Alginate-Encapsulated Mycobacteriophage: A Potential Approach for the Management of Intestinal Mycobacterial Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Bacterial Strains, and Phages
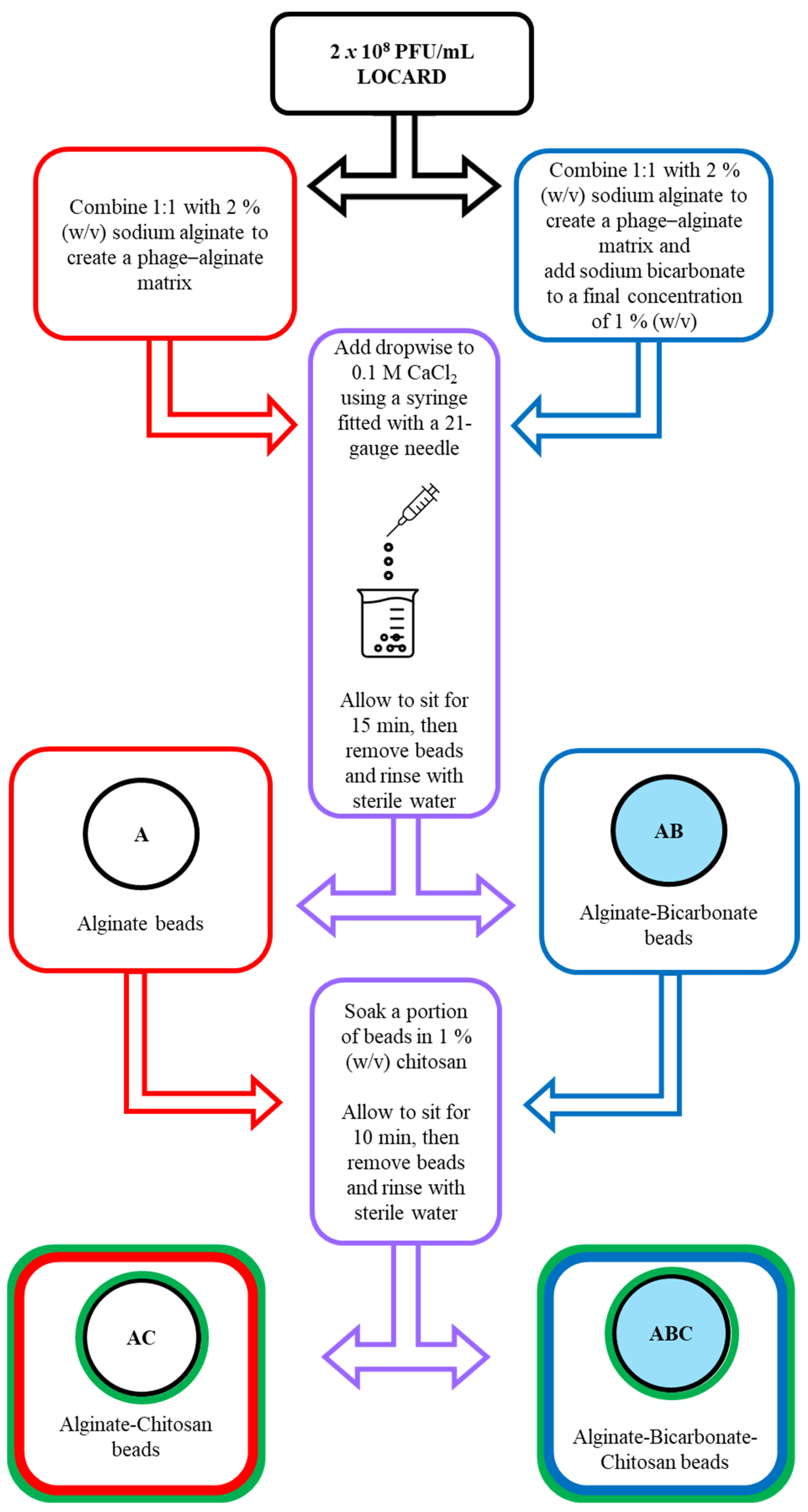
2.2. Encapsulation of LOCARD in Alginate
2.3. Introducing Sodium Bicarbonate and Chitosan to the Alginate Beads to Reinforce Encapsulation
2.4. Examining the Quantity of Viable LOCARD Particles Retained in Each Variety of Beads
2.5. Stability of the Encapsulated LOCARD during Short-Term Storage
2.6. Release of LOCARD Particles in SIF
2.7. Exposure of Encapsulated LOCARD to SGF and Release of Phages from Gastrically Treated Beads in SIF
2.8. Reduction of Mycobacterial Numbers by Phages Released from Alginate Beads in SIF
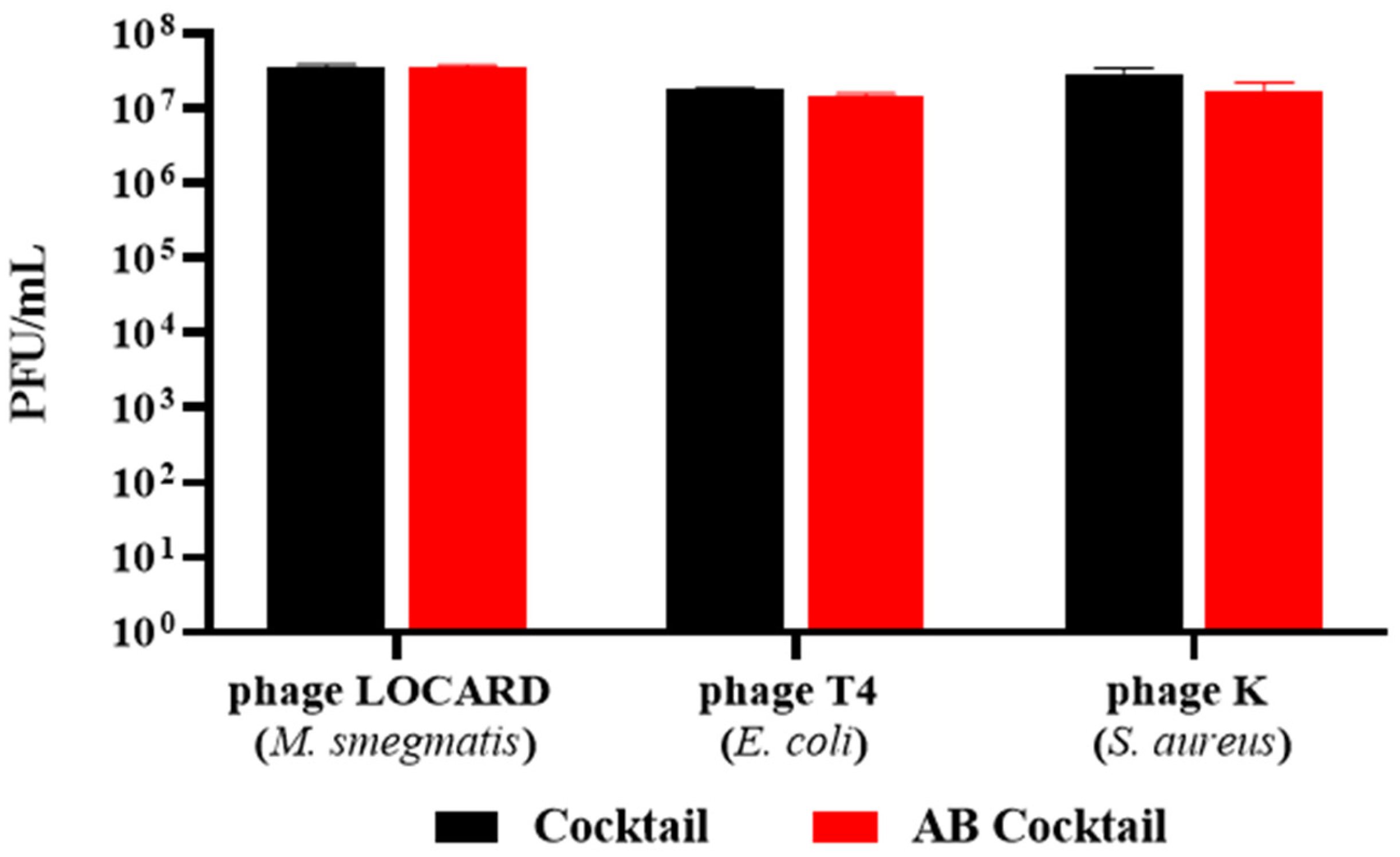
2.9. Encapsulation of a Phage Cocktail as a Preliminary Demonstration of Multivalent Phage Beads
2.10. Statistical Analyses and Presentation of Data
3. Results
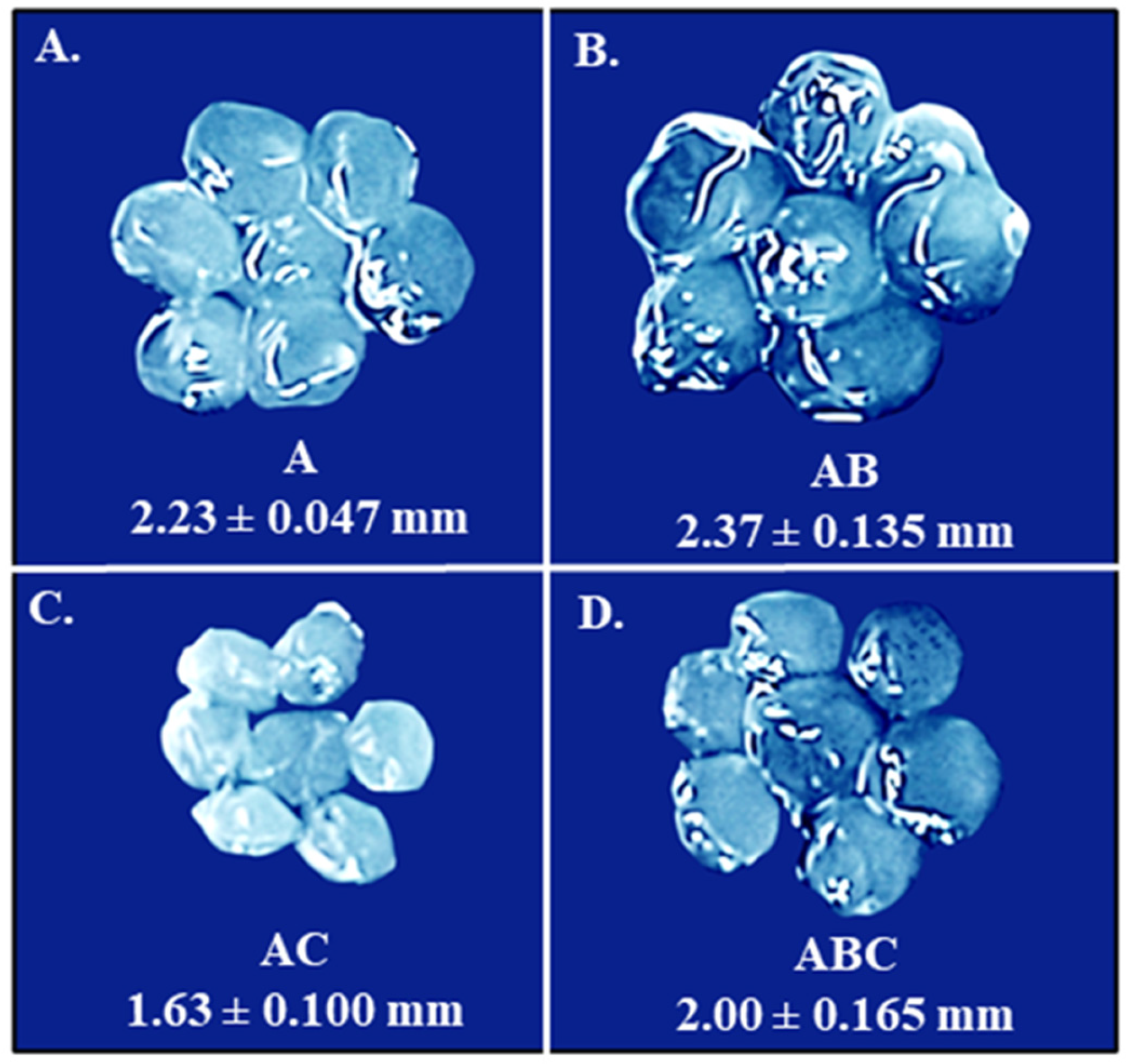
3.1. Encapsulation of LOCARD in an Alginate-Based Matrix
3.2. Introducing Sodium Bicarbonate and Chitosan to the Alginate Beads in an Effort to Reinforce Encapsulation
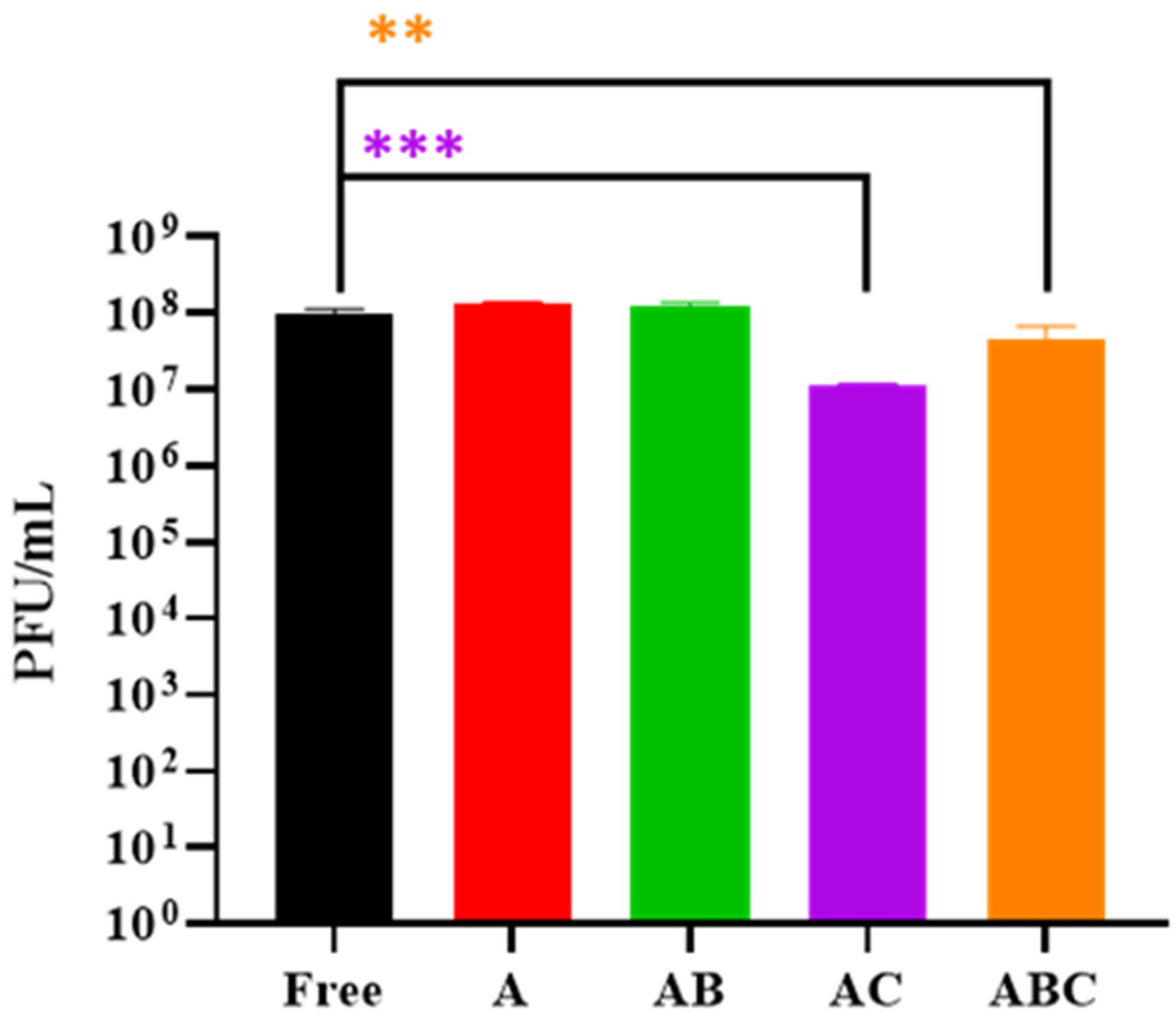
3.3. Examining the Quantity of Viable LOCARD Particles Retained in Each Variety of Beads
3.4. Stability of the Encapsulated LOCARD during Short-Term Storage
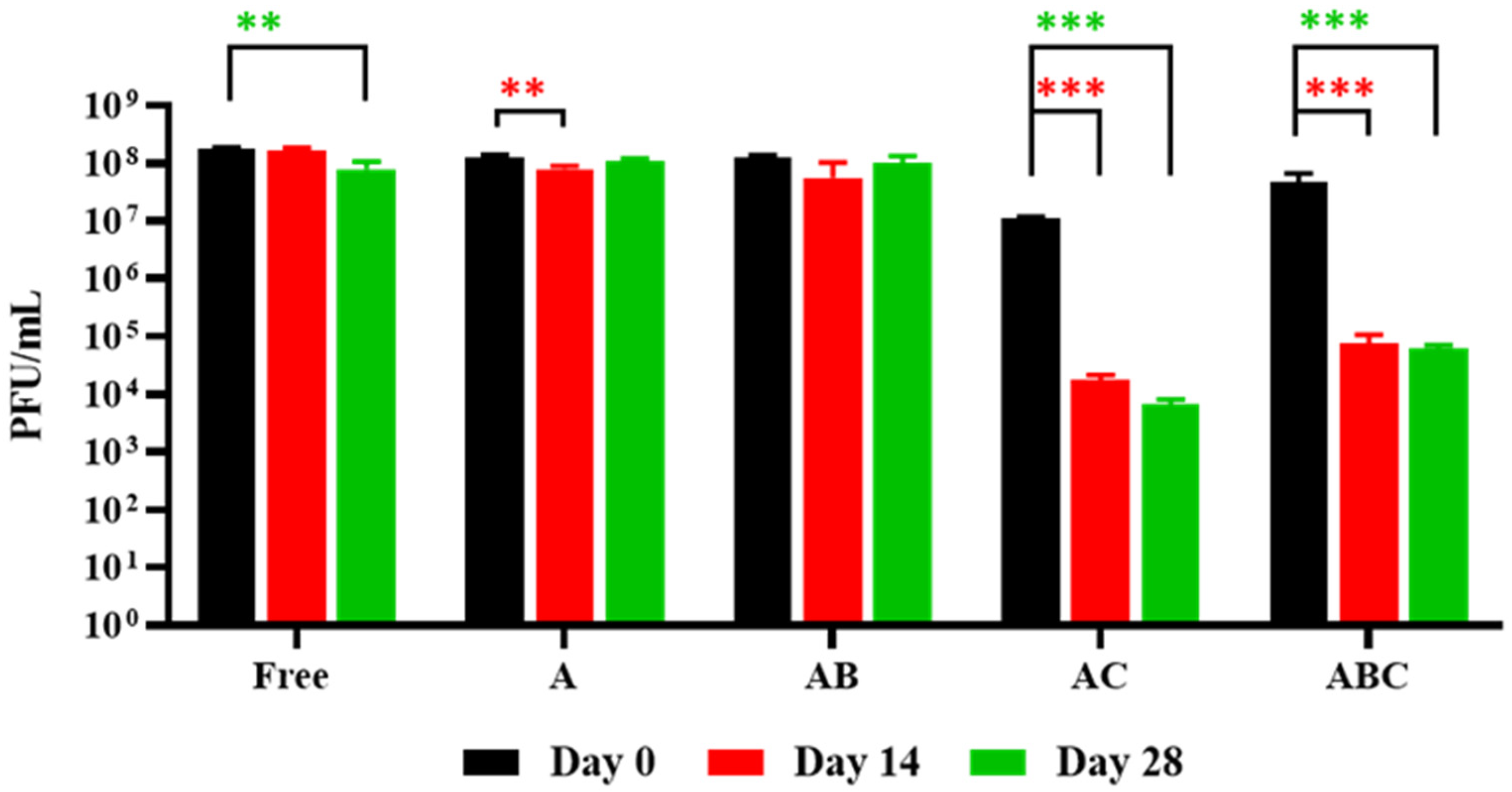
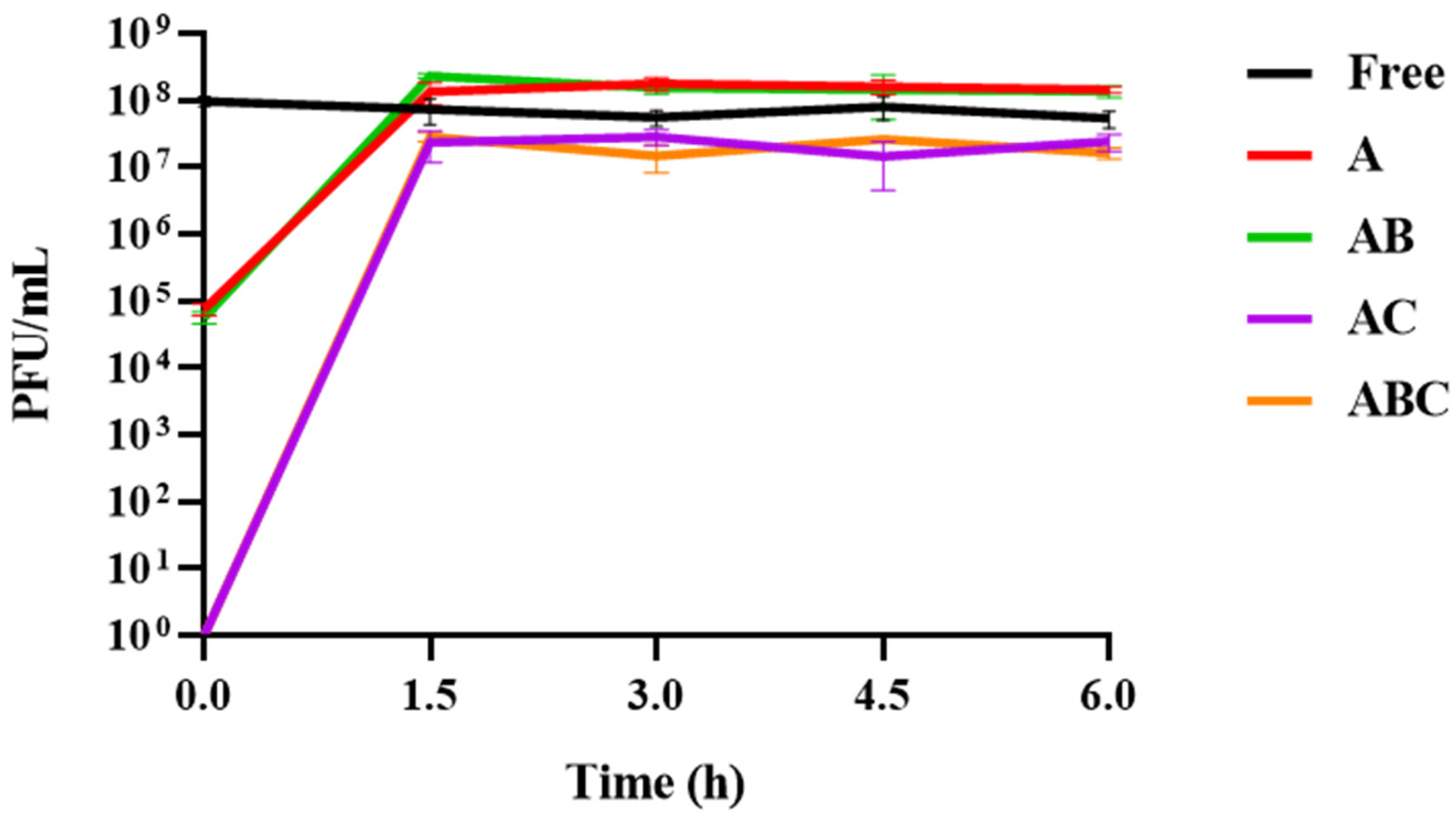
3.5. Release of Phages from Each Bead Type When Exposed to SIF
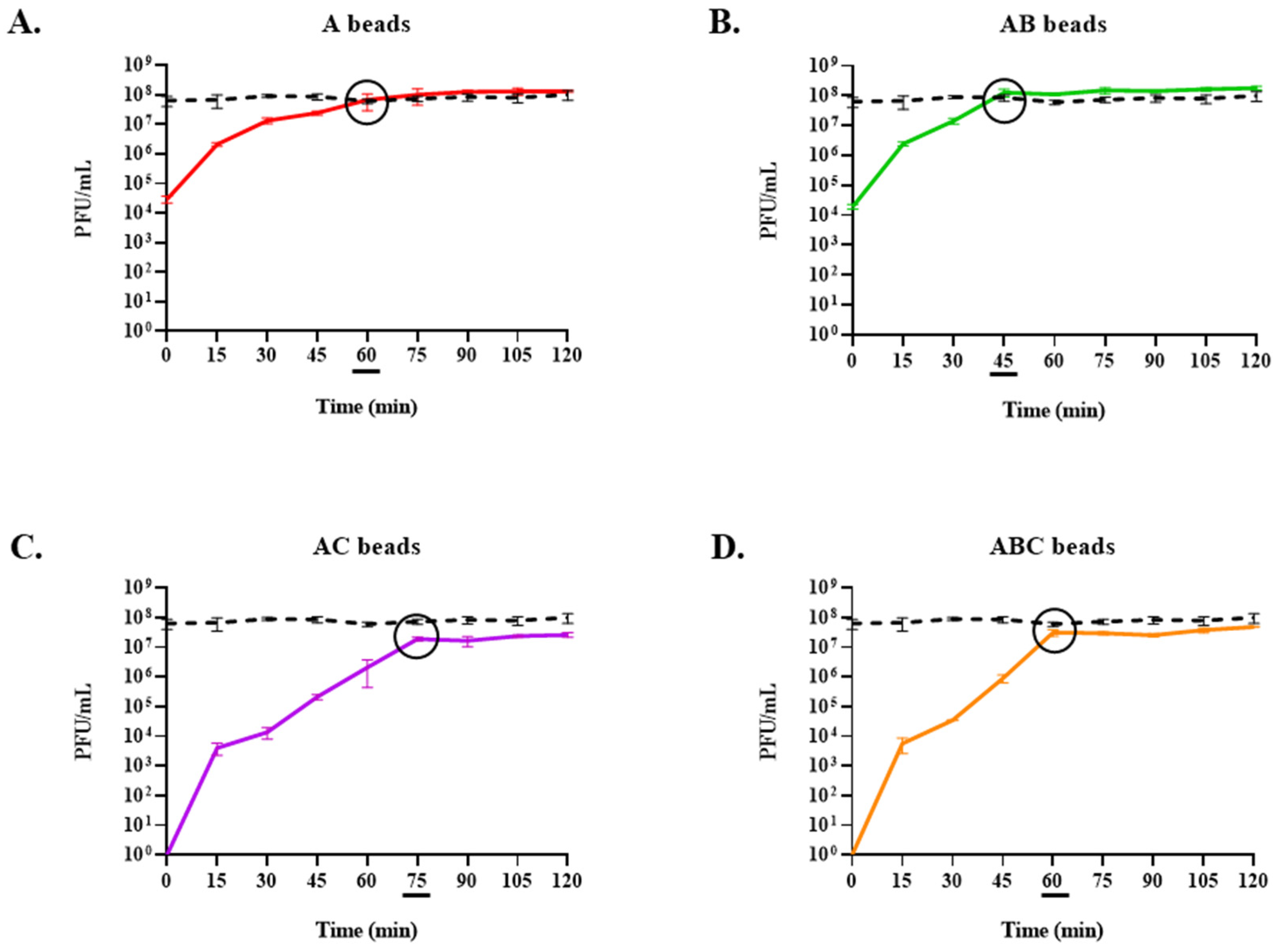
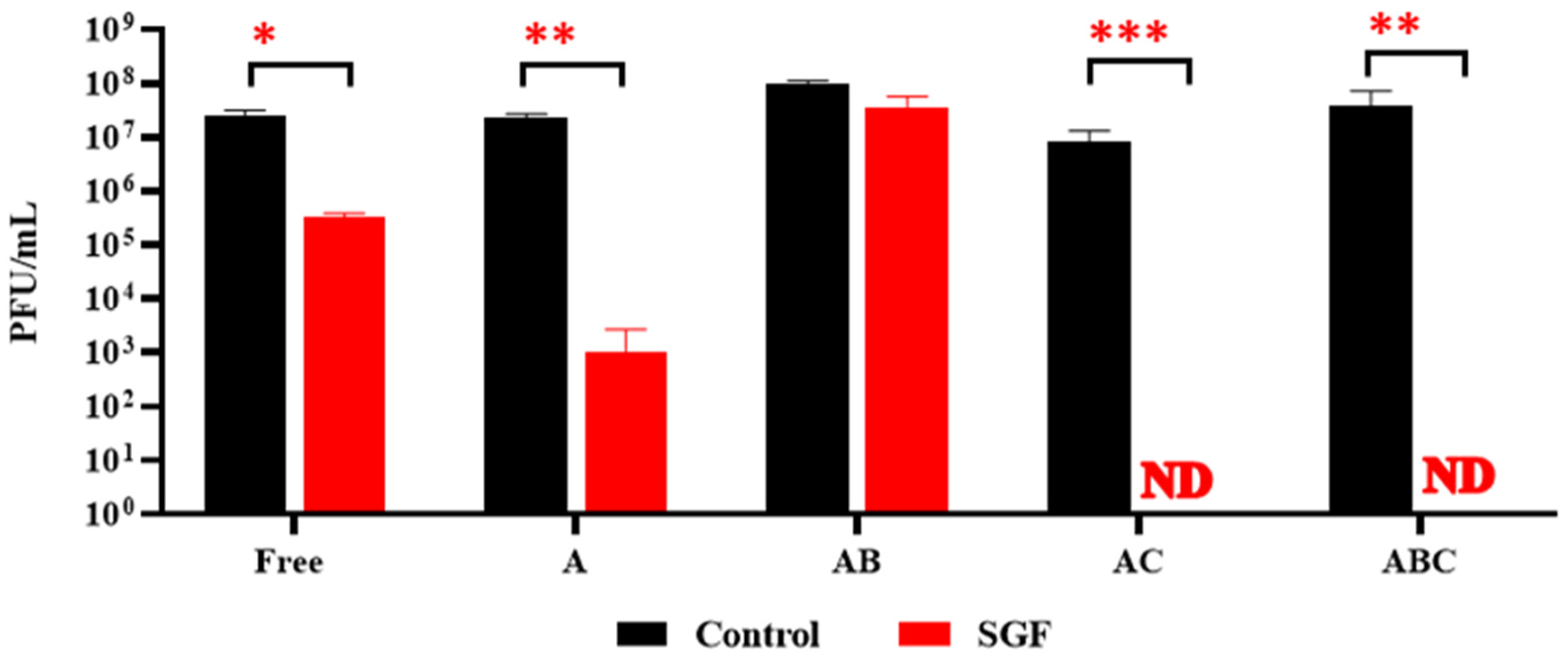
3.6. Exposure of Encapsulated LOCARD to SGF and Release of Phages from Gastrically Treated Beads (GAB) in SIF
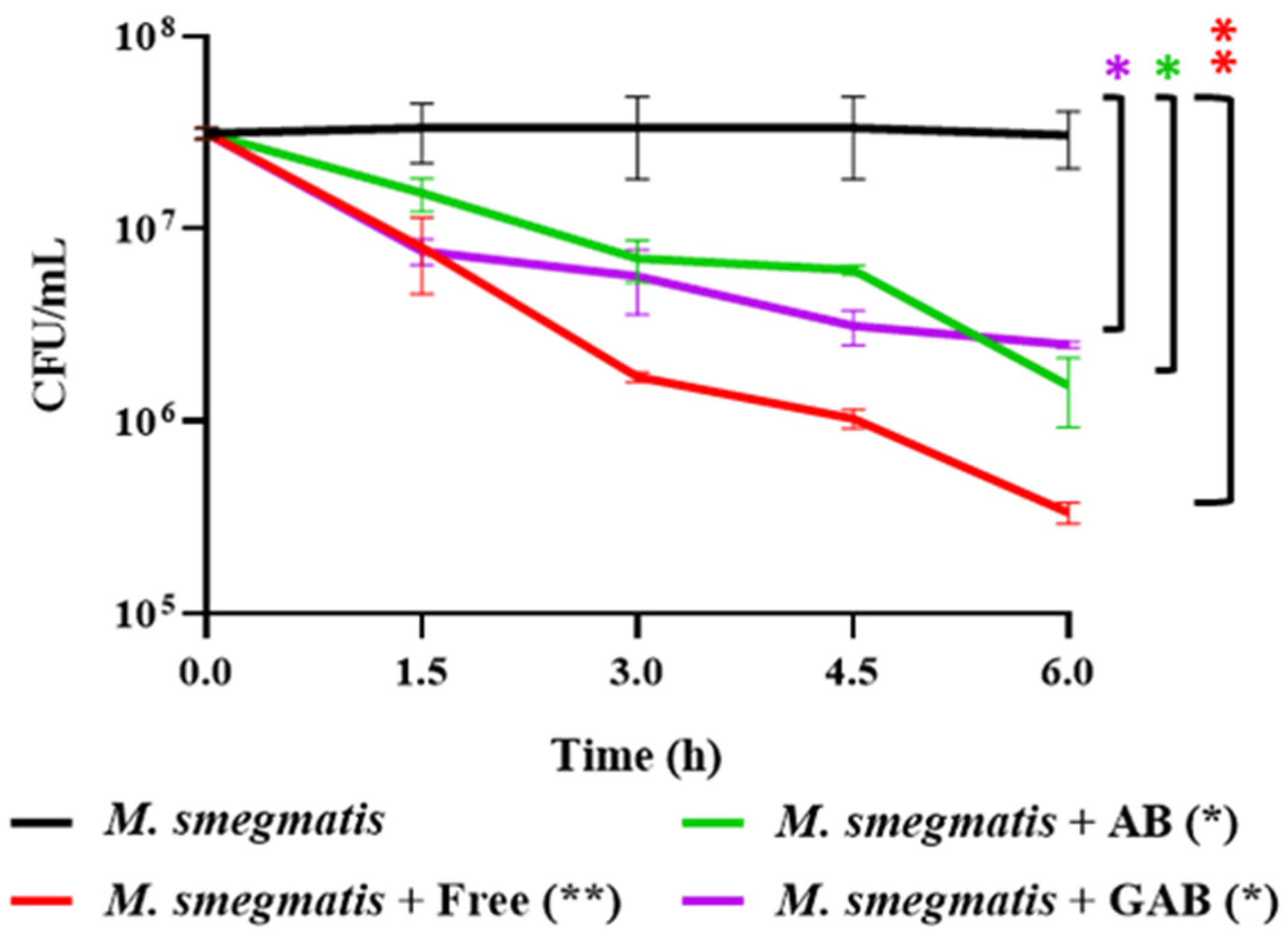
3.7. Reduction of M. smegmatis in SIF by Free and Encapsulated LOCARD
3.8. Encapsulation of a Phage Cocktail as a Preliminary Demonstration of Multivalent Phage Beads
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage therapy of Mycobacterium Infections: Compassionate use of phages in 20 patients with drug-resistant mycobacterial disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Sieiro, C.; Areal-Hermida, L.; Pichardo-Gallardo, Á.; Almuiña-González, R.; de Miguel, T.; Sánchez, S.; Sánchez-Pérez, Á.; Villa, T.G. A hundred years of bacteriophages: Can phages replace antibiotics in agriculture and aquaculture? Antibiotics 2020, 9, 493. [Google Scholar] [CrossRef]
- Nunney, E.; Crotta, M.; van Winden, S.; Bond, K.; Green, M.; Guitian, J. Unravelling transmission of Mycobacterium avium subspecies paratuberculosis to dairy calves: Results of a lifelong longitudinal study. Prev. Vet. Med. 2023, 219, 106022. [Google Scholar] [CrossRef]
- O’Connell, L.M.; Coffey, A.; O’Mahony, J.M. Alternatives to antibiotics in veterinary medicine: Considerations for the management of Johne’s disease. Anim. Health Res. Rev. 2023, 24, 12–27. [Google Scholar] [CrossRef]
- Kralik, P.; Pribylova-Dziedzinska, R.; Kralova, A.; Kovarcik, K.; Slana, I. Evidence of passive faecal shedding of Mycobacterium avium subsp. paratuberculosis in a Limousin cattle herd. Vet. J. 2014, 201, 91–94. [Google Scholar] [CrossRef]
- Espeschit, I.F.; Bastos, D.S.S.; Fonseca Junior, A.; Cardoso, S.A.; Ferrari, M.L.A.; Moreira, M.A.S. Mycobacterium avium subsp. paratuberculosis and Crohn’s disease: Characterisation of the interaction with different aspects of the disease. Braz. J. Microbiol. 2023, 54, 1239–1249. [Google Scholar] [CrossRef]
- Sousa, T.; Costa, M.; Sarmento, P.; Manso, M.C.; Abreu, C.; Bull, T.J.; Cabeda, J.; Sarmento, A. DNA-based detection of Mycobacterium avium subsp. paratuberculosis in domestic and municipal water from Porto (Portugal), an area of high IBD prevalence. AIMS Microbiol. 2021, 7, 163–174. [Google Scholar] [CrossRef]
- Patel, D.; Danelishvili, L.; Yamazaki, Y.; Alonso, M.; Paustian, M.L.; Bannantine, J.P.; Meunier-Goddik, L.; Bermudez, L.E. The ability of Mycobacterium avium subsp. paratuberculosis to enter bovine epithelial cells is influenced by preexposure to a hyperosmolar environment and intracellular passage in bovine mammary epithelial cells. Infect. Immun. 2006, 74, 2849–2855. [Google Scholar] [CrossRef]
- Bor, S.; Kalkan, İ.H.; Çelebi, A.; Dinçer, D.; Akyüz, F.; Dettmar, P.; Özen, H. Alginates: From the ocean to gastroesophageal reflux disease treatment. Turk. J. Gastroenterol. 2019, 30 (Suppl. S2), 109–136. [Google Scholar] [CrossRef]
- Aburub, A.; Fischer, M.; Camilleri, M.; Semler, J.R.; Fadda, H.M. Comparison of pH and motility of the small intestine of healthy subjects and patients with symptomatic constipation using the wireless motility capsule. Int. J. Pharm. 2018, 544, 158–164. [Google Scholar] [CrossRef]
- Moghtader, F.; Eğri, S.; Piskin, E. Phages in modified alginate beads. Artif. Cells Nanomed. Biotechnol. 2017, 45, 357–363. [Google Scholar] [CrossRef]
- Abdelsattar, A.S.; Abdelrahman, F.; Dawoud, A.; Connerton, I.F.; El-Shibiny, A. Encapsulation of E. coli phage ZCEC5 in chitosan-alginate beads as a delivery system in phage therapy. AMB Express 2019, 9, 87. [Google Scholar] [CrossRef]
- Ahmadi, H.; Wang, Q.; Lim, L.T.; Balamurugan, S. Encapsulation of Listeria Phage A511 by alginate to improve its thermal stability. Methods Mol. Biol. 2018, 1681, 89–95. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, D.; Yu, H.; Han, J.; Liu, W.; Qu, D. Encapsulation of Salmonella phage SL01 in alginate/carrageenan microcapsules as a delivery system and its application in vitro. Front. Microbiol. 2022, 13, 906103. [Google Scholar] [CrossRef]
- Del Gaudio, P.; Colombo, P.; Colombo, G.; Russo, P.; Sonvico, F. Mechanisms of formation and disintegration of alginate beads obtained by prilling. Int. J. Pharm. 2005, 302, 1–9. [Google Scholar] [CrossRef]
- Pan, C.T.; Yu, R.S.; Yang, C.J.; Chen, L.R.; Wen, Z.H.; Chen, N.Y.; Ou, H.Y.; Yu, C.Y.; Shiue, Y.L. Sustained-Release and pH-Adjusted Alginate Microspheres-Encapsulated Doxorubicin Inhibit the Viabilities in Hepatocellular Carcinoma-Derived Cells. Pharmaceutics 2021, 13, 1417. [Google Scholar] [CrossRef]
- Kim, H.M. Raft formation of sodium alginate in the stomach. J. Neurogastroenterol. Motil. 2016, 22, 705–706. [Google Scholar] [CrossRef][Green Version]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef]
- Carrigy, N.B.; Larsen, S.E.; Reese, V.; Pecor, T.; Harrison, M.; Kuehl, P.J.; Hatfull, G.F.; Sauvageau, D.; Baldwin, S.L.; Finlay, W.H.; et al. Prophylaxis of Mycobacterium tuberculosis H37Rv infection in a preclinical mouse model via inhalation of nebulised bacteriophage D29. Antimicrob. Agents Chemother. 2019, 63, e00871-19. [Google Scholar] [CrossRef]
- Nazareth, N.; Magro, F.; Machado, E.; Ribeiro, T.G.; Martinho, A.; Rodrigues, P.; Alves, R.; Macedo, G.N.; Gracio, D.; Coelho, R.; et al. Prevalence of Mycobacterium avium subsp. paratuberculosis and Escherichia coli in blood samples from patients with inflammatory bowel disease. Med. Microbiol. Immunol. 2015, 204, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Briot, T.; Kolenda, C.; Ferry, T.; Medina, M.; Laurent, F.; Leboucher, G.; Pirot, F. Paving the way for phage therapy using novel drug delivery approaches. J. Control. Release 2022, 347, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Diós, P.; Nagy, S.; Pál, S.; Pernecker, T.; Kocsis, B.; Budán, F.; Horváth, I.; Szigeti, K.; Bölcskei, K.; Máthé, D.; et al. Preformulation studies and optimization of sodium alginate based floating drug delivery system for eradication of Helicobacter pylori. Eur. J. Pharm. Biopharm. 2015, 96, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, D.K.; Kang, D.H. Evaluation of micro-organism-detaching efficacy from meat samples by spindle or stomacher treatment and quality analysis of suspensions. J. Appl. Microbiol. 2016, 120, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tian, F.; Hu, Y.; Lin, W.; Liu, Y.; Zhao, F.; Ren, H.; Pan, Q.; Shi, T.; Tong, Y. Characterisation and genomic analysis of BUCT549, a novel bacteriophage infecting Vibrio alginolyticus with flagella as receptor. Front. Microbiol. 2021, 12, 668319. [Google Scholar] [CrossRef]
- Li, Y.; Fortner, L.; Kong, F. Development of a Gastric Simulation Model (GSM) incorporating gastric geometry and peristalsis for food digestion study. Food Res. Int. 2019, 125, 108598. [Google Scholar] [CrossRef]
- Lawrence, G.W.; McCarthy, N.; Walsh, C.J.; Kunyoshi, T.M.; Lawton, E.M.; O’Connor, P.M.; Begley, M.; Cotter, P.D.; Guinane, C.M. Effect of a bacteriocin-producing Streptococcus salivarius on the pathogen Fusobacterium nucleatum in a model of the human distal colon. Gut Microbes 2022, 14, 2100203. [Google Scholar] [CrossRef]
- Alomari, M.M.M.; Dec, M.; Nowaczek, A.; Puchalski, A.; Wernicki, A.; Kowalski, C.; Urban-Chmiel, R. Therapeutic and prophylactic effect of the experimental bacteriophage treatment to control diarrhea caused by E. coli in newborn calves. ACS Infect. Dis. 2021, 7, 2093–2101. [Google Scholar] [CrossRef]
- Hua, S. Physiological and pharmaceutical considerations for rectal drug formulations. Front. Pharmacol. 2019, 10, 1196. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connell, L.M.; Coffey, A.; O’Mahony, J. Alginate-Encapsulated Mycobacteriophage: A Potential Approach for the Management of Intestinal Mycobacterial Disease. Viruses 2023, 15, 2290. https://doi.org/10.3390/v15122290
O’Connell LM, Coffey A, O’Mahony J. Alginate-Encapsulated Mycobacteriophage: A Potential Approach for the Management of Intestinal Mycobacterial Disease. Viruses. 2023; 15(12):2290. https://doi.org/10.3390/v15122290
Chicago/Turabian StyleO’Connell, Laura Michelle, Aidan Coffey, and Jim O’Mahony. 2023. "Alginate-Encapsulated Mycobacteriophage: A Potential Approach for the Management of Intestinal Mycobacterial Disease" Viruses 15, no. 12: 2290. https://doi.org/10.3390/v15122290
APA StyleO’Connell, L. M., Coffey, A., & O’Mahony, J. (2023). Alginate-Encapsulated Mycobacteriophage: A Potential Approach for the Management of Intestinal Mycobacterial Disease. Viruses, 15(12), 2290. https://doi.org/10.3390/v15122290






