Epidemiology of Infectious Bursal Disease Virus in Poland during 2016–2022
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Detection and Identification of IBDV
2.3. Phylogenetic Analysis
2.4. Virus Isolation
3. Results
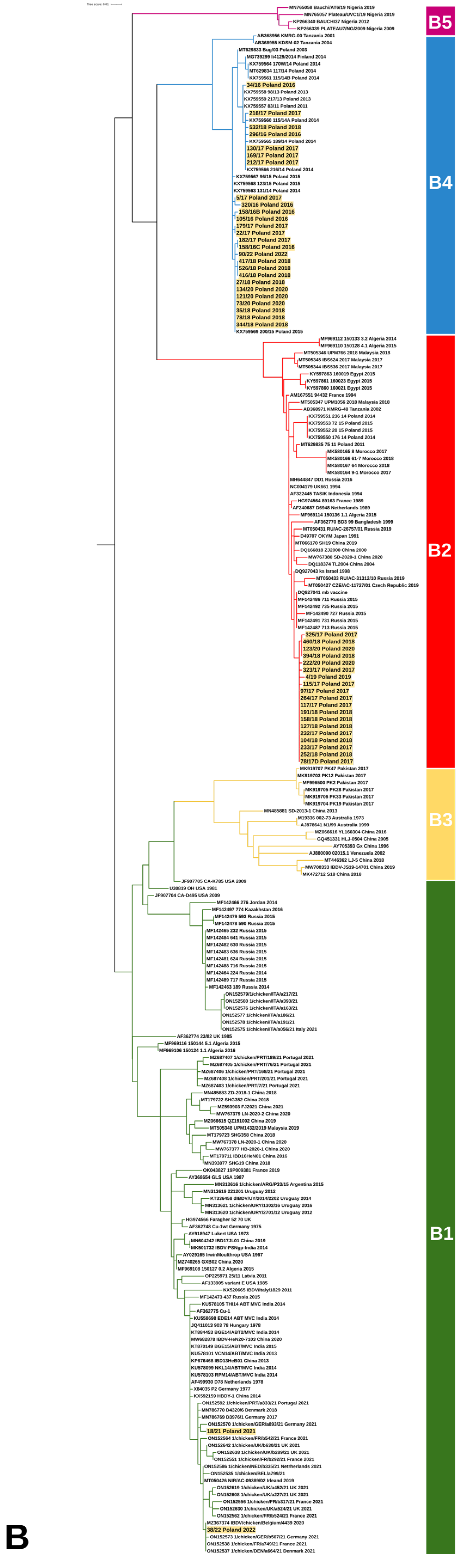
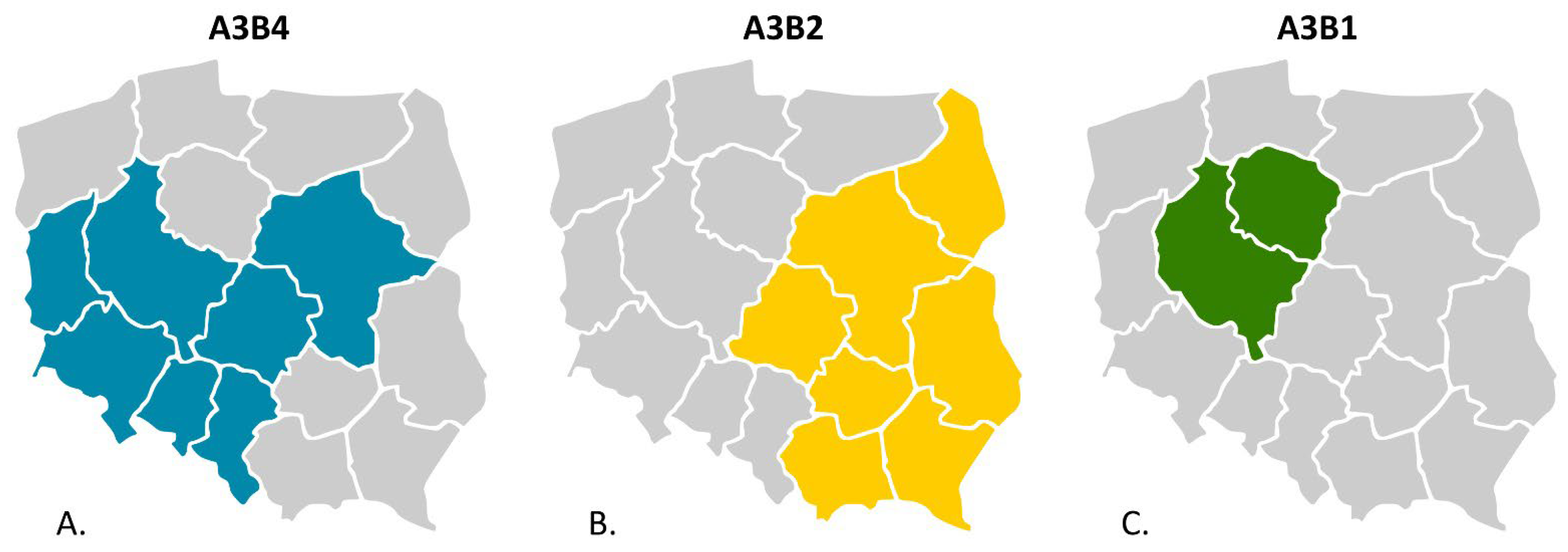
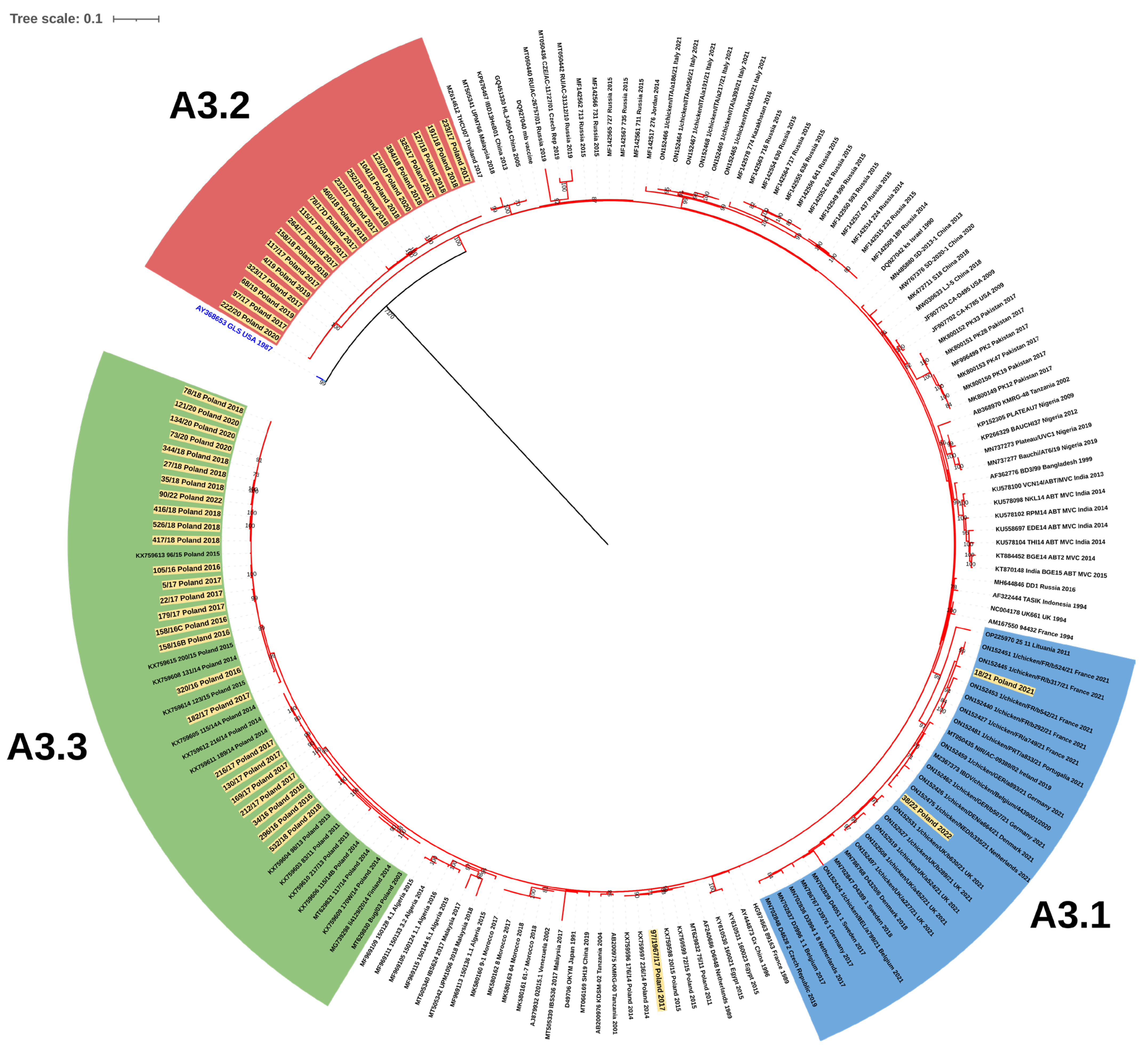
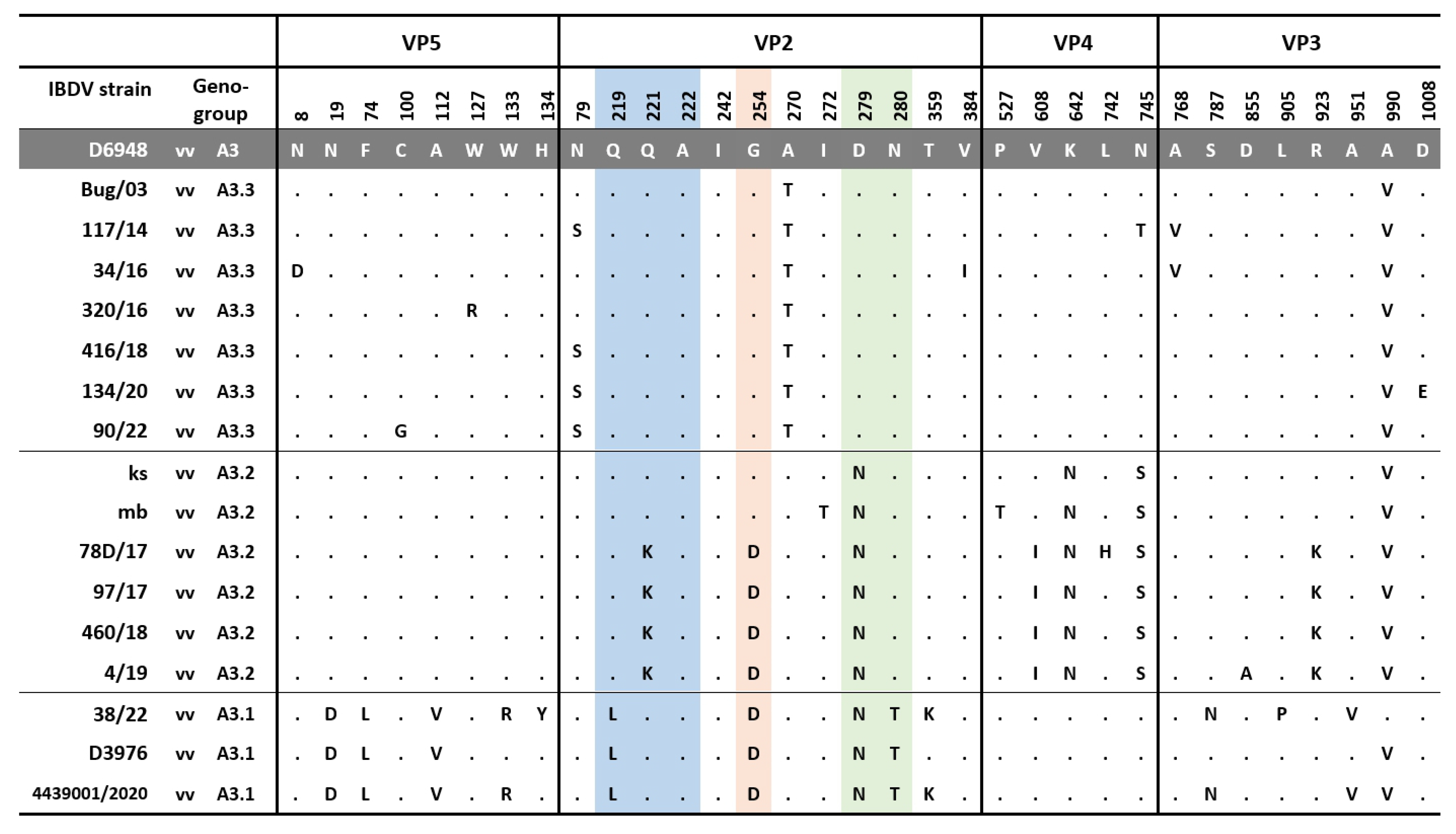
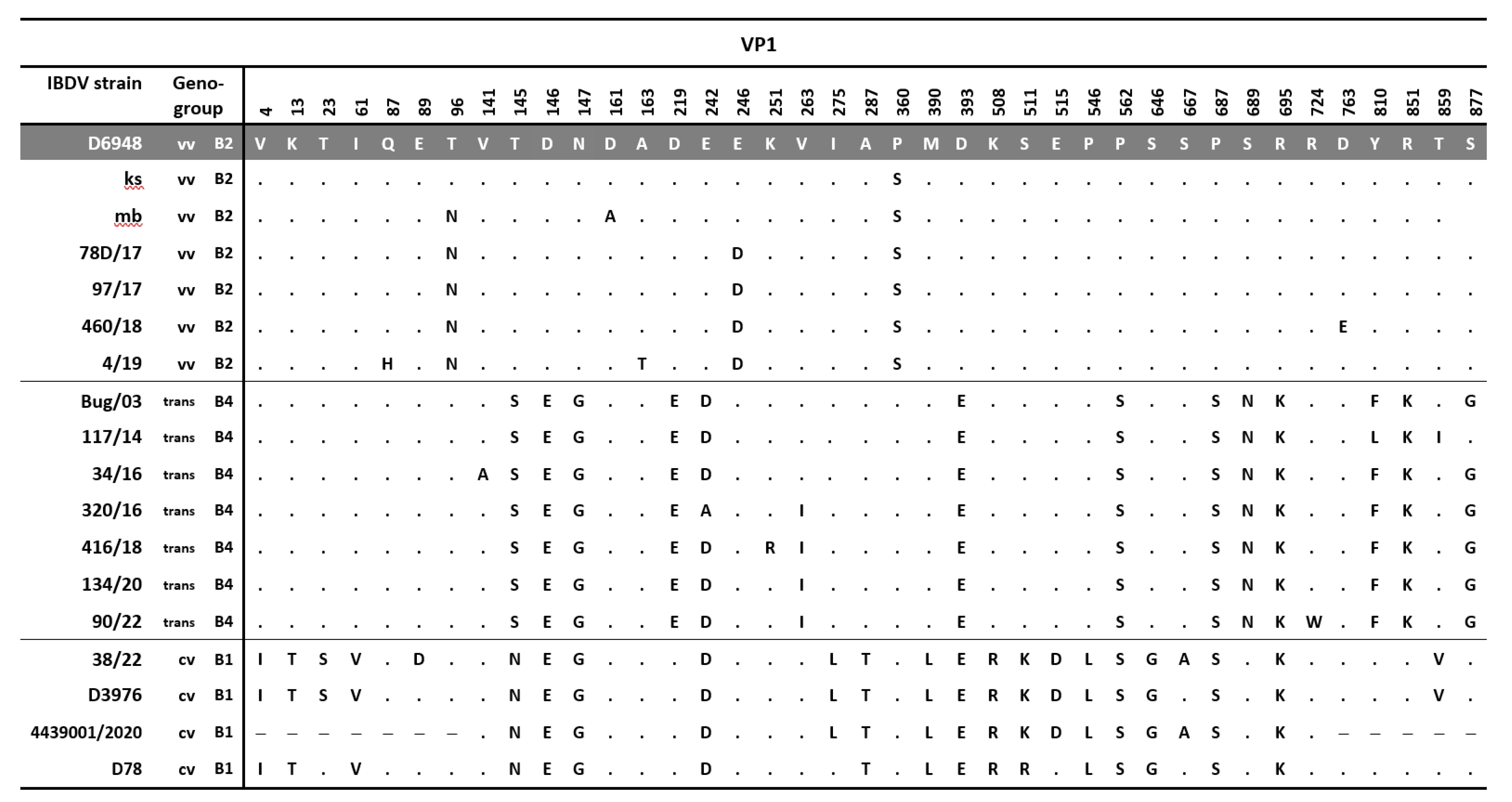
3.1. Molecular Detection, Genotyping, and Characterization of IBDV
3.2. Virus Isolation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirai, K.; Funakoshi, T.; Nakai, T.; Shimakura, S. Sequential changes in the number of surface immunoglobulin-bearing B lymphocytes in infectious bursal disease virus-infected chickens. Avian Dis. 1981, 25, 484–496. [Google Scholar] [CrossRef]
- Dey, S.; Pathak, D.C.; Ramamurthy, N.; Maity, H.K.; Chellappa, M.M. Infectious bursal disease virus in chickens: Prevalence, impact, and management strategies. Vet. Med. 2019, 10, 85–97. [Google Scholar] [CrossRef]
- Dobos, P.; Hill, B.J.; Hallett, R.; Kells, D.T.; Becht, H.; Teninges, D. Biophysical and biochemical characterization of five animal viruses with bisegmented double-stranded RNA genomes. J. Virol. 1979, 32, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Lejal, N.; Da Costa, B.; Huet, J.C.; Delmas, B. Role of Ser-652 and Lys-692 in the protease activity of infectious bursal disease virus VP4 and identification of its substrate cleavage sites. J. Gen. Virol. 2000, 81, 983–992. [Google Scholar] [CrossRef]
- Schnitzler, D.; Bernstein, F.; Muller, H.; Becht, H. The Genetic-Basis for the Antigenicity of the Vp2-Protein of the Infectious Bursal Disease Virus. J. Gen. Virol. 1993, 74, 1563–1571. [Google Scholar] [CrossRef]
- Van Loon, A.; de Haas, N.; Zeyda, I.; Mundt, E. Alteration of amino acids in VP2 of very virulent infectious bursal disease virus results in tissue culture adaptation and attenuation in chickens. J. Gen. Virol. 2002, 83, 121–129. [Google Scholar] [CrossRef]
- Von Einem, U.I.; Gorbalenya, A.E.; Schirrmeier, H.; Behrens, S.E.; Letzel, T.; Mundt, E. VP1 of infectious bursal disease virus is an RNA-dependent RNA polymerase. J. Gen. Virol. 2004, 85, 2221–2229. [Google Scholar] [CrossRef]
- Ye, C.; Wang, Y.; Zhang, E.; Han, X.; Yu, Z.; Liu, H. VP1 and VP3 Are Required and Sufficient for Translation Initiation of Uncapped Infectious Bursal Disease Virus Genomic Double-Stranded RNA. J. Virol. 2018, 92, e01345-17. [Google Scholar] [CrossRef] [PubMed]
- Le Nouen, C.; Toquin, D.; Muller, H.; Raue, R.; Kean, K.M.; Langlois, P.; Cherbonnel, M.; Eterradossi, N. Different Domains of the RNA Polymerase of Infectious Bursal Disease Virus Contribute to Virulence. PLoS ONE 2012, 7, e28064. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Nooruzzaman, M.; Rahman, T.; Mumu, T.T.; Rahman, M.M.; Chowdhury, E.H.; Eterradossi, N.; Muller, H. A unified genotypic classification of infectious bursal disease virus based on both genome segments. Avian Pathol. 2021, 50, 190–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Fan, L.J.; Jiang, N.; Gao, L.; Li, K.; Gao, Y.L.; Liu, C.J.; Cui, H.Y.; Pan, Q.; Zhang, Y.P.; et al. An improved scheme for infectious bursal disease virus genotype classification based on both genome-segments A and B. J. Integr. Agric. 2021, 20, 1372–1381. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Y.; Zhang, Y.; Qiao, Y.; Deng, Q.; Chen, R.; Chen, J.; Huang, T.; Wei, T.; Mo, M.; et al. The emerging naturally reassortant strain of IBDV (genotype A2dB3) having segment A from Chinese novel variant strain and segment B from HLJ 0504-like very virulent strain showed enhanced pathogenicity to three-yellow chickens. Transbound. Emerg. Dis. 2021, 69, e566–e579. [Google Scholar] [CrossRef]
- Legnardi, M.; Franzo, G.; Tucciarone, C.M.; Koutoulis, K.; Duarte, I.; Silva, M.; Le Tallec, B.; Cecchinato, M. Detection and molecular characterization of a new genotype of infectious bursal disease virus in Portugal. Avian Pathol. 2022, 51, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Wang, X.M.; Gao, Y.L.; Qi, X.L. The Over-40-Years-Epidemic of Infectious Bursal Disease Virus in China. Viruses 2022, 14, 2253. [Google Scholar] [CrossRef] [PubMed]
- Thai, T.N.; Jang, I.; Kim, H.A.; Kim, H.S.; Kwon, Y.K.; Kim, H.R. Characterization of antigenic variant infectious bursal disease virus strains identified in South Korea. Avian Pathol. 2021, 50, 174–181. [Google Scholar] [CrossRef]
- Wang, W.; He, X.; Zhang, Y.; Qiao, Y.; Shi, J.; Chen, R.; Chen, J.; Xiang, Y.; Wang, Z.; Chen, G.; et al. Analysis of the global origin, evolution and transmission dynamics of the emerging novel variant IBDV (A2dB1b): The accumulation of critical aa-residue mutations and commercial trade contributes to the emergence and transmission of novel variants. Transbound. Emerg. Dis. 2022, 69, e2832–e2851. [Google Scholar] [CrossRef]
- Myint, O.; Suwanruengsri, M.; Araki, K.; Izzati, U.Z.; Pornthummawat, A.; Nueangphuet, P.; Fuke, N.; Hirai, T.; Jackwood, D.J.; Yamaguchi, R. Bursa atrophy at 28 days old caused by variant infectious bursal disease virus has a negative economic impact on broiler farms in Japan. Avian Pathol. 2021, 50, 6–17. [Google Scholar] [CrossRef]
- Legnardi, M.; Franzo, G.; Tucciarone, C.M.; Koutoulis, K.; Cecchinato, M. Infectious bursal disease virus in Western Europe: The rise of reassortant strains as the dominant field threat. Avian Pathol. 2022, 52, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Mato, T.; Tatar-Kis, T.; Felfoldi, B.; Jansson, D.S.; Homonnay, Z.; Banyai, K.; Palya, V. Occurrence and spread of a reassortant very virulent genotype of infectious bursal disease virus with altered VP2 amino acid profile and pathogenicity in some European countries. Vet. Microbiol. 2020, 245, 108663. [Google Scholar] [CrossRef] [PubMed]
- Drissi Touzani, C.; Fellahi, S.; Fihri, O.F.; Gaboun, F.; Khayi, S.; Mentag, R.; Lico, C.; Baschieri, S.; El Houadfi, M.; Ducatez, M. Complete genome analysis and time scale evolution of very virulent infectious bursal disease viruses isolated from recent outbreaks in Morocco. Infect. Genet. Evol. 2020, 77, 104097. [Google Scholar] [CrossRef]
- Lachheb, J.; Jbenyeni, A.; Nsiri, J.; Larbi, I.; Ammouna, F.; El Behi, I.; Ghram, A. Full-length genome sequencing of a very virulent infectious bursal disease virus isolated in Tunisia. Poult. Sci. 2021, 100, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Arowolo, O.A.; George, U.E.; Luka, P.D.; Maurice, N.A.; Atuman, Y.J.; Shallmizhili, J.J.; Shittu, I.; Oluwayelu, D.O. Infectious bursal disease in Nigeria: Continuous circulation of reassortant viruses. Trop. Anim. Health Prod. 2021, 53, 271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Qi, X.L.; Gao, H.L.; Gao, Y.L.; Lin, H.; Song, X.Q.; Pei, L.; Wang, X.M. Comparative study of the replication of infectious bursal disease virus in DF-1 cell line and chicken embryo fibroblasts evaluated by a new real-time RT-PCR. J. Virol. Methods 2009, 157, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Pikuła, A.; Domanska-Blicharz, K.; Cepulis, R.; Smietanka, K. Identification of infectious bursal disease virus with atypical VP2 amino acid profile in Latvia. J. Vet. Res. 2017, 61, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Pikuła, A.; Lisowska, A.; Jasik, A.; Perez, L.J. The Novel Genetic Background of Infectious Bursal Disease Virus Strains Emerging from the Action of Positive Selection. Viruses 2021, 13, 396. [Google Scholar] [CrossRef]
- Tammiranta, N.; Ek-Kommonen, C.E.; Rossow, L.; Huovilainen, A. Circulation of very virulent avian infectious bursal disease virus in Finland. Avian Pathol. 2018, 47, 520–525. [Google Scholar] [CrossRef]
- Van Borm, S.; Steensels, M.; Mathijs, E.; Vandenbussche, F.; van den Berg, T.; Lambrecht, B. Metagenomic sequencing determines complete infectious bronchitis virus (avian Gammacoronavirus) vaccine strain genomes and associated viromes in chicken clinical samples. Virus Genes 2021, 57, 529–540. [Google Scholar] [CrossRef]
- Lazarus, D.; Pasmanik-Chor, M.; Gutter, B.; Gallili, G.; Barbakov, M.; Krispel, S.; Pitcovski, J. Attenuation of very virulent infectious bursal disease virus and comparison of full sequences of virulent and attenuated strains. Avian Pathol. 2008, 37, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. Production of Meat: Poultry. Available online: https://ec.europa.eu/eurostat/databrowser/view/tag00043/default/table?lang=en (accessed on 24 October 2022).
- Minta, Z.; Daniel, A. Infectious bursal disease virus in Poland: Current situation and vaccinal control. In Proceedings of the 1st International Symposium on Infectious Bursal Disease and Chicken Infectious Anemia, Rauischholzhausen, Germany, 21–24 June 1994; pp. 208–214. [Google Scholar]
- Pikuła, A.; Śmietanka, K.; Perez, L.J. Emergence and expansion of novel pathogenic reassortant strains of infectious bursal disease virus causing acute outbreaks of the disease in Europe. Transbound. Emerg. Dis. 2020, 67, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Molinet, A.; Courtillon, C.; Le Men, M.; Amenna-Bernard, N.; Retaux, C.; Leroux, A.; Lucas, P.; Blanchard, Y.; Nooruzzaman, M.; Islam, M.R.; et al. Full-Length Genome Sequence of a Novel European Antigenic Variant Strain of Infectious Bursal Disease Virus. Microbiol. Resour. Announc. 2022, 11, e0010222. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.L.; Cao, Y.; Yu, T.; Mo, W.C. Adaptation of very virulent infectious bursal disease virus to chicken embryonic fibroblasts by site-directed mutagenesis of residues 279 and 284 of viral coat protein VP2. J. Virol. 1999, 73, 2854–2862. [Google Scholar] [CrossRef] [PubMed]
- Toroghi, R.; Kataria, J.M.; Verma, K.C.; Kataria, R.S.; Tiwari, A.K. Amino acid changes in the variable region of VP2 in three infectious bursal disease viruses with different virulence, originating from a common ancestor. Avian Pathol. 2001, 30, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Mato, T.; Medveczki, A.; Kiss, I. Research Note: “Hidden” infectious bursal disease virus infections in Central Europe. Poult. Sci. 2022, 101, 101958. [Google Scholar] [CrossRef]
- Tomás, G.; Marandino, A.; Courtillon, C.; Amelot, M.; Keita, A.; Pikuła, A.; Hernández, M.; Hernández, D.; Vagnozzi, A.; Panzera, Y.; et al. Antigenicity, pathogenicity and immunosuppressive effect caused by a South American isolate of infectious bursal disease virus belonging to the “distinct” genetic lineage. Avian Pathol. 2019, 48, 245–254. [Google Scholar] [CrossRef]


| Primers | Positions 1 | Nucleotide Sequence (5′→3′) | Amplicon Size (bp) |
|---|---|---|---|
| Segment A | |||
| VP2-F | 512 | ACCTTCCAAGGAAGCCTGAGTG | 739 |
| VP2-R | 1251 | ATCAGCTCGAAGTTGCTCACC | |
| SegA0_F | 1 | GGATACGATCGGTCTGACCCCGGGGGAG | 3260 |
| SegA0_R | 3260 | GGGGACCCGCGAACGGATCCAAT | |
| Segment B | |||
| VP1.154F | 154 | TCAGCAGCGTTCGGCATAAAGC | 790 |
| VP1.998R | 998 | TTTGTCCCTGCACCCTGCTTCA | |
| SegB0_F | 1 | GGATACGATGGGTCTGACCCTCTG | 2827 |
| SegB0_R | 2827 | GGGGGCCCCCGCAGGCGAAG | |
| Strain | Genotype | Collection | Chicken | Vaccination | GenBank Accession no. | ||
|---|---|---|---|---|---|---|---|
| Date | Age | Type | (Vaccine Strain) | VP1 | VP2 | ||
| 34/16 | A3B4 | February 2016 | 5w | broiler | LIBDV | OP978030 | OP978040 |
| 105/16 | A3B4 | April 2016 | 42d | broiler | Lukert | OP978050 | OP978090 |
| 158/16B | A3B4 | June 2016 | 6w | broiler | NA | OP978051 | OP978091 |
| 158/16C | A3B4 | June 2016 | 4w | broiler | NA | OP978052 | OP978092 |
| 296/16 | A3B4 | November 2016 | 31d | broiler | W2512 & CH/80 | OP978053 | OP978093 |
| 320/16 | A3B4 | December 2016 | 6w | broiler | D78 | OP978031 | OP978041 |
| 5/17 | A3B4 | January 2017 | 4w | broiler | LC75 | OP978054 | OP978094 |
| 22/17 | A3B4 | January 2017 | 18w | layer | LIBDV | OP978055 | OP978095 |
| H287/17 | A0Bx | March 2017 | NA | broiler | NA | OP978056 | - |
| 78/17 | A3B2 | March 2017 | 43d | broiler | LC75 | OP978032 | OP978042 |
| 97/17 | A3B2 | April 2017 | NA | broiler | D78 | OP978033 | OP978043 |
| 97/1967/17 | A3Bx | April 2017 | NA | broiler | NA | OP978057 | - |
| 115/17 | A3B2 | April 2017 | 5.5w | broiler | GM97 | OP978058 | OP978096 |
| 117/17 | A3B2 | April 2017 | NA | broiler | W2512 & D78 | OP978059 | OP978097 |
| 130/17K10 | A3B4 | April 2017 | 31d | broiler | W2512 & CH/80 | OP978060 | OP978098 |
| 130/17K11 | A3B4 | April 2017 | 31d | broiler | W2512 & CH/80 | OP978061 | OP978098 |
| 169/17 | A3B4 | May 2017 | 25d | broiler | NA | OP978061 | OP978099 |
| 179/17 | A3B4 | June 2017 | 9w | layer | NA | OP978062 | OP978100 |
| 182/17 | A3B4 | June 2017 | 6w | broiler | Faragher 52/70 | OP978063 | OP978101 |
| 212/17 | A3B4 | June 2017 | NA | NA | NA | OP978064 | OP978102 |
| 216/17 | A3B4 | June 2017 | NA | NA | GM97 | OP978065 | OP978103 |
| 232/17 | A3B2 | July 2017 | 6w | broiler | GM97 | OP978066 | OP978104 |
| 233/17 | A3B2 | July 2017 | 6w | broiler | GM97 | OP978067 | OP978105 |
| 264/17 | A3B2 | August 2017 | 39d | broiler | GM97 & CH/80 | OP978068 | OP978106 |
| 323/17 | A3B2 | October 2017 | 41d | broiler | LIBDV | OP978069 | OP978107 |
| 325/17 | A3B2 | October 2017 | 39d | broiler | GM97 | OP978070 | OP978108 |
| 27/18 | A3B4 | February 2018 | 31d | broiler | NA | OP978071 | OP978109 |
| 35/18 | A3B4 | February 2018 | 5w | broiler | NA | OP978072 | OP978110 |
| 78/18 | A3B4 | March 2018 | 5w | broiler | NA | OP978073 | OP978111 |
| 104/18K5 | A3B2 | April 2018 | 5.5w | broiler | NA | OP978074 | OP978112 |
| 104/18K7 | A3B2 | April 2018 | 5w | broiler | NA | OP978074 | OP978112 |
| 127/18 | A3B2 | April 2018 | 49d | broiler | NA | OP978075 | OP978113 |
| 158/18 | A3B2 | May 2018 | 40d | layer | No vac1 | OP978076 | OP978114 |
| 191/18 | A3B2 | June 2018 | 32d | broiler | GM97 | OP978077 | OP978115 |
| 252/18 | A3B2 | July 2018 | NA | broiler | NA | OP978078 | OP978116 |
| 344/18 | A3B4 | July 2018 | 6w | layer | Faragher 52/70 | OP978079 | OP978117 |
| 394/18 | A3B2 | August 2018 | 32d | broiler | NA | OP97808 | OP978118 |
| 416/18 | A3B4 | September 2018 | 36d | broiler | W2512 & LIBDV | OP978034 | OP978044 |
| 417/18 | A3B4 | September 2018 | 32d | broiler | W2512 & LIBDV | OP978081 | OP978119 |
| 460/18 | A3B2 | October 2018 | 35d | broiler | W2512 | OP978035 | OP978045 |
| 526/18 | A3B4 | November 2018 | 7w | layer | CH/80 | OP978082 | OP978120 |
| 532/18 | A3B4 | November 2018 | 4.5w | broiler | W2512 | OP978083 | OP978121 |
| 4/19 | A3B2 | January 2019 | 5w | broiler | NA | OP978036 | OP978046 |
| 68/19 | A3Bx | February 2019 | NA | broiler | GM97 | OP978084 | - |
| 73/20 | A3B4 | April 2020 | 6w | layer | D78 | OP978085 | OP978122 |
| 121/20 | A3B4 | May 2020 | 39d | broiler | No vac1 | OP978086 | OP978123 |
| 123/20 | A3B2 | May 2020 | 6w | broiler | NA | OP97808 | OP978124 |
| 134/20 | A3B4 | June 2020 | NA | broiler | NA | OP978037 | OP978047 |
| 222/20 | A3B2 | August 2020 | 35d | broiler | NA | OP978088 | OP978125 |
| 18/21 | A3B1 | August 2021 | 42d | broiler | NA | OP978089 | OP978126 |
| 38/22 | A3B1 | March 2022 | 31d | broiler | LIBDV | OP978038 | OP978048 |
| 90/22 | A3B4 | June 2022 | 4w | layer | 1/65/PV | OP978039 | OP978049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pikuła, A.; Lisowska, A.; Domańska-Blicharz, K. Epidemiology of Infectious Bursal Disease Virus in Poland during 2016–2022. Viruses 2023, 15, 289. https://doi.org/10.3390/v15020289
Pikuła A, Lisowska A, Domańska-Blicharz K. Epidemiology of Infectious Bursal Disease Virus in Poland during 2016–2022. Viruses. 2023; 15(2):289. https://doi.org/10.3390/v15020289
Chicago/Turabian StylePikuła, Anna, Anna Lisowska, and Katarzyna Domańska-Blicharz. 2023. "Epidemiology of Infectious Bursal Disease Virus in Poland during 2016–2022" Viruses 15, no. 2: 289. https://doi.org/10.3390/v15020289
APA StylePikuła, A., Lisowska, A., & Domańska-Blicharz, K. (2023). Epidemiology of Infectious Bursal Disease Virus in Poland during 2016–2022. Viruses, 15(2), 289. https://doi.org/10.3390/v15020289






