses. Viruses. 2023; 15(11):2194. https://doi.org/10.3390/v15112194
Chicago/Turabian StyleSaha, Amrita, Badri Narayan Acharya, Manmohan Parida, Nandita Saxena, Jaya Rajaiya, and Paban Kumar Dash. 2023. "Identification of 2,4-Diaminoquinazoline Derivative as a Potential Small-Molecule Inhibitor against Chikungunya and Ross River Viruses" Viruses 15, no. 11: 2194. https://doi.org/10.3390/v15112194






