Viral RNA in Mosquitoes (Diptera: Culicidae) Collected between 2019 and 2021 in Germany
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Collection
2.2. Mosquito Identification
2.3. Virus RNA Screening
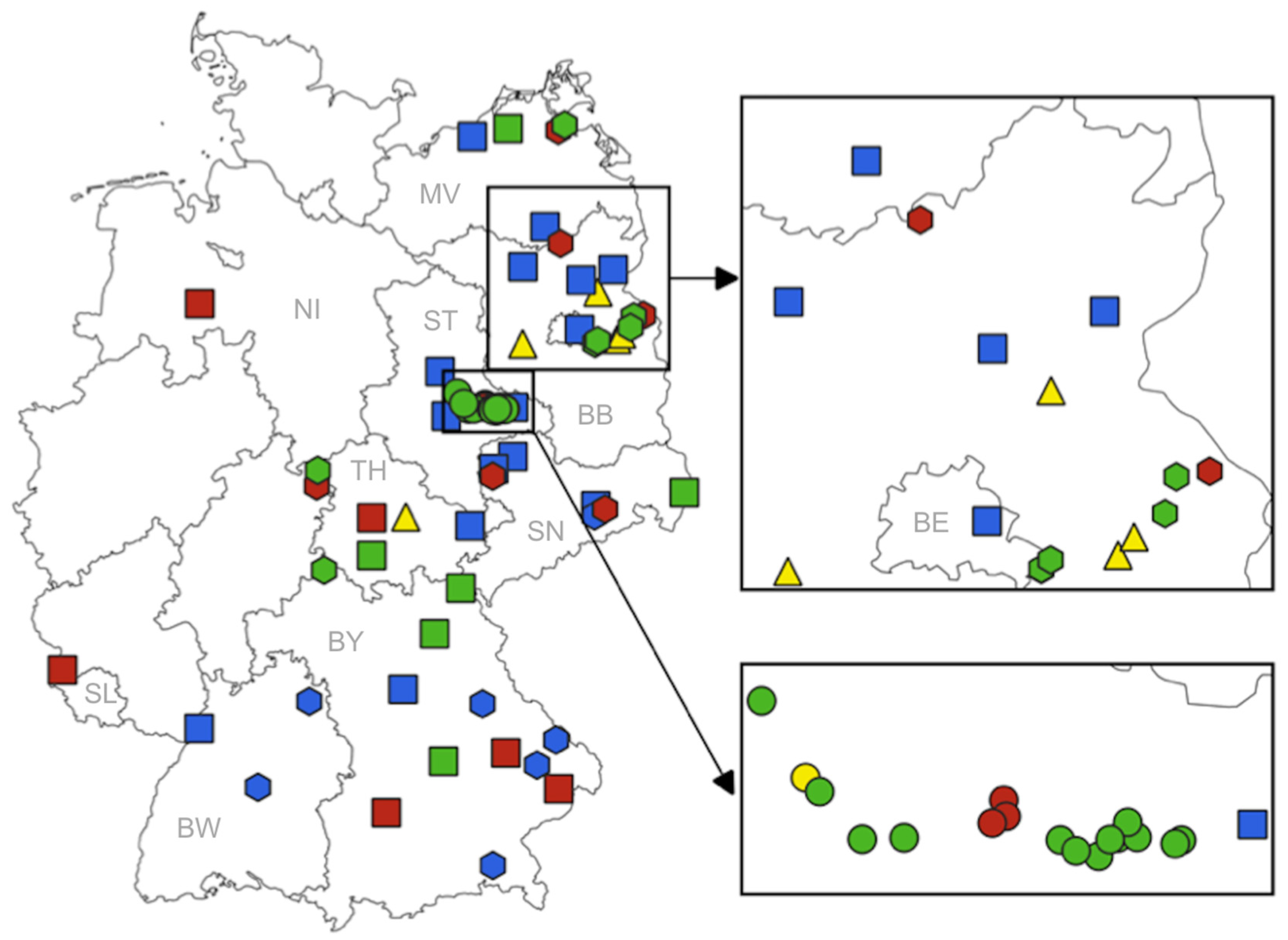
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boualam, M.A.; Pradines, B.; Drancourt, M.; Barbieri, R. Malaria in Europe: A historical perspective. Front. Med. 2021, 8, 691095. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Kowalczyk, S.; Kampen, W. Nine years of mosquito monitoring in Germany, 2011–2019, with an updated inventory of German culicid species. Parasitol. Res 2020, 119, 2765–2774. [Google Scholar] [CrossRef] [PubMed]
- Kuhlisch, C. Discovery of Aedes (Ochlerotatus) pionips Dyar, 1919 (Diptera, Culicidae) in Germany. Check List. 2022, 18, 897–906. [Google Scholar] [CrossRef]
- Kampen, H.; Walther, D. Vector potential of mosquito species (Diptera: Culicidae) occurring in Central Europe. In Parasitology Research Monographs: Mosquito-Borne Diseases; Benelli, G., Mehlhorn, H., Eds.; Springer Nature: Cham, Switzerland, 2018; Volume 10, pp. 41–68. [Google Scholar] [CrossRef]
- Suk, J. Preparedness for mosquito-borne diseases in Europe: The ECDC perspective. Eur. J. Public. Health 2017, 27, 230–231. [Google Scholar] [CrossRef]
- ECDC (European Centre for Disease Prevention and Control). Autochthonous Transmission of Chikungunya Virus in Mainland EU/EEA, 2007-Present. ECDC, Stockholm. 2023. Available online: https://www.ecdc.europa.eu/en/infectious-disease-topics/z-disease-list/chikungunya-virus-disease/surveillance-threats-and (accessed on 9 October 2023).
- ECDC (European Centre for Disease Prevention and Control). Autochthonous Vectorial Transmission of Dengue Virus in Mainland EU/EEA, 2010-Present. ECDC, Stockholm. 2023. Available online: https://www.ecdc.europa.eu/en/all-topics-z/dengue/surveillance-and-disease-data/autochthonous-transmission-dengue-virus-eueea (accessed on 9 October 2023).
- Sousa, C.A.; Clairouin, M.; Seixas, G.; Viveiros, B.; Novo, M.T.; Silva, A.C.; Escoval, M.T.; Economopoulou, A. Ongoing outbreak of dengue type 1 in the Autonomous Region of Madeira, Portugal: Preliminary report. Eurosurveillance 2012, 17, 20333. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Triana, L.M.; Jeffries, C.L.; Mansfield, K.L.; Carnell, G.; Fooks, A.R.; Johnson, N. Emergence of West Nile virus lineage 2 in Europe: A review on the introduction and spread of a mosquito-borne disease. Front. Public. Health 2014, 2, 271. [Google Scholar] [CrossRef] [PubMed]
- Vidaña, B.; Busquets, N.; Napp, S.; Pérez-Ramírez, E.; Jiménez-Clavero, M.A.; Johnson, N. The role of birds of prey in West Nile virus epidemiology. Vaccines 2020, 8, 550. [Google Scholar] [CrossRef]
- Giesen, C.; Herrador, Z.; Fernandez-Martinez, B.; Figuerola, J.; Gangoso, L.; Vazquez, A.; Gómez-Barroso, D. A systematic review of environmental factors related to WNV circulation in European and Mediterranean countries. One Health 2023, 16, 100478. [Google Scholar] [CrossRef]
- Camp, J.V.; Nowotny, N. The knowns and unknowns of West Nile virus in Europe: What did we learn from the 2018 outbreak? Expert Rev. Anti Infect. Ther. 2020, 18, 145–154. [Google Scholar] [CrossRef]
- Ziegler, U.; Lühken, R.; Keller, M.; Cadar, D.; van der Grinten, E.; Michel, F.; Albrecht, K.; Eiden, M.; Rinder, M.; Lachmann, L.; et al. West Nile virus epizootic in Germany, 2018. Antivir. Res. 2019, 162, 39–43. [Google Scholar] [CrossRef]
- Kampen, H.; Werner, D. Arthropod vectors and their growing importance in Europe. In Parasitology Research Monographs: Progress in Parasitology; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 259–282. [Google Scholar] [CrossRef]
- Pilaski, J.; Mackenstein, H. Isolation of Tahyna virus from mosquitoes in two different European natural foci. ZBL Bakt. P. 1985, 180, 394–420. (In German) [Google Scholar]
- Jöst, H.; Bialonski, A.; Storch, V.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation and phylogenetic analysis of Sindbis viruses from mosquitoes in Germany. J. Clin. Microbiol. 2010, 48, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, D.E.; Schäfer, M.; Eiden, M.; Heym, E.C.; Ziegler, U.; Walther, D.; Schmidt-Chanasit, J.; Keller, M.; Groschup, M.H.; Kampen, H. Detection of Usutu, Sindbis, and Batai viruses in mosquitoes (Diptera: Culicidae) collected in Germany, 2011–2016. Viruses 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Heym, E.C.; Kampen, H.; Krone, O.; Schäfer, M.; Werner, D. Molecular detection of vector-borne pathogens from mosquitoes collected in two zoological gardens in Germany. Parasitol. Res. 2019, 118, 2097–2105. [Google Scholar] [CrossRef] [PubMed]
- Jöst, H.; Bialonski, A.; Maus, D.; Sambri, V.; Eiden, M.; Groschup, M.H.; Günther, S.; Becker, N.; Schmidt-Chanasit, J. Isolation of Usutu virus in Germany. Am. J. Trop. Med. Hyg. 2011, 85, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Kampen, H.; Holicki, C.M.; Ziegler, U.; Groschup, M.H.; Tews, B.A.; Werner, D. West Nile virus mosquito vectors (Diptera: Culicidae) in Germany. Viruses 2020, 12, 493. [Google Scholar] [CrossRef] [PubMed]
- Kampen, H.; Tews, B.A.; Werner, D. First evidence of West Nile virus overwintering in mosquitoes in Germany. Viruses 2021, 13, 2463. [Google Scholar] [CrossRef] [PubMed]
- Hubálek, Z. Mosquito-borne viruses in Europe. Parasitol. Res. 2008, 103, 29–43. [Google Scholar] [CrossRef]
- Adouchief, S.; Smura, T.; Sane, J.; Vapalahti, O.; Kurkela, S. Sindbis virus as a human pathogen—Epidemiology, clinical picture and pathogenesis. Rev. Med. Virol. 2016, 26, 221–241. [Google Scholar] [CrossRef]
- Skogh, M.; Espmark, Å. Ockelbo disease: Epidemic arthritis-exanthema syndrome in Sweden caused by Sindbis-virus like agent. Lancet 1982, 1, 795–796. [Google Scholar] [CrossRef]
- Brummer-Korvenkontio, M.; Vapalahti, O.; Kuusisto, P.; Saikku, P.; Manni, T.; Koskela, P.; Nygren, T.; Brummer-Korvenkontio, H.; Vaheri, A. Epidemiology of Sindbis virus infections in Finland 1981–96: Possible factors explaining a peculiar disease pattern. Epidemiol. Infect. 2002, 129, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Hubalek, Z.; Sebesta, O.; Pesko, J.; Betasova, L.; Blazejova, H.; Venclikova, K.; Rudolf, I. Isolation of Tahyna virus (California encephalitis group) from Anopheles hyrcanus (Diptera, Culicidae), a mosquito species new to, and expanding in, Central Europe. J. Med. Entomol. 2014, 51, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.V.; Haider, R.; Porea, D.; Oslobanu, L.E.; Forgách, P.; Nowotny, N. Serological surveillance for Tahyna virus (California encephalitis orthobunyavirus, Peribunyaviridae) neutralizing antibodies in wild ungulates in Austria, Hungary and Romania. Zoonoses Public Health 2018, 65, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, V.; Vilibic-Cavlek, T.; Savic, V.; Klobucar, A.; Kovac, S.; Posavec, M.C.; Petrinic, S.; Bogdanic, M.; Santini, M.; Tesic, V.; et al. Surveillance of Tahyna orthobunyavirus in urban areas in Croatia—The “One Health” approach. Trop. Med. Infect. Dis. 2022, 7, 320. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, A.; Jiménez-Clavero, M.A.; Franco, L.; Donoso-Mantke, O.; Sambri, V.; Niedrig, M.; Zeller, H.; Tenorio, A. Usutu virus—Potential risk of human disease in Europe. Eurosurveillance 2011, 16, 19935. [Google Scholar] [CrossRef] [PubMed]
- Grottola, A.; Marcacci, M.; Tagliazucchi, S.; Gennari, W.; Di Gennaro, A.; Orsini, M.; Monaco, F.; Marchegiano, P.; Marini, V.; Meacci, M.; et al. Usutu virus infection in humans: Retrospective analysis in the municipality of Modena, Italy. Clin. Microbiol. Infect. 2017, 23, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Weissenböck, H.; Kolodziejek, J.; Fragner, K.; Kuhn, R.; Pfeffer, M.; Nowotny, N. Usutu virus activity in Austria, 2001–2002. Microbes Infect. 2003, 5, 1132–1136. [Google Scholar] [CrossRef]
- Becker, N.; Jöst, H.; Ziegler, U.; Eiden, M.; Höper, D.; Emmerich, P.; Schmidt-Chanasit, J. Epizootic emergence of Usutu virus in wild and captive birds in Germany. PLoS ONE 2012, 7, e32604. [Google Scholar] [CrossRef]
- Cadar, D.; Bosch, S.; Jöst, H.; Börstler, J.; Garigliany, M.M.; Becker, N.; Schmidt-Chanasit, J. Putative lineage of novel African Usutu virus, central Europe. Emerg. Infect. Dis. 2015, 21, 1647. [Google Scholar] [CrossRef]
- Ziegler, U.; Jöst, H.; Müller, K.; Fischer, D.; Rinder, M.; Tietze, D.T.; Danner, K.J.; Becker, N.; Skuballa, J.; Hamann, H.P.; et al. Epidemic spread of Usutu virus in southwest Germany in 2011 to 2013 and monitoring of wild birds for Usutu and West Nile viruses. Vector Borne Zoonotic Dis. 2015, 15, 481–488. [Google Scholar] [CrossRef]
- Ziegler, U.; Fast, C.; Eiden, M.; Bock, S.; Schulze, C.; Hoeper, D.; Ochs, A.; Schlieben, P.; Keller, M.; Zielke, D.E.; et al. Evidence for an independent third Usutu virus introduction into Germany. Vet. Microbiol. 2016, 192, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cadar, D.; Lühken, R.; van der Jeugd, H.; Garigliany, M.; Ziegler, U.; Keller, M.; Lahoreau, J.; Lachmann, L.; Becker, N.; Kik, M.; et al. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Eurosurveillance 2017, 22, 30452. [Google Scholar] [CrossRef] [PubMed]
- Sieg, M.; Schmidt, V.; Ziegler, U.; Keller, M.; Höper, D.; Heenemann, K.; Vahlenkamp, T.W. Outbreak and cocirculation of three different Usutu virus strains in eastern Germany. Vector Borne Zoonotic Dis. 2017, 17, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Michel, F.; Sieg, M.; Fischer, D.; Keller, M.; Eiden, M.; Reuschel, M.; Ziegler, U. Evidence for West Nile virus and Usutu virus infections in wild and resident birds in Germany, 2017 and 2018. Viruses 2019, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Werner, D.; Kampen, H. Zoos and wildlife parks: A laboratory for the study of mosquito-borne wildlife diseases. In Ecology of Diseases Transmitted by Mosquitoes to Wildlife—Ecology and Control of Vector-Borne Diseases; Gutiérrez-López, R., Logan, J.G., Martínez-de la Puente, J., Eds.; Academic Publishers: Wageningen, The Netherlands, 2022; Volume 7, pp. 81–93. [Google Scholar] [CrossRef]
- Rossi, S.L.; Ross, T.M.; Evans, J.D. West Nile virus. Clin. Lab. Med. 2010, 30, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef]
- Pietsch, C.; Michalski, D.; Münch, J.; Petros, S.; Bergs, S.; Trawinski, H.; Lübbert, C.; Liebert, U. Autochthonous West Nile virus infection outbreak in humans, Leipzig, Germany, August to September 2020. Eurosurveillance 2020, 25, 2001786. [Google Scholar] [CrossRef]
- Ziegler, U.; Bergmann, F.; Fischer, D.; Müller, K.; Holicki, C.M.; Sadeghi, B.; Sieg, M.; Keller, M.; Schwehn, R.; Reuschel, M.; et al. Spread of West Nile virus and Usutu virus in the German bird population, 2019–2020. Microorganisms 2022, 10, 807. [Google Scholar] [CrossRef]
- Ziegler, U.; Santos, P.D.; Groschup, M.H.; Hattendorf, C.; Eiden, M.; Höper, D.; Eisermann, P.; Keller, M.; Michel, F.; Klopfleisch, R.; et al. West Nile virus epidemic in Germany triggered by epizootic emergence, 2019. Viruses 2020, 12, 448. [Google Scholar] [CrossRef]
- Sauer, F.; Jaworski, L.; Lühken, R.; Kiel, E. Impacts of sampling rhythm and exposition on the effectiveness of artificial resting shelters for mosquito collection in northern Germany. J. Vector. Ecol. 2020, 45, 142–146. [Google Scholar] [CrossRef]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes: Identification, Ecology and Control, 3rd ed.; Springer Nature: Cham, Switzerland, 2020; pp. 103–122. [Google Scholar]
- Ries, C.; Sharav, T.; Tseren-Ochir, E.O.; Beer, M.; Hoffmann, B. Putative novel serotypes 33 and 35 in clinically healthy small ruminants in Mongolia expand the group of atypical BTV. Viruses 2020, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Proft, J.; Maier, W.A.; Kampen, H. Identification of six sibling species of the Anopheles maculipennis complex (Diptera: Culicidae) by a polymerase chain reaction assay. Parasitol. Res. 1999, 85, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Kronefeld, M.; Werner, D.; Kampen, H. PCR identification and distribution of Anopheles daciae (Diptera, Culicidae) in Germany. Parasitol. Res. 2014, 113, 2079–2086. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, M.; Czajka, C.; Börstler, J.; Melaun, C.; Jöst, H.; Thien, H.; von Badusche, M.; Becker, N.; Schmidt-Chanasit, J.; Krüger, A.; et al. First nationwide surveillance of Culex pipiens complex and Culex torrentium mosquitoes demonstrated the presence of Culex pipiens biotype pipiens/molestus hybrids in Germany. PLoS ONE 2013, 8, e71832. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 5, 294–299. [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Vina-Rodriguez, A.; Sachse, K.; Ziegler, U.; Chaintoutis, S.C.; Keller, M.; Groschup, M.H.; Eiden, M. A novel pan-Flavivirus detection and identification assay based on RT-qPCR and microarray. BioMed. Res. Int. 2017, 2017, 4248756. [Google Scholar] [CrossRef]
- Lambert, A.J.; Lanciotti, R.S. Consensus amplification and novel multiplex sequencing method for S segment species identification of 47 viruses of the Orthobunyavirus, Phlebovirus, and Nairovirus genera of the family Bunyaviridae. J. Clin. Microbiol. 2009, 47, 2398–2404. [Google Scholar] [CrossRef]
- Eshoo, M.W.; Whitehouse, C.A.; Zoll, S.T.; Massire, C.; Pennella, T.D.; Blyn, L.B.; Sampath, R.; Hall, T.A.; Ecker, J.A.; Desai, A.; et al. Direct broad-range detection of alphaviruses in mosquito extracts. Virology 2007, 368, 286–295. [Google Scholar] [CrossRef]
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). West Nile Virus-Resources. Available online: http://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html (accessed on 9 October 2023).
- Eiden, M.; Ziegler, U.; Keller, M.; Müller, K.; Granzow, H.; Jöst, H.; Schmidt-Chanasit, J.; Groschup, M.H. Isolation of Sindbis virus from a hooded crow in Germany. Vector Borne Zoonotic Dis. 2014, 14, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Fischer, D.; Eiden, M.; Reuschel, M.; Müller, K.; Schwehn, R.; Schmidt, V.; Groschup, M.H.; Keller, M. Sindbis virus—A wild bird associated zoonotic arbovirus circulates in Germany. Vet. Microbiol. 2019, 239, 108453. [Google Scholar] [CrossRef] [PubMed]
- Jansen, S.; Lühken, R.; Helms, M.; Pluskota, B.; Pfitzner, W.P.; Oerther, S.; Becker, N.; Schmidt-Chanasit, J.; Heitmann, A. Vector competence of mosquitoes from Germany for Sindbis virus. Viruses 2022, 14, 2644. [Google Scholar] [CrossRef] [PubMed]
- Modlmaier, M.; Kuhn, R.; Kaaden, O.R.; Pfeffer, M. Transmission studies of a European Sindbis virus in the floodwater mosquito Aedes vexans (Diptera: Culicidae). Int. J. Med. Microbiol. 2002, 3, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A.C.; Mueller, S.; Guenther, K.; Shult, P. Assessing the dilution effect of specimen pooling on the sensitivity of SARS-CoV-2 PCR tests. J. Med. Virol. 2021, 93, 1568–1572. [Google Scholar] [CrossRef] [PubMed]
- Bergman, A.; Dahl, E.; Lundkvist, Å.; Hesson, J.C. Sindbis virus infection in non-blood-fed hibernating Culex pipiens mosquitoes in Sweden. Viruses 2020, 12, 1441. [Google Scholar] [CrossRef] [PubMed]
- Hesson, J.C.; Verner-Carlsson, J.; Larsson, A.; Ahmed, R.; Lundkvist, Å.; Lundström, J.O. Culex torrentium mosquito role as major enzootic vector defined by rate of Sindbis virus infection, Sweden, 2009. Emerg. Infect. Dis. 2015, 21, 875. [Google Scholar] [CrossRef] [PubMed]
- Holicki, C.M.; Scheuch, D.E.; Ziegler, U.; Lettow, J.; Kampen, H.; Werner, D.; Groschup, M.H. German Culex pipiens biotype molestus and Culex torrentium are vector-competent for Usutu virus. Parasit. Vectors 2020, 13, 625. [Google Scholar] [CrossRef]
- Körsten, C.; Al-Hosary, A.A.; Holicki, C.M.; Schäfer, M.; Tews, B.A.; Vasić, A.; Ziegler, U.; Groschup, M.H.; Silaghi, C. Simultaneous coinfections with West Nile virus and Usutu virus in Culex pipiens and Aedes vexans mosquitoes. Transbound. Emerg. Dis. 2023, 2023, 6305484. [Google Scholar] [CrossRef]
- Frank, C.; Offergeld, R.; Lachmann, R.; Stark, K.; Schmidt-Chanasit, J. Saison stechmückenübertragener Krankheitserreger beginnt. Epidemiol. Bull. 2023, 22, 3–7. [Google Scholar] [CrossRef]
- Jaworski, L.; Sauer, F.; Jansen, S.; Tannich, E.; Schmidt-Chanasit, J.; Kiel, E.; Lühken, R. Artificial resting sites: An alternative sampling method for adult mosquitoes. Med. Vet. Entomol. 2022, 36, 139–148. [Google Scholar] [CrossRef] [PubMed]

| Primer/Probe | Sequence | Amplicon Length [bp] | Target Region | Virus Target | Reference |
|---|---|---|---|---|---|
| PFlav-fAAR | 5′-TACAACATGATGGGAAAGAGAGAGAARAA-3′ | 266 | NS5 | Flaviviridae | [53] |
| PFlav-rKR | 5′-GTGTCCCAKCCRGCTGTGTCATC-3′ | ||||
| WN-LCV-F1 | 5′-GTGATCCATGTAAGCCCTCAGAA-3′ | 76 | 3′-UTR | WNV line-ages 1 and 2 | [56] |
| WN-LCV-R1 | 5′-GTCTGACATTGGGCTTTGAAGTTA-3′ | ||||
| USU-F | 5′-ACGGCCCAAGCGAACAGAC-3′ | 91 | 3′-UTR | USUV | |
| USU-R | 5′-GGCTTGGGCCGCACCTAA-3′ | ||||
| WN-LCV-S1 | 5′-FAM-AGGACCCCACATGTT-3′MGB | – | WNV lineage 1 | ||
| WN-LCV-S2 | 5′-VIC-AGGACCCCACGTGCT-3′MGB | – | WNV lineage 2 | ||
| USU-S | 5′-CY5-CGAACTGTTCGTGGAAGG-3′BBQ | – | USUV | ||
| Cal/Bwa forward | 5′-GCAAATGGATTTGATCCTGATGCAG-3′ | 210 | N ORF | Peribunya-viridae | [54] |
| Cal/Bwa reverse | 5′-TTGTTCCTGTTTGCTGGAAAATGAT-3′ | ||||
| Bun forward | 5′-CTGCTAACACCAGCAGTACTTTTGAC-3′ | 250 | |||
| Bun reverse | 5′-TGGAGGGTAAGACCATCGTCAGGAACTG-3′ | ||||
| Wyeomyia forward | 5′-ATGTCTGAAATTGTATTTGATGATATTGG-3′ | 230 | |||
| Wyeomyia reverse | 5′-TATTTCGATTCCCCGGAAAGT-3′ | ||||
| Oropouche forward | 5′-GGCCCATGGTTGACCTTACTTT-3′ | 300 | |||
| Oropouche reverse | 5′-ACCAAAGGGAAGAAAGTGAAT-3′ | ||||
| VIR2052F | 5′-TGGCGCTATGATGAAATCTGGAATGTT-3′ | 139 | nsP4 | Alphavirus (Togaviridae) | [55] |
| VIR2052R | 5′-TACGATGTTGTCGTCGCCGATGAA-3′ |
| Year | Location | Total no. of Tested Mosquitoes | No. of Tested Single Mosquitoes | No. of Tested Pools | Virus-RNA Found | Number of Positive Specimens/Pools | Minimum Infection Rate |
|---|---|---|---|---|---|---|---|
| 2019 | Beerfelde | 30 | 12 | 9 | SINV | 1 | 33.3 |
| Berlin | 83 | 24 | 14 | SINV | 3 | 36.1 | |
| Dannenreich | 75 | 4 | 13 | SINV | 1 | 13.3 | |
| Groß Kreutz | 340 | 4 | 60 | SINV | 1 | 2.9 | |
| Groß Kreutz | 340 | 4 | 60 | USUV | 1 | 2.9 | |
| 2020 | Berlin | 238 | 26 | 25 | SINV | 1 | 4.2 |
| Eggenstein-Leopoldshafen | 815 | 628 | 35 | SINV | 1 | 1.2 | |
| Kunsterspring | 91 | 25 | 12 | SINV | 1 | 11.0 | |
| Moos | 1318 | 763 | 111 | SINV | 2 | 1.5 | |
| Berlin | 238 | 26 | 25 | USUV | 1 | 4.2 | |
| Dresden | 68 | 11 | 20 | USUV | 1 | 14.7 | |
| Schorfheide | 67 | 25 | 13 | USUV | 1 | 14.9 | |
| Bernburg | 319 | 87 | 32 | WNV | 1 | 3.1 | |
| Dresden | 68 | 11 | 20 | WNV | 1 | 14.7 | |
| Magdeburg | 66 | 8 | 12 | WNV | 1 | 15.2 | |
| 2021 | Aken | 388 | 18 | 47 | SINV | 1 | 2.6 |
| Bernburg | 574 | 133 | 61 | SINV | 1 | 1.7 | |
| Eggenstein-Leopoldshafen | 837 | 66 | 90 | SINV | 2 | 2.4 | |
| Gera | 84 | 4 | 14 | SINV | 1 | 11.9 | |
| Goldenstedt | 1184 | 78 | 154 | SINV | 2 | 1.7 | |
| Irgenöd | 488 | 122 | 45 | SINV | 1 | 2.1 | |
| Kunsterspring | 131 | 22 | 22 | SINV | 1 | 7.6 | |
| Neustrelitz | 78 | 20 | 13 | SINV | 1 | 12.8 | |
| Angermünde | 839 | 43 | 111 | USUV | 1 | 1.2 | |
| Berlin | 245 | 23 | 34 | USUV | 1 | 4.1 | |
| Magdeburg | 606 | 21 | 76 | WNV | 7 | 11.6 | |
| Wittenberg | 2374 | 29 | 172 | WNV | 1 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rau, J.; Köchling, K.; Schäfer, M.; Tews, B.A.; Wylezich, C.; Schaub, G.A.; Werner, D.; Kampen, H. Viral RNA in Mosquitoes (Diptera: Culicidae) Collected between 2019 and 2021 in Germany. Viruses 2023, 15, 2298. https://doi.org/10.3390/v15122298
Rau J, Köchling K, Schäfer M, Tews BA, Wylezich C, Schaub GA, Werner D, Kampen H. Viral RNA in Mosquitoes (Diptera: Culicidae) Collected between 2019 and 2021 in Germany. Viruses. 2023; 15(12):2298. https://doi.org/10.3390/v15122298
Chicago/Turabian StyleRau, Janine, Katharina Köchling, Mandy Schäfer, Birke A. Tews, Claudia Wylezich, Günter A. Schaub, Doreen Werner, and Helge Kampen. 2023. "Viral RNA in Mosquitoes (Diptera: Culicidae) Collected between 2019 and 2021 in Germany" Viruses 15, no. 12: 2298. https://doi.org/10.3390/v15122298





