Red and Blue Light Induce Soybean Resistance to Soybean Mosaic Virus Infection through the Coordination of Salicylic Acid and Jasmonic Acid Defense Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Virus Inoculation, and Light Treatment
2.2. Determination of the Phenotypic and Physiological Parameters
2.3. Sample Collection and Sequencing
2.4. Read Alignment and Expression Analysis
2.5. Functional Enrichment Analysis of DEGs
2.6. Validation of Gene Expression via qRT-PCR
2.7. Statistical Analysis
3. Results and Discussion
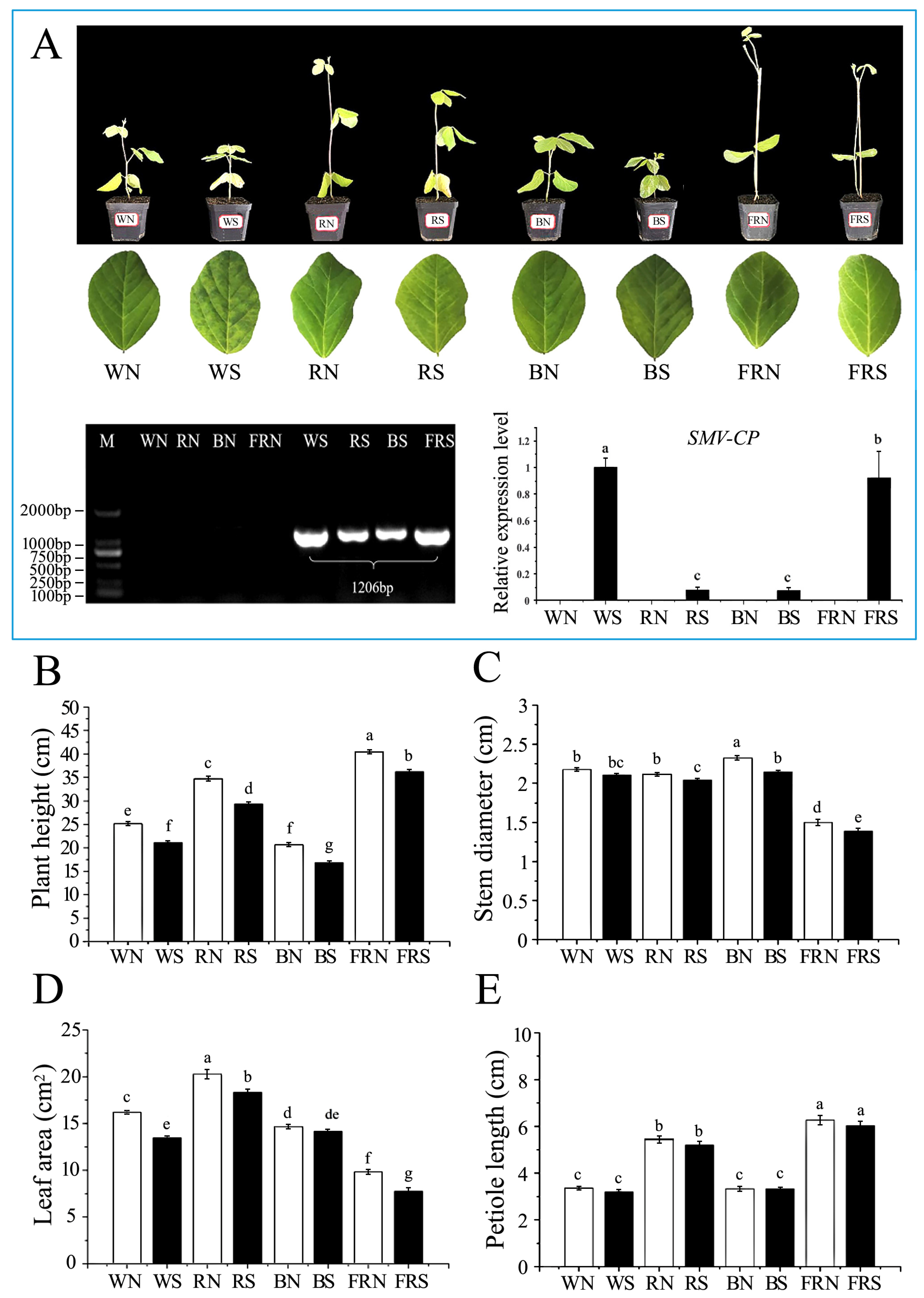
3.1. Phenotype and Virus Content of Infected Soybeans
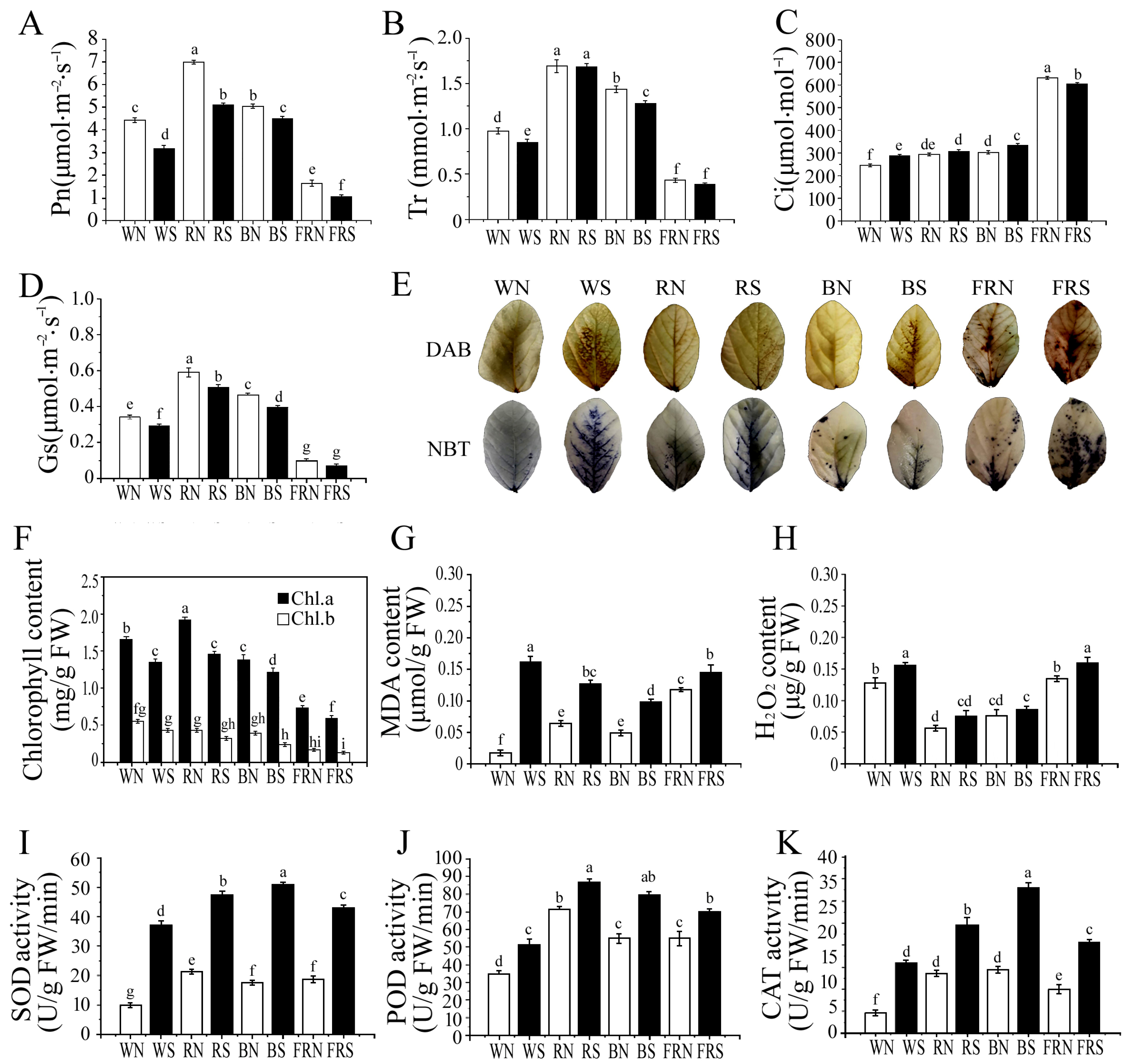
3.2. Determination of Photosynthetic Parameters and Chlorophyll Content
3.3. Histochemical Staining and Membrane Damage Index Determination
3.4. Determination of Enzyme Activity
3.5. Statistical Analysis of Differentially Expressed Genes
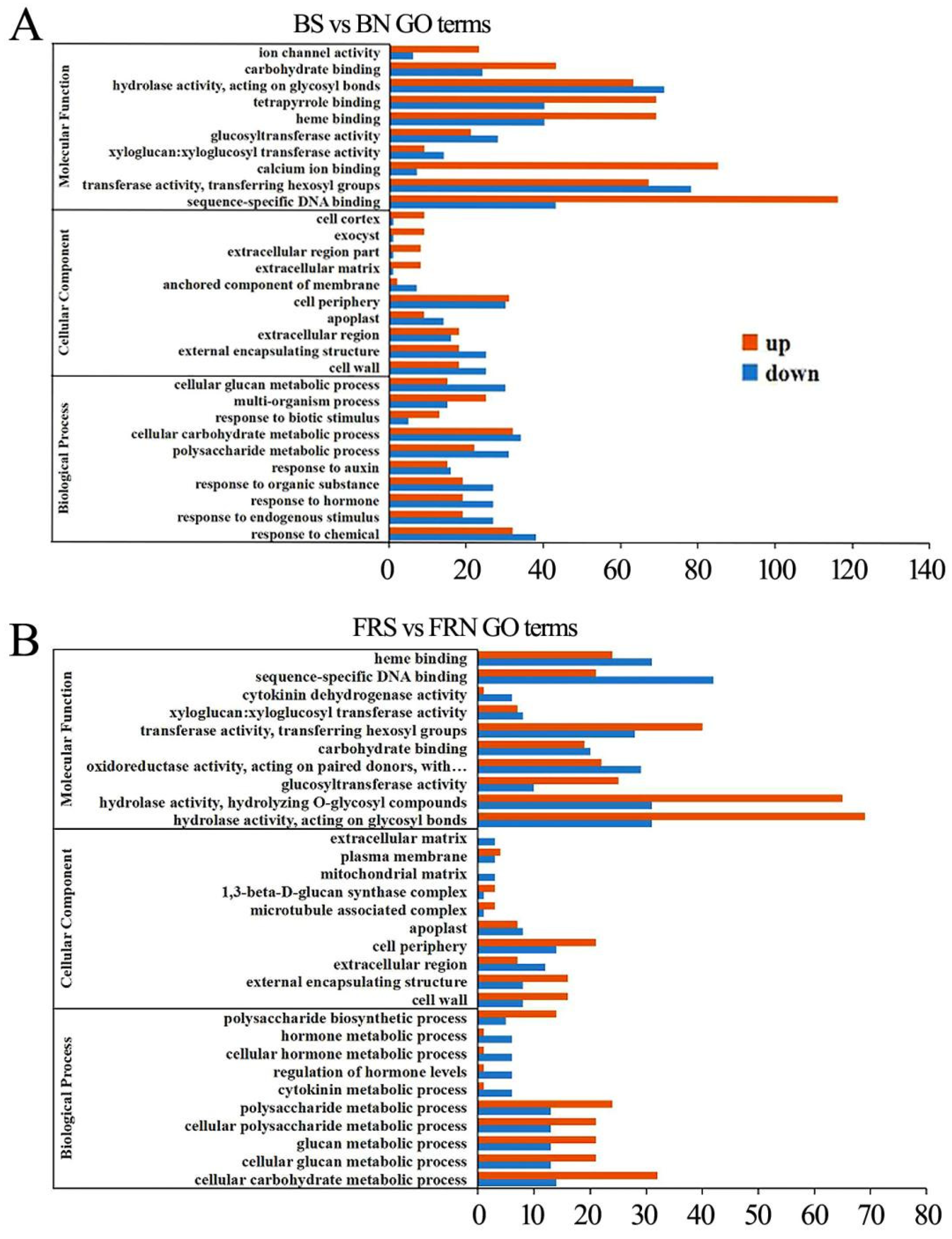
3.6. GO Function Enrichment Analysis of DEGs
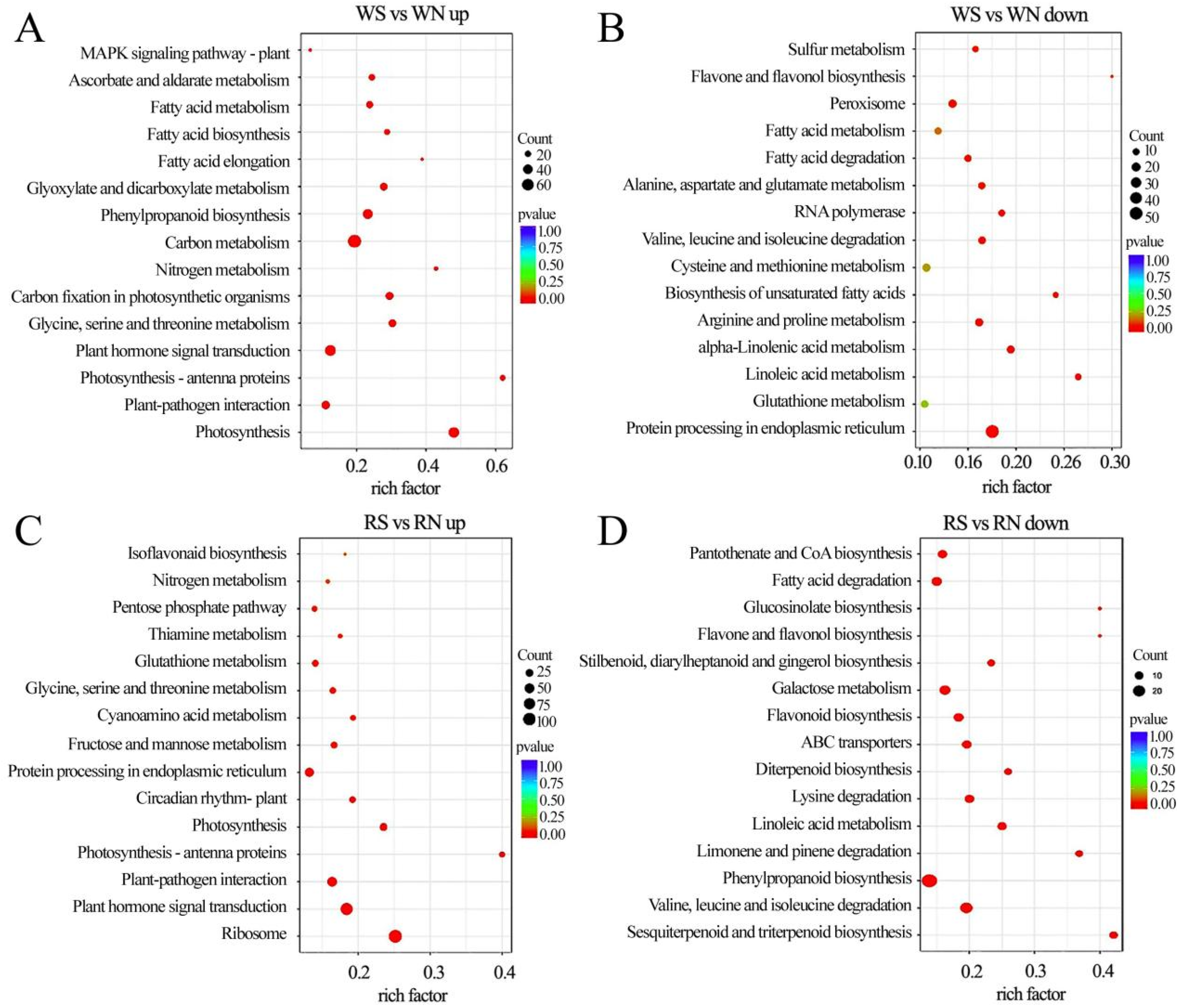
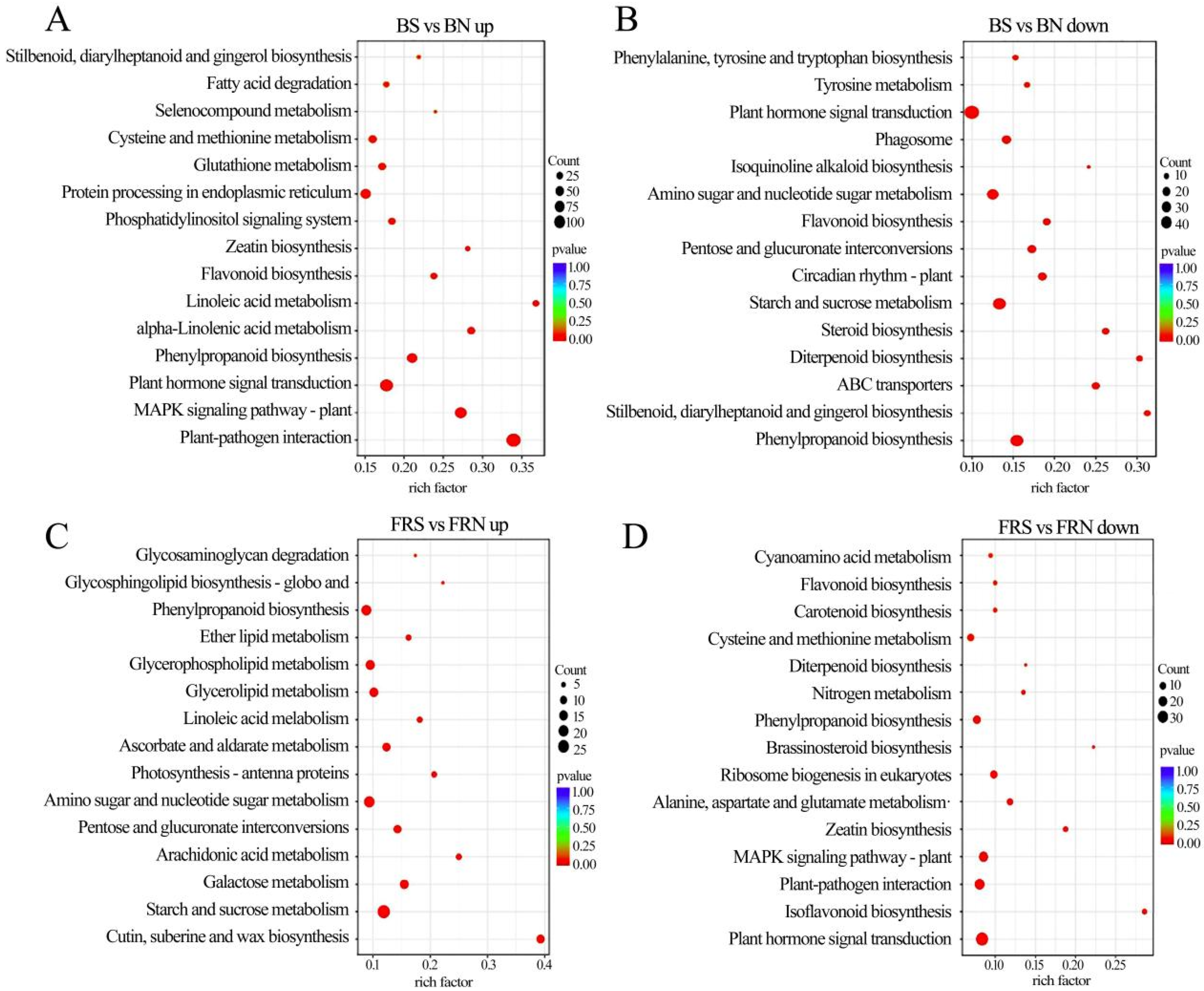
3.7. KEGG Pathway Enrichment Analysis of DEGs
3.8. DEGs Involved in Plant–Pathogen Interaction
3.9. DEGs Involved in Plant Hormone Signal Transduction
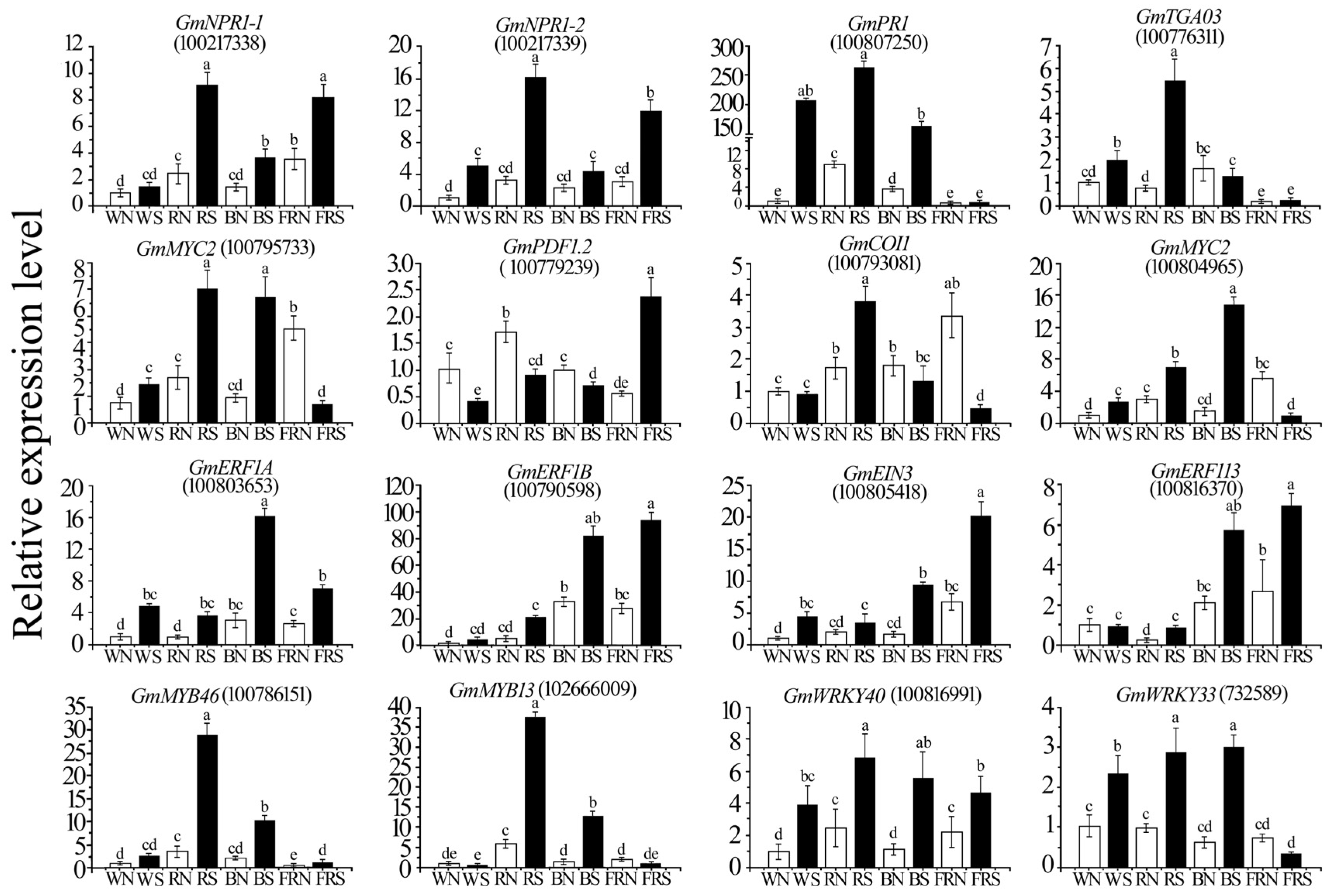
3.10. Validation of RNA-Seq Data by qRT-PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Z.; Huang, Y.; Yang, J.; Yao, S.; Zhao, K.; Wang, D.; Qin, Q.; Bian, Z.; Li, Y.; Lan, Y.; et al. Jasmonate signaling enhances RNA silencing and antiviral defense in rice. Cell Host Microbe. 2020, 28, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, F.; Meng, Z.; Lin, H.H.; Xi, D.H. Effects of light quality on the interaction between Cucumber mosaic virus and Nicotiana tabacum. J. Phytopathol. 2015, 163, 1002–1013. [Google Scholar] [CrossRef]
- Kook, H.S.; Park, S.H.; Jang, Y.J.; Lee, G.W.; Kim, J.S.; Kim, H.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Blue LED (light-emitting diodes)-mediated growth promotion and control of Botrytis disease in lettuce. Acta Agric. Scand. Sect. B 2013, 63, 271–277. [Google Scholar]
- Hui, X.U.; Yan-Nan, F.U.; Tian-Lai, L.I.; Wang, R. Effects of different LED light wavelengths on the resistance of tomato against Botrytis cinerea and the corresponding physiological mechanisms. J. Int. Agricul. 2017, 16, 106–114. [Google Scholar]
- Yu, S.M.; Ramkumar, G.; Lee, Y.H. Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plants. J. Appl. Microbiol. 2013, 115, 509–516. [Google Scholar] [CrossRef] [PubMed]
- de Wit, M.; Spoel, S.H.; Sanchez-Perez, G.F.; Gommers, C.M.M.; Pieterse, C.M.J.; Voesenek, L.A.C.J.; Pierik, R. Perception of low red:Far-red ratio compromises both salicylic acid- and jasmonic acid-dependent pathogen defences in Arabidopsis thaliana. Plant J. 2013, 75, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, Y.P.; Yu, H.J.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q. Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur. J. Plant Pathol. 2010, 127, 125–135. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Xing, Q.; Zhao, Y.; Yu, B.; Ma, Y.; Wang, F.; Qi, H.Y. PIF8-WRKY42-mediated salicylic acid synthesis modulates red light induced powdery mildew resistance in oriental melon. Plant Cell Environ. 2023, 46, 1726–1742. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S.; Stryjewski, E.C. Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. 1997, 79, 273–282. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Honda, Y.; Arase, S. Red-light-induced resistance in broad bean (Vicia faba L.) to leaf spot disease caused by Alternaria tenuissima. J. Phytopathol. 2003, 151, 86–91. [Google Scholar] [CrossRef]
- Islam, S.; Babadoost, M.; Honda, Y. Effect of red light treatment of seedlings of pepper, pumpkin and tomato on the occurrence of Phytophthora damping-off. HortScience 2002, 37, 678–681. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Khanam, H.; Ueno, M.; Kihara, J.; Honda, Y.; Arase, S. Suppression by red light irradiation of Corynespora leaf spot of cucumber caused by Corynespora cassiicola. J. Phytopathol. 2010, 158, 378–381. [Google Scholar] [CrossRef]
- Khanam, N.N.; Ueno, M.; Kihara, J.; Honda, Y.; Arase, S. Suppression of red light-induced resistance in broad beans to Botrytis cinerea by salicylic acid. Physiol. Mol. Plant Pathol. 2005, 66, 20–29. [Google Scholar] [CrossRef]
- Montes, N.; Pagán, I. Light Intensity modulates the efficiency of virus seed transmission through modifications of plant tolerance. Plants 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Chen, X.; Zhai, Y.; Cui, W.; Ai, X.; Rao, S.; Chen, J.; Yan, F. Downregulation of light-harvesting complex II induces ROS-mediated defense against Turnip mosaic virus infection in Nicotiana benthamiana. Front. Microbiol. 2021, 12, 690988. [Google Scholar] [CrossRef] [PubMed]
- Jeong, R.D.; Chandra-Shekara, A.C.; Barman, S.R.; Navarre, D.; Klessig, D.F.; Kachroo, A.; Kachroo, P. Cryptochrome 2 and phototropin 2 regulate resistance protein-mediated viral defense by negatively regulating an E3 ubiquitin ligase. Proc. Natl. Acad. Sci. USA 2010, 107, 13538–13543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, D.; Zhao, P.; Sun, Y.; Fang, R.X.; Ye, J. Near-infrared light and PIF4 promote plant antiviral defense by enhancing RNA interference. Plant Commun. 2023, 30, 100644. [Google Scholar] [CrossRef]
- Breen, S.; McLellan, H.; Birch, P.R.J.; Gilroy, E.M. Tuning the Wavelength: Manipulation of Light Signaling to Control Plant Defense. Int. J. Mol. Sci. 2023, 24, 3803. [Google Scholar] [CrossRef]
- Sanders, D.; Kehoe, R.; Tiley, K.; Bennie, J.; Cruse, D.; Davies, T.W.; Frank van Veen, F.J.; Gaston, K.J. Artificial nighttime light changes aphid-parasitoid population dynamics. Sci. Rep. 2015, 5, 15232. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Shekoofa, A.; Pessarakli, M. Reactive Oxygen Species (ROS) Generation and Detoxifying in Plants. J. Plant Nutr. 2014, 37, 1573–1585. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Jing, X.; Wang, H.; Gong, B.; Liu, S.; Wei, M.; Ai, X.; Li, Y.; Shi, Q. Secondary and sucrose metabolism regulated by different light quality combinations involved in melon tolerance to powdery mildew. Plant Physiol. Biochem. 2018, 124, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Wu, C.; Ahammed, G.J.; Wu, C.; Yang, Z.M.; Wan, C.P.; Chen, J.Y. Red light-Induced systemic resistance against root-knot nematode is mediated by a coordinated regulation of salicylic acid, jasmonic acid and redox signaling in watermelon. Front. Plant Sci. 2018, 9, 899. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Ren, H.; Deng, X.G.; Li, Y.N.; Cha, W.Q.; Lin, H.H.; Xi, D.H. Effects of light intensity on the susceptibility of Nicotiana tabacum to Cucumber mosaic virus. J. Gen. Plant Pathol. 2015, 81, 399–408. [Google Scholar] [CrossRef]
- Ádám, A.L.; Nagy, Z.Á.; Kátayet, G.; Mergenthaler, E.; Viczián, O. Signals of Systemic Immunity in Plants: Progress and open questions. Int. J. Mol. Sci. 2018, 19, 1146. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, R.; Park, E.; Lee, S.W.; Baek, K.H. Soybean (Glycine max L. Merr.) sprouts germinated under red light irradiation induce disease resistance against bacterial rotting disease. PLoS ONE 2015, 10, e0117712. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. NPR1, all things considered. Curr. Opin. Plant Biol. 2004, 7, 547–552. [Google Scholar] [CrossRef]
- Ballaré, C.L. Light regulation of plant defense. Annu. Rev. Plant Biol. 2014, 65, 335–363. [Google Scholar] [CrossRef]
- Taheri, P.; Tarighi, S. Riboflavin induces resistance in rice against Rhizoctonia solani via jasmonate-mediated priming of phenylpropanoid pathway. J. Plant Physiol. 2010, 167, 201–208. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Cheng, Y.; Feng, L.; Wu, X.; Fan, Y.; Raza, M.A.; Wang, X.; Yong, T.; Liu, W.; et al. Low red/far-red ratio as a signal promotes carbon assimilation of soybean seedlings by increasing the photosynthetic capacity. BMC Plant Biol. 2020, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Delauré, S.L.; De Bolle, F.C.; Cammue, B.P. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006, 44, 393–416. [Google Scholar] [CrossRef] [PubMed]
- Groen, S.C.; Whiteman, N.K. The evolution of ethylene signaling in plant chemical ecology. J. Chem. Ecol. 2014, 40, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Molina, A.; Solano, R. Constitutive expression of ethylene-response-facctor1 in Arabidopsis thaliana confers resistance to several necrotrophic fungi. Plant J. 2002, 29, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Molina, A. Ethylene response factor 1 mediates Arabidopsis thaliana resistance to the soilborne fungus Fusarium oxysporum. Mol. Plant Microbe. Interact. 2004, 17, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Cerrudo, I.; Keller, M.M.; Cargnel, M.D.; Demkura, P.V.; de Wit, M.; Patitucci, M.S. Low red/far-red ratios reduce Arabidopsis thaliana resistance to Botrytis cinerea and jasmonate responses via a COI1-JAZ10-dependent, salicylic acid-independent mechanism. Plant Physiol. 2012, 158, 2042–2052. [Google Scholar] [CrossRef]
- Shang, J.; Xi, D.H.; Xu, F.; Wang, S.D.; Cao, S.; Xu, M.Y.; Zhao, P.P.; Wang, J.H.; Jia, S.D.; Zhang, Z.W.; et al. A broad-spectrum, efficient and nontransgenic approach to control plant viruses by application of salicylic acid and jasmonic acid. Planta 2011, 233, 299–308. [Google Scholar] [CrossRef]
- Shang, J.; Zhang, L.; Jia, Q.; Tang, Z.Q.; Yuan, S.; Yang, H.; Zhang, M.; Huang, Y. Early ROS accumulation in chloroplasts of nicotiana glutinosa infected by Cucumber mosaic virus. Int. J. Agric. Biol. 2019, 6, 149–154. [Google Scholar]
- Zhang, L.; Shang, J.; Wang, W.; Du, J.; Li, K.; Wu, X.; Yu, L.; Liu, C.; Khaskheli, M.I.; Yang, W. Comparison of transcriptome differences in soybean response to Soybean mosaic virus under normal light and in the shade. Viruses 2019, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Vásquez, A.; Salinas, P.; Holuigue, L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front. Plant Sci. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Chaouch, S.; Mhamdi, A.; Queval, G.; Zechmann, B.; Noctor, G. Functional analysis of Arabidopsis thaliana mutants points to novel roles for glutathione in coupling H2O2 to activation of salicylic acid accumulation and signaling. Antioxid. Redox Signal. 2013, 18, 2106–2121. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Q.; Shang, J.; Zhang, L.; Du, J.B.; Yang, H.; Zeng, S.H.; Li, P.L.; Bawa, G.; Yu, L.; Hou, X.X.; et al. Characterization of synergy between Cucumber mosaic virus and alternaria in Nicotiana tabacum. Physiol. Mol. Plant Pathol. 2019, 108, 10140–101404. [Google Scholar] [CrossRef]
- Yu, D.; Chen, C.; Chen, Z. Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 2001, 13, 1527–1540. [Google Scholar] [CrossRef]
- Courbier, S.; Snoek, B.L.; Kajala, K.; Li, L.; van Wees, S.C.M.; Pierik, R. Mechanisms of far-red light-mediated dampening of defense against Botrytis cinerea in tomato leaves. Plant Physiol. 2021, 187, 1250–1266. [Google Scholar] [CrossRef]
- Ndamukong, I.; Abdallat, A.A.; Thurow, C.; Fode, B.; Zander, M.; Weigel, R.; Gatz, C. SA-inducible Arabidopsis thaliana glutaredoxin interacts with TGA factors and suppresses JA-responsive PDF1.2 transcription. Plant J. 2007, 50, 128–139. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, H.; Sun, H.; Li, A.; Liu, S.; Yu, R.; Cui, X.; Zhang, D.; Wuriyanghan, H. Salicylic acid and broad spectrum of NBS-LRR family genes are involved in SMV-soybean interactions. Plant Physiol. Biochem. 2018, 123, 132–140. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, J.; Zhang, S.; Du, J.; Wang, W.; Li, K.; Yang, W. Red and Blue Light Induce Soybean Resistance to Soybean Mosaic Virus Infection through the Coordination of Salicylic Acid and Jasmonic Acid Defense Pathways. Viruses 2023, 15, 2389. https://doi.org/10.3390/v15122389
Shang J, Zhang S, Du J, Wang W, Li K, Yang W. Red and Blue Light Induce Soybean Resistance to Soybean Mosaic Virus Infection through the Coordination of Salicylic Acid and Jasmonic Acid Defense Pathways. Viruses. 2023; 15(12):2389. https://doi.org/10.3390/v15122389
Chicago/Turabian StyleShang, Jing, Siqi Zhang, Junbo Du, Wenming Wang, Kai Li, and Wenyu Yang. 2023. "Red and Blue Light Induce Soybean Resistance to Soybean Mosaic Virus Infection through the Coordination of Salicylic Acid and Jasmonic Acid Defense Pathways" Viruses 15, no. 12: 2389. https://doi.org/10.3390/v15122389
APA StyleShang, J., Zhang, S., Du, J., Wang, W., Li, K., & Yang, W. (2023). Red and Blue Light Induce Soybean Resistance to Soybean Mosaic Virus Infection through the Coordination of Salicylic Acid and Jasmonic Acid Defense Pathways. Viruses, 15(12), 2389. https://doi.org/10.3390/v15122389





