An Old Acquaintance: Could Adenoviruses Be Our Next Pandemic Threat?
Abstract
:1. Introduction
2. History of HAdVs
3. HAdV Classification (Types)
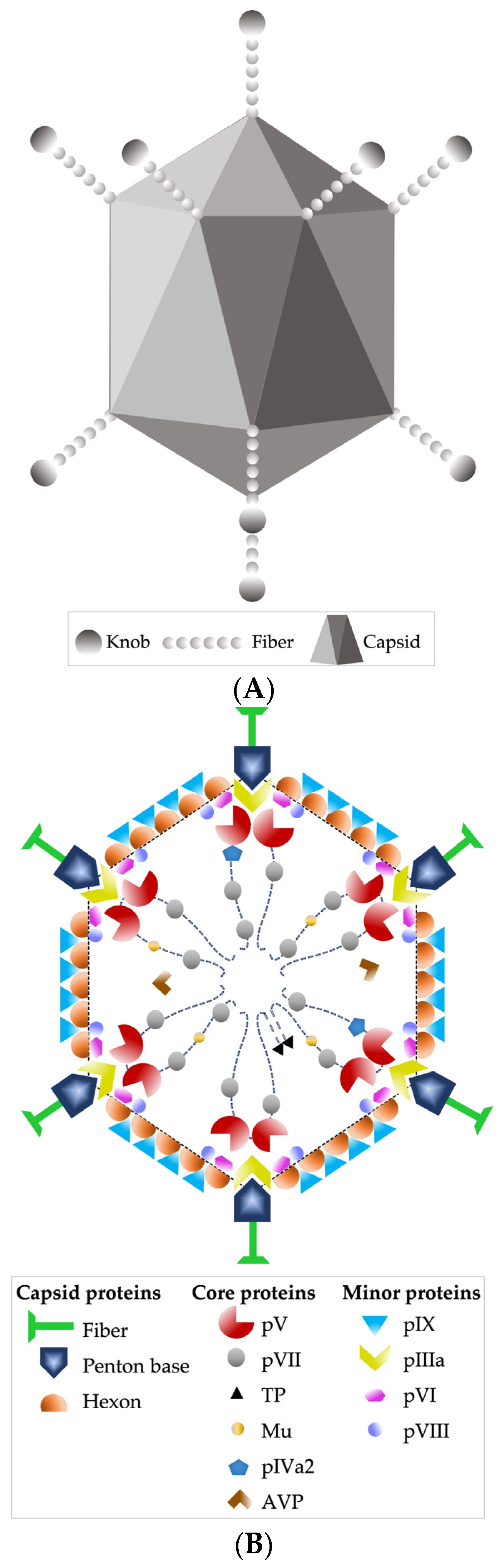
4. Virological Characteristics of HAdV and Its Pathogenesis
5. Microbiological Diagnosis
6. Application of HAdV Genotyping
7. Viral Type and Disease Association
8. HAdV Genetic Variability
| HAdV Genotype | Name | Accession # | Year (Publication) | Penton Base | Hexon | Fiber |
|---|---|---|---|---|---|---|
| HAdV-B55 | P14H11F14/2006/CHN | FJ643676 | 2009 | 14 | 11 | 14 |
| HAdV-B66 | P66H7F3/1987/ARG | JN860676 | 2012 | 66 | 7 | 3 |
| HAdV-B68 | P16H3F16/2004/ARG | JN860678 | 2011 | 16 | 3 | 16 |
| HAdV-B77 | P35H34F7/1985/DEU | KF268328 | 2013 | 35 | 34 | 7 |
| HAdV-B78 | P11H11F7/2013/USA | KT970441 | 2016 | 11 | 11 | 7 |
| HAdV-C89 | P89H2F2/2015/DEU | MH121097 | 2019 | 89 | 2 | 2 |
| HAdV-C104 | P1H1F2/2017/CHN | MH558113 | 2021 | 1 | 1 | 2 |
| HAdV-C108 | P1H2F2 | N/A | 2014 | 1 | 2 | 2 |
9. Association of Types, Infected Tissues and Severity of the Infection
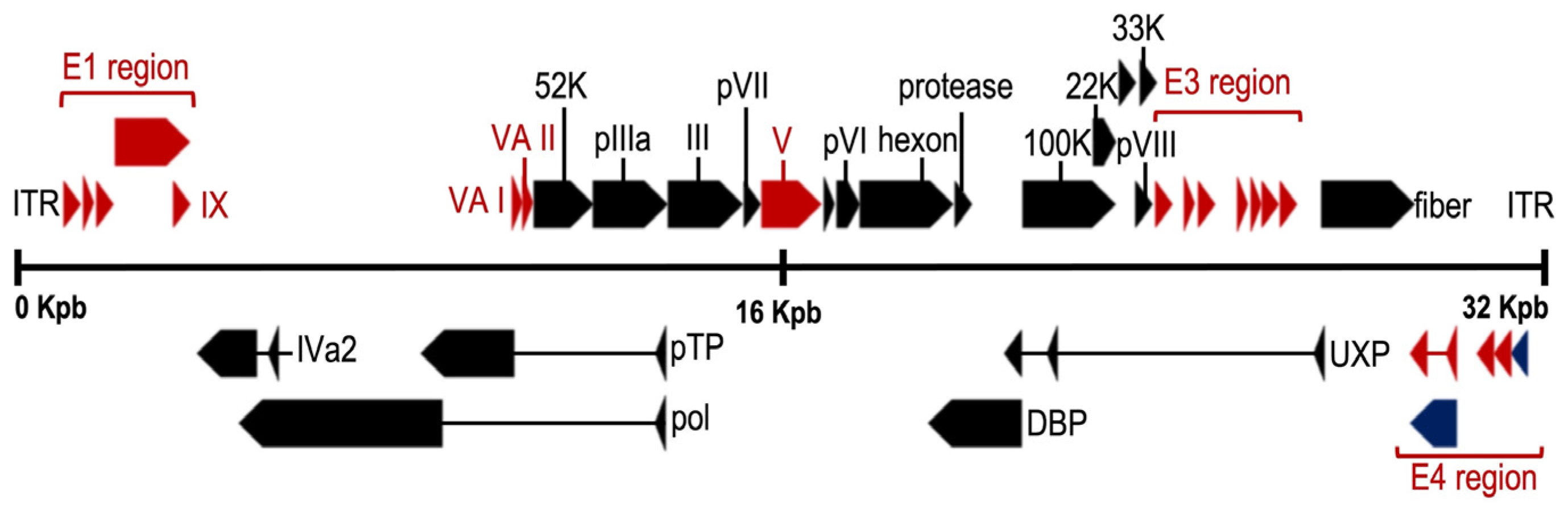
10. Relevant Characteristics of the HAdV Genome
11. Social Determinants of Health and Their Impact on HAdV
12. Treatment
13. Adenovirus-Based Vaccines as a SARS-CoV-2 Prevention Strategy
14. Strategies for the Prevention of HAdV
15. Why Is Genomic Surveillance of HAdV Important? Establishment of SARS-CoV-2 Genomic Surveillance
15.1. Genomic Surveillance Overview
15.2. Surveillance in HAdV
15.3. HAdV Surveillance in Wastewater
15.4. Surveillance and Monitoring of Zoonoses
16. Future Projections of HAdV Study in the 21st Century
16.1. Unknown Hepatitis in COVID-19 Outbreak
16.2. Universal Vaccination for SARS-CoV-2 with Recombinant Vaccines. What Will Be the Effect on Circulating HAdV in the Community?
16.3. HAdV as a Vector for Other Vaccine Models
16.4. Utility of Recognizing HAdV Genotypes. One More Step towards Genomic Surveillance
16.5. Genomic Surveillance
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Chapter 18—Adenoviruses. In Fenner and White’s Medical Virology, 5th ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 263–271. ISBN 9780123751560. [Google Scholar] [CrossRef]
- Ginsberg, H.S. The life and times of adenoviruses. Adv. Virus Res. 1999, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Slifkin, M.; Merkow, L.; Rapoza, N.P. Tumor induction by simian adenovirus 30 and establishment of tumor cell lines. Cancer Res. 1968, 28, 1173–1179. [Google Scholar]
- Tsetlin, E.M.; Levenbuk, I.S.; Al’tshtein, A.D. Onkogennost’ adenovirusa obez’ian SA7(C8) dlia myshei i krys [Oncogenicity of simian adenovirus SA7(C8) for mice and rats]. Vopr. Onkol. 1972, 18, 37–42. [Google Scholar] [PubMed]
- Irlin, I.S.; Ter-Grigorov, V.S.; Al’tshteĭn, A.D.; Dodonova, N.N.; Biriulina, T.I. Induktsiia opukholeĭ pecheni u mysheĭ adenovirusom SA (C8) obez’ian [Induction of liver tumors in mice by simian adenovirus SA (C8)]. Vopr. Onkol. 1971, 17, 76–80. [Google Scholar] [PubMed]
- Nishida, T.; Mukai, N.; Solish, S.P. Complement-dependent cytotoxicity in rats bearing human adenovirus type 12-induced, primary retinoblastoma-like tumor in the eye. Curr. Eye Res. 1981, 1, 53–55. [Google Scholar] [CrossRef]
- Tessier, T.M.; Dodge, M.J.; MacNeil, K.M.; Evans, A.M.; Prusinkiewicz, M.A.; Mymryk, J.S. Almost famous: Human adenoviruses (and what they have taught us about cancer). Tumour Virus Res. 2021, 12, 200225. [Google Scholar] [CrossRef] [PubMed]
- Lion, T. Adenovirus persistence, reactivation, and clinical management. FEBS Lett. 2019, 593, 3571–3582. [Google Scholar] [CrossRef] [Green Version]
- Avendaño, L.F. Infeccion respiratoria por adenovirus en pediatría: De ayer a hoy. Neumol. Pediátrica 2019, 14, 12–18. [Google Scholar] [CrossRef]
- Parra-Lucares, A.; Segura, P.; Rojas, V.; Pumarino, C.; Saint-Pierre, G.; Toro, L. Emergence of SARS-CoV-2 Variants in the World: How Could This Happen? Life 2022, 12, 194. [Google Scholar] [CrossRef]
- Fu, Y.; Tang, Z.; Ye, Z.; Mo, S.; Tian, X.; Ni, K.; Ren, L.; Liu, E.; Zang, N. Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect. Dis. 2019, 19, 36. [Google Scholar] [CrossRef]
- Kumthip, K.; Khamrin, P.; Ushijima, H.; Maneekarn, N. Enteric and non-enteric adenoviruses associated with acute gastroenteritis in pediatric patients in Thailand, 2011 to 2017. PLoS ONE 2019, 14, e0220263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, L.R.; de Almeida, S.M.; Cavalli, B.M.; Dieckmann, T.G.; Raboni, S.M.; Salvador, G.L.O.; Pereira, L.A.; Rotta, I.; Nogueira, M.B. Human adenovirus meningoencephalitis: A 3-years’ overview. J. Neurovirol. 2019, 25, 589–596. [Google Scholar] [CrossRef]
- Adrian, T.; Schäfer, G.; Cooney, M.K.; Fox, J.P.; Wigand, R. Persistent enteral infections with adenovirus types 1 and 2 in infants: No evidence of reinfection. Epidemiol. Infect. 1988, 101, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proenca-Modena, J.L.; de Souza Cardoso, R.; Criado, M.F.; Milanez, G.P.; de Souza, W.M.; Parise, P.L.; Bertol, J.W.; de Jesus, B.L.S.; Prates, M.C.M.; Silva, M.L.; et al. Human adenovirus replication and persistence in hypertrophic adenoids and palatine tonsils in children. J. Med. Virol. 2019, 91, 1250–1262. [Google Scholar] [CrossRef]
- Parcell, B.J.; McIntyre, P.G.; Yirrell, D.L.; Fraser, A.; Quinn, M.; Templeton, K.; Christie, S.; Romanes, F. Prison and community outbreak of severe respiratory infection due to adenovirus type 14p1 in Tayside, UK. J. Public Health 2015, 37, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Huang, W.; Zhou, X.; Zhao, Q.; Wang, Q.; Jia, B. Seroprevalence of neutralizing antibodies to human adenoviruses type-5 and type-26 and chimpanzee adenovirus type-68 in healthy Chinese adults. J. Med. Virol. 2013, 85, 1077–1084. [Google Scholar] [CrossRef]
- Schilham, M.W.; Claas, E.C.; van Zaane, W.; Heemskerk, B.; Vossen, J.M.; Lankester, A.C.; Toes, R.E.; Echavarria, M.; Kroes, A.C.; van Tol, M.J. High levels of adenovirus DNA in serum correlate with fatal outcome of adenovirus infection in children after allogeneic stem-cell transplantation. Clin. Infect. Dis. 2002, 35, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef] [Green Version]
- Akello, J.O.; Kamgang, R.; Barbani, M.T.; Suter-Riniker, F.; Leib, S.L.; Ramette, A. Epidemiology of Human Adenoviruses: A 20-Year Retrospective Observational Study in Hospitalized Patients in Bern, Switzerland. Clin. Epidemiol. 2020, 12, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.X.; Zou, X.H.; Jiang, S.Y.; Lu, N.N.; Han, M.; Zhao, J.H.; Guo, X.J.; Zhao, S.C.; Lu, Z.Z. Prevalence of serum neutralizing antibodies to adenovirus type 5 (Ad5) and 41 (Ad41) in children is associated with age and sanitary conditions. Vaccine 2016, 34, 5579–5586. [Google Scholar] [CrossRef]
- Mennechet, F.J.D.; Paris, O.; Ouoba, A.R.; Salazar, S.; Sirima, S.B.; Takoudjou, G.R.; Diarra, A.; Traore, I.T.; Kania, D.; Eichholz, K.; et al. A review of 65 years of human adenovirus seroprevalence. Expert Rev. Vaccines 2019, 18, 597–613. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Adenovirus Transmission; National Center for Immunization and Respiratory Diseases, Division of Viral Diseases: Atlanta, GA, USA, 2018. Available online: https://www.cdc.gov/adenovirus/about/transmission.html (accessed on 26 November 2022).
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on Adenoviruses: Epidemiology, Pathogenicity, and Gene Therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Centros para el Control de Enfermedades (CDC). Brote de fiebre faringoconjuntival en un campamento de verano—Carolina del Norte, 1991. MMWR Morb. Mortal. Semanal. Rep. 1992, 41, 342–344. [Google Scholar]
- Payne, S.B.; Grilli, E.A.; Smith, A.J.; Hoskins, T.W. Investigation of an outbreak of adenovirus type 3 infection in a boys’ boarding school. J. Hyg. (Lond). 1984, 93, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ison, M.G.; Hayden, R.T. Adenovirus. Microbiol. Spectr. 2016, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rowe, W.P.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.H.; Ward, T.G. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 1953, 84, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.O.; England, B.; Mauris, C.; Shuey, H.E.; Lennette, E.H. Etiology Of Acute Respiratory Disease Among Service Personnel At Fort Ord, California. Am. J. Hyg. 1955, 62, 283–294. [Google Scholar] [CrossRef]
- Mukai, N.; Kalter, S.S.; Cummins, L.B.; Matthews, V.A.; Nishida, T.; Nakajima, T. Retinal tumor induced in the babuino by human adenovirus 12. Science 1980, 210, 1023–1025. [Google Scholar] [CrossRef]
- Pereira, H.G.; Huebner, R.J.; Ginsberg, H.S.; Van Der Veen, J. A Short Description Of The Adenovirus Group. Virology 1963, 20, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Ip, W.-H.; Dobner, T. Animal Models in Human Adenovirus Research. Biology 2021, 10, 1253. [Google Scholar] [CrossRef]
- Benkő, M.; Aoki, K.; Arnberg, N.; Davison, A.J.; Echavarría, M.; Hess, M.; Jones, M.S.; Kaján, G.L.; Kajon, A.E.; Mittal, S.K.; et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J. Gen. Virol. 2022, 103, 001721. [Google Scholar] [CrossRef]
- HAdV Working Group. Criteria for a New HAdV Type. Available online: http://hadvwg.gmu.edu/index.php/criteria-for-a-new-hadv-type/ (accessed on 28 November 2022).
- Wang, Y.; Li, Y.; Lu, R.; Zhao, Y.; Xie, Z.; Shen, J.; Tan, W. Phylogenetic evidence for intratypic recombinant events in a novel human adenovirus C that causes severe acute respiratory infection in children. Sci. Rep. 2016, 6, 23014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganime, A.C.; Carvalho-Costa, F.A.; Santos, M.; Costa Filho, R.; Leite, J.P.; Miagostovich, M.P. Viability of human adenovirus from hospital fomites. J. Med. Virol. 2014, 86, 2065–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Nishikawaji, Y.; Kawakami, H.; Kosai, K.I. Adenovirus Biology, Recombinant Adenovirus, and Adenovirus Usage in Gene Therapy. Viruses 2021, 13, 2502. [Google Scholar] [CrossRef] [PubMed]
- Gingeras, T.R.; Sciaky, D.; Gelinas, R.E.; Bing-Dong, J.; Yen, C.E.; Kelly, M.M.; Bullock, P.A.; Parsons, B.L.; O’Neill, K.E.; Roberts, R.J. Nucleotide sequences from the adenovirus-2 genome. J. Biol. Chem. 1982, 257, 13475–13491. [Google Scholar] [CrossRef]
- Tessier, J.; Chadeuf, G.; Nony, P.; Avet-Loiseau, H.; Moullier, P.; Salvetti, A. Characterization of adenovirus-induced inverted terminal repeat-independent amplification of integrated adeno-associated virus rep-cap sequences. J. Virol. 2001, 75, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Furuse, Y.; Ornelles, D.A.; Cullen, B.R. Persistently adenovirus-infected lymphoid cells express micrornas derived from the viral vai and especially vaii rna. Virology 2013, 447, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Radke, J.R.; Cook, J.L. Human adenovirus infections: Update and consideration of mechanisms of viral persistence. Curr. Opin. Infect. Dis. 2018, 31, 251–256. [Google Scholar] [CrossRef]
- Tseng, C.C.; Chang, L.Y.; Li, C.S. Detection of airborne viruses in a pediatrics department measured using real-time qPCR coupled to an air-sampling filter method. J. Env. Health 2010, 73, 22–28. [Google Scholar]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Outbreak of pharyngoconjunctival fever at a summer camp--North Carolina, 1991. MMWR Morb. Mortal. Wkly. Rep. 1992, 41, 342–344. [Google Scholar]
- Siegel, J.D.; Rhinehart, E.; Jackson, M.; Chiarello, L.; The Healthcare Infection Control Practices Advisory Committee. Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings. 2007. Available online: https://www.cdc.gov/infectioncontrol/guidelines/isolation/index.html (accessed on 10 January 2023).
- Chughtai, A.A.; Khan, W. Use of personal protective equipment to protect against respiratory infections in Pakistan: A systematic review. J. Infect. Public Health 2020, 13, 385–390. [Google Scholar] [CrossRef]
- Dai, M.; Wu, Y.; Tan, H.; Deng, J.; Hou, M.; Peng, W.; Chen, G.; Li, Y.; Li, H.; Pan, P.; et al. Cross-infection of adenovirus among medical staff: A warning from the intensive care unit in a tertiary care teaching hospital in China. Int. J. Infect. Dis. 2020, 98, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Walsh, M.P.; Chintakuntlawar, A.; Robinson, C.M.; Madisch, I.; Harrach, B.; Hudson, N.R.; Schnurr, D.; Heim, A.; Chodosh, J.; Seto, D.; et al. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS ONE 2009, 4, e5635. [Google Scholar] [CrossRef]
- Gonzalez, G.; Yawata, N.; Aoki, K.; Kitaichi, N. Challenges in management of epidemic keratoconjunctivitis with emerging recombinant human adenoviruses. J. Clin. Virol. 2019, 112, 1–9. [Google Scholar] [CrossRef]
- Corvalán, L.P.; Arias, B.G.; Morales, S.P.; González, M.R.; Inostroza, S.J.; Fuenzalida, I.L. Inmunofluorescencia indirecta versus reacción de polimerasa en cadena para el diagnóstico de virus respiratorios en niños ingresados en un hospital de la Región Metropolitana [Indirect immunofluorescence technique versus polymerase chain reaction for the diagnosis of respiratory viruses in children admitted to a hospital in the Metropolitan Region]. Rev. Chilena Infectol. 2019, 36, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.R.; Sharp, I.R.; Caul, E.O.; Paul, I.; Bailey, A.S.; Hawkins, M.; Pugh, S.; Treharne, J.; Stevenson, S. Rapid detection and serotyping of adenovirus by direct immunofluorescence. J. Med. Virol. 1997, 51, 198–201. [Google Scholar] [CrossRef]
- Ko, G.; Cromeans, T.L.; Sobsey, M.D. Detection of infectious adenovirus in cell culture by mRNA reverse transcription-PCR. Appl. Environ. Microbiol. 2003, 69, 7377–7384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takimoto, S.; Grandien, M.; Ishida, M.A.; Pereira, M.S.; Paiva, T.M.; Ishimaru, T.; Makita, E.M.; Martinez, C.H. Comparison of enzyme-linked immunosorbent assay, indirect immunofluorescence assay, and virus isolation for detection of respiratory viruses in nasopharyngeal secretions. J. Clin. Microbiol. 1991, 29, 470–474. [Google Scholar] [CrossRef] [Green Version]
- Calico-Bosch, I. Diagnóstico De Las Infecciones Por Adenovirus; Control de Calidad de la SEIMC: Madrid, Spain, 2002. [Google Scholar]
- Tsutsumi, H.; Ouchi, K.; Ohsaki, M.; Yamanaka, T.; Kuniya, Y.; Takeuchi, Y.; Nakai, C.; Meguro, H.; Chiba, S. Immunochromatography test for rapid diagnosis of adenovirus respiratory tract infections: Comparison with virus isolation in tissue culture. J. Clin. Microbiol. 1999, 37, 2007–2009. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.N.; Fowler, R.; Balada-Llasat, J.M.; Carroll, A.; Stone, H.; Akerele, O.; Buchan, B.; Windham, S.; Hopp, A.; Ronen, S.; et al. Multicenter Evaluation of the BioFire FilmArray Pneumonia/Pneumonia Plus Panel for Detection and Quantification of Agents of Lower Respiratory Tract Infection. J. Clin. Microbiol. 2020, 58, e00128-20. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L.; Everhart, K.; Daly, J.A.; Cullison, J.; Daly, J.; Holt, S.; Lephart, P.; Salimnia, H.; Schreckenberger, P.C.; DesJarlais, S.; et al. Multicenter Evaluation of BioFire FilmArray Respiratory Panel 2 for Detection of Viruses and Bacteria in Nasopharyngeal Swab Samples. J. Clin. Microbiol. 2018, 56, e01945-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, C.; Hirsch, H.H. Comparing Luminex NxTAG-Respiratory Pathogen Panel and RespiFinder-22 for multiplex detection of respiratory pathogens. J. Med. Virol. 2016, 88, 1319–1324. [Google Scholar] [CrossRef]
- Räty, R.; Kleemola, M.; Melén, K.; Stenvik, M.; Julkunen, I. Efficacy of PCR and other diagnostic methods for the detection of respiratory adenoviral infections. J. Med. Virol. 1999, 59, 66–72. [Google Scholar] [CrossRef]
- Marcone, D.N.; Carballal, G.; Ricarte, C.; Echavarria, M. Diagnóstico de virus respiratorios utilizando un sistema automatizado de PCR múltiples (FilmArray) y su comparación con métodos convencionales [Respiratory viral diagnosis by using an automated system of multiplex PCR (FilmArray) compared to conventional methods]. Rev. Argent. Microbiol. 2015, 47, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Pinsky, B.A.; Hayden, R.T. Cost-Effective Respiratory Virus Testing. J. Clin. Microbiol. 2019, 5, e00373-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcone, D.N.; Culasso, A.C.A.; Reyes, N.; Kajon, A.; Viale, D.; Campos, R.H.; Carballal, G.; Echavarria, M. Genotypes and phylogenetic analysis of adenovirus in children with respiratory infection in Buenos Aires, Argentina (2000–2018). PLoS ONE 2021, 16, e0248191. [Google Scholar] [CrossRef]
- De Jong, J.C.; Osterhaus, A.D.; Jones, M.S.; Harrach, B. Human adenovirus type 52: A type 41 in disguise? J. Virol. 2008, 82, 3809–3810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadell, G.; Varsányi, T.M.; Lord, A.; Sutton, R.N. Epidemic outbreaks of adenovirus 7 with special reference to the pathogenicity of adenovirus genome type 7b. Am. J. Epidemiol. 1980, 112, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Sarantis, H.; Johnson, G.; Brown, M.; Petric, M.; Tellier, R. Comprehensive detection and serotyping of human adenoviruses by PCR and sequencing. J. Clin. Microbiol. 2004, 42, 3963–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Erdman, D.D. Molecular typing of human adenoviruses by PCR and sequencing of a partial region of the hexon gene. Arch. Virol. 2006, 151, 1587–1602. [Google Scholar] [CrossRef] [PubMed]
- Allard, A.; Albinsson, B.; Wadell, G. Rapid typing of human adenoviruses by a general PCR combined with restriction endonuclease analysis. J. Clin. Microbiol. 2001, 39, 498–505. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhang, J.; Lan, W.; Quan, L.; Ou, J.; Zhao, W.; Wu, J.; Woo, P.C.; Seto, D.; Zhang, Q. Molecular Typing and Rapid Identification of Human Adenoviruses Associated With Respiratory Diseases Using Universal PCR and Sequencing Primers for the Three Major Capsid Genes: Penton Base, Hexon, and Fiber. Front. Microbiol. 2022, 13, 911694. [Google Scholar] [CrossRef]
- Okada, M.; Ogawa, T.; Kubonoya, H.; Yoshizumi, H.; Shinozaki, K. Detection and sequence-based typing of human adenoviruses using sensitive universal primer sets for the hexon gene. Arch. Virol. 2007, 152, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, Z.; Zhang, G.; Sai, L. Molecular and epidemiological characterization of human adenoviruses infection among children with acute diarrhea in Shandong Province, China. Virol. J. 2021, 18, 195. [Google Scholar] [CrossRef]
- Moyo, S.J.; Hanevik, K.; Blomberg, B.; Kommedal, O.; Nordbø, S.A.; Maselle, S.; Langeland, N. Prevalence and molecular characterisation of human adenovirus in diarrhoeic children in Tanzania; a case control study. BMC Infect. Dis. 2014, 14, 666. [Google Scholar] [CrossRef]
- Lu, X.; Trujillo-Lopez, E.; Lott, L.; Erdman, D.D. Quantitative real-time PCR assay panel for detection and type-specific identification of epidemic respiratory human adenoviruses. J. Clin. Microbiol. 2013, 51, 1089–1093. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Erdman, D.D. Quantitative real-time PCR assays for detection and type-specific identification of the endemic species C human adenoviruses. J. Virol Methods. 2016, 237, 174–178. [Google Scholar] [CrossRef]
- Echavarría, M. Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008, 21, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benko, M. Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., Van Regenmortel, M.H.V., Eds.; Academic Press Inc.: Oxford, UK, 2008; pp. 25–29. [Google Scholar]
- Ylihärsilä, M.; Harju, E.; Arppe, R.; Hattara, L.; Hölsä, J.; Saviranta, P.; Soukka, T.; Waris, M. Genotyping of clinically relevant human adenoviruses by array-in-well hybridization assay. Clin. Microbiol. Infect. 2013, 19, 551–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrero, P.R.; Valinotto, L.E.; Tittarelli, E.; Mistchenko, A.S. Molecular typing of adenoviruses in pediatric respiratory infections in Buenos Aires, Argentina (1999–2010). J. Clin. Virol. 2012, 53, 145–150. [Google Scholar] [CrossRef]
- Garnett, C.T.; Talekar, G.; Mahr, J.A.; Huang, W.; Zhang, Y.; Ornelles, D.A.; Gooding, L.R. Latent species C adenoviruses in human tonsil tissues. J. Virol. 2009, 83, 2417–2428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akello, J.O.; Kamgang, R.; Barbani, M.T.; Suter-Riniker, F.; Aebi, C.; Beuret, C.; Paris, D.H.; Leib, S.L.; Ramette, A. Genomic analyses of human adenoviruses unravel novel recombinant genotypes associated with severe infections in pediatric patients. Sci. Rep. 2021, 11, 24038. [Google Scholar] [CrossRef] [PubMed]
- Kosulin, K.; Geiger, E.; Vécsei, A.; Huber, W.D.; Rauch, M.; Brenner, E.; Wrba, F.; Hammer, K.; Innerhofer, A.; Pötschger, U.; et al. Persistence and reactivation of human adenoviruses in the gastrointestinal tract. Clin. Microbiol. Infect. 2016, 22, 381.e1–381.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houldcroft, C.J.; Beale, M.A.; Sayeed, M.A.; Qadri, F.; Dougan, G.; Mutreja, A. Identification of novel adenovirus genotype 90 in children from Bangladesh. Microb. Genom. 2018, 4, e000221. [Google Scholar] [CrossRef]
- Kaján, G.L.; Affranio, I.; Tóthné Bistyák, A.; Kecskeméti, S.; Benkő, M. An emerging new fowl adenovirus genotype. Heliyon 2019, 5, e01732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Risso-Ballester, J.; Cuevas, J.M.; Sanjuán, R. Genome-Wide Estimation of the Spontaneous Mutation Rate of Human Adenovirus 5 by High-Fidelity Deep Sequencing. PLoS Pathog. 2016, 12, e1006013. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.; Grodzicker, T.; Sharp, P.; Sambrook, J. Adenovirus recombination: Physical mapping of crossover events. Cell 1975, 4, 113–119. [Google Scholar] [CrossRef]
- Boursnell, M.E.; Mautner, V. Recombination in adenovirus: Crossover sites in intertypic recombinants are located in regions of homology. Virology 1981, 112, 198–209. [Google Scholar] [CrossRef]
- Mautner, V.; Mackay, N. Recombination in adenovirus: Analysis of crossover sites in intertypic overlap recombinants. Virology 1984, 139, 43–52. [Google Scholar] [CrossRef]
- Dhingra, A.; Hage, E.; Ganzenmueller, T.; Böttcher, S.; Hofmann, J.; Hamprecht, K.; Obermeier, P.; Rath, B.; Hausmann, F.; Dobner, T.; et al. Molecular Evolution of Human Adenovirus (HAdV) Species, C. Sci. Rep. 2019, 9, 1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, X.; Wu, H.; Zhou, R. Molecular evolution of human adenovirus type 16 through multiple recombination events. Virus Genes 2019, 55, 769–778. [Google Scholar] [CrossRef]
- Lukashev, A.N.; Ivanova, O.E.; Eremeeva, T.P.; Iggo, R.D. Evidence of frequent recombination among human adenoviruses. J. Gen. Virol. 2008, 89, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Kajon, A.E.; Lamson, D.M.; St George, K. Emergence and re-emergence of respiratory adenoviruses in the United States. Curr. Opin. Virol. 2019, 34, 63–69. [Google Scholar] [CrossRef]
- Yang, J.; Mao, N.; Zhang, C.; Ren, B.; Li, H.; Li, N.; Chen, J.; Zhang, R.; Li, H.; Zhu, Z.; et al. Human adenovirus species C recombinant virus continuously circulated in China. Sci. Rep. 2019, 9, 9781. [Google Scholar] [CrossRef] [Green Version]
- Lichtenstein, D.L.; Toth, K.; Doronin, K.; Tollefson, A.E.; Wold, W.S. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 2004, 23, 75–111. [Google Scholar] [CrossRef]
- Cheng, Z.; Yan, Y.; Jing, S.; Li, W.G.; Chen, W.W.; Zhang, J.; Li, M.; Zhao, S.; Cao, N.; Ou, J.; et al. Comparative Genomic Analysis of Re-emergent Human Adenovirus Type 55 Pathogens Associated With Adult Severe Community-Acquired Pneumonia Reveals Conserved Genomes and Capsid Proteins. Front. Microbiol. 2018, 9, 1180. [Google Scholar] [CrossRef] [Green Version]
- HAdV Working Group. Available online: http://hadvwg.gmu.edu/ (accessed on 28 November 2022).
- Yu, Z.; Zeng, Z.; Zhang, J.; Pan, Y.; Chen, M.; Guo, Y.; Yu, N.; Chodosh, J.; Fu, N.; Che, X.; et al. Fatal Community-acquired Pneumonia in Children Caused by Re-emergent Human Adenovirus 7d Associated with Higher Severity of Illness and Fatality Rate. Sci. Rep. 2016, 6, 37216. [Google Scholar] [CrossRef] [Green Version]
- Bastug, A.; Altas, A.B.; Koc, B.T.; Bayrakdar, F.; Korukluoglu, G.; Bodur, H.; Oguzoglu, T.C. Molecular characterization of human adenoviruses associated with respiratory infection in Turkey. APMIS 2021, 129, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.H.; Wang, C.; Wei, T.L.; Wang, H.; Ma, F.L.; Zheng, L.S. Human adenovirus among hospitalized children with respiratory tract infections in Beijing, China, 2017–2018. Virol. J. 2019, 16, 78. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Hong, J.Y.; Lee, H.J.; Shin, S.H.; Kim, Y.K.; Inada, T.; Hashido, M.; Piedra, P.A. Genome type analysis of adenovirus types 3 and 7 isolated during successive outbreaks of lower respiratory tract infections in children. J. Clin. Microbiol. 2003, 41, 4594–4599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, F.E.; Mesquita, J.R.; Portes, S.A.; Ramos, E.A.; Siqueira, M.M. Caracterización antigénica y genómica de adenovirus asociados a infecciones respiratorias en niños residentes en el Nordeste de Brasil. Mem. Inst. Oswaldo Cruz 2007, 102, 937–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.H.; Lin, Y.C.; Chen, H.L.; Ke, G.M.; Chiang, C.J.; Hwang, K.P.; Chu, P.Y.; Lin, J.H.; Liu, D.P.; Chen, H.Y. A two decade survey of respiratory adenovirus in Taiwan: The reemergence of adenovirus types 7 and 4. J. Med. Virol. 2004, 73, 274–279. [Google Scholar] [CrossRef]
- Arnberg, N. Adenovirus E3 protein modulates leukocyte functions. Proc. Natl. Acad. Sci. USA 2013, 110, 19976–19977. [Google Scholar] [CrossRef] [Green Version]
- Houldcroft, C.J.; Roy, S.; Morfopoulou, S.; Margetts, B.K.; Depledge, D.P.; Cudini, J.; Shah, D.; Brown, J.R.; Romero, E.Y.; Williams, R.; et al. Use of Whole-Genome Sequencing of Adenovirus in Immunocompromised Pediatric Patients to Identify Nosocomial Transmission and Mixed-Genotype Infection. J. Infect. Dis. 2018, 218, 1261–1271. [Google Scholar] [CrossRef]
- Miro, E.; Del Cuerpo, M.; Rubio, M.; Berengua, C.; Español, M.; Marin, P.; Vela, J.I.; Pomar, V.; Gutierrez, C.; Navarro, F.; et al. Whole-genome analysis to describe a human adenovirus D8 conjunctivitis outbreak in a tertiary hospital. J. Med. Virol. 2021, 93, 4840–4845. [Google Scholar] [CrossRef]
- Li, Y.; Kang, J.; Friedman, J.; Tarassishin, L.; Ye, J.; Kovalenko, A.; Wallach, D.; Horwitz, M.S. Identification of a cell protein (FIP-3) as a modulator of NF-kappaB activity and as a target of an adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis. Proc. Natl. Acad. Sci. USA 1999, 96, 1042–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsing, A.; Burgert, H.G. The adenovirus E3/10.4K-14.5K proteins down-modulate the apoptosis receptor Fas/Apo-1 by inducing its internalization. Proc. Natl. Acad. Sci. USA 1998, 95, 10072–10077. [Google Scholar] [CrossRef] [Green Version]
- Tollefson, A.E.; Scaria, A.; Hermiston, T.W.; Ryerse, J.S.; Wold, L.J.; Wold, W.S. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J. Virol. 1996, 70, 2296–2306. [Google Scholar] [CrossRef] [Green Version]
- Windheim, M.; Southcombe, J.H.; Kremmer, E.; Chaplin, L.; Urlaub, D.; Falk, C.S.; Claus, M.; Mihm, J.; Braithwaite, M.; Dennehy, K.; et al. A unique secreted adenovirus E3 protein binds to the leukocyte common antigen CD45 and modulates leukocyte functions. Proc. Natl. Acad. Sci. USA 2013, 110, E4884–E4893. [Google Scholar] [CrossRef] [Green Version]
- Vidal, D.; Chamblas, I.; Zavala, M.; Muller, R.; Rodriguez, M.C.; Chavez, A. Social Determinants Of Health And Lifestyles In Adult Population Concepción, Chile. Ciencia Y Enfermeria 2014, 20, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Braveman, P.; Gottlieb, L. The social determinants of health: It’s time to consider the causes of the causes. Public Health Rep. 2014, 129 (Suppl. 2), 19–31. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Herbert, C.; Ward, D.V.; Simin, K.; McCormick, B.A.; Ellison, R.T.; Zai, A.H. COVID-19 Variant Surveillance and Social Determinants in Central Massachusetts: Development Study. JMIR Form. Res. 2022, 6, e3785. [Google Scholar] [CrossRef]
- Burström, B.; Tao, W. Social determinants of health and inequalities in COVID-19. Eur. J. Public Health 2020, 30, 617–618. [Google Scholar] [CrossRef]
- Anderson, G.; Frank, J.W.; Naylor, C.D.; Wodchis, W.; Feng, P. Using socioeconomics to counter health disparities arising from the covid-19 pandemic. BMJ 2020, 369, m2149. [Google Scholar] [CrossRef] [PubMed]
- Pagel, C. There is a real danger that covid-19 will become entrenched as a disease of poverty. BMJ 2021, 373, n986. [Google Scholar] [CrossRef] [PubMed]
- Bahar Özvarış, Ş.; Kayı, İ.; Mardin, D.; Sakarya, S.; Ekzayez, A.; Meagher, K.; Patel, P. COVID-19 barriers and response strategies for refugees and undocumented migrants in Turkey. J. Migr. Health 2020, 1, 100012. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.; Ozbilgin, M. Age, industry, and unemployment risk during a pandemic lockdown. J. Econ. Dyn. Control 2021, 133, 104233. [Google Scholar] [CrossRef]
- Ahdam, N. Rapid Response: Poverty predisposes populations to contracting COVID-19. BMJ 2021, 373, n986. [Google Scholar]
- Wang, M.; Barasheed, O.; Rashid, H.; Booy, R.; El Bashir, H.; Haworth, E.; Ridda, I.; Holmes, E.C.; Dwyer, D.E.; Nguyen-Van-Tam, J.; et al. A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims. J. Epidemiol. Glob. Health 2015, 5, 181–189. [Google Scholar] [CrossRef]
- Shieh, W.J. Human adenovirus infections in pediatric population—An update on clinico-pathologic correlation. Biomed. J. 2022, 45, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.J. What is the risk of a deadly adenovirus pandemic? PLoS Pathog. 2021, 17, e1009814. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, H.; Sera, N.; Gonzalez, G.; Hanaoka, N.; Fujimoto, T. First isolation of a new type of human adenovirus (genotype 79), species Human mastadenovirus B (B2) from sewage water in Japan. J. Med. Virol. 2017, 89, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.C. Human adenoviruses in water: Occurrence and health implications: A critical review. Environ. Sci. Technol. 2006, 40, 7132–7140. [Google Scholar] [CrossRef] [PubMed]
- Pfortmueller, C.A.; Barbani, M.T.; Schefold, J.C.; Hage, E.; Heim, A.; Zimmerli, S. Severe acute respiratory distress syndrome (ARDS) induced by human adenovirus B21: Report on 2 cases and literature review. J. Crit. Care 2019, 51, 99–104. [Google Scholar] [CrossRef]
- Xu, N.; Chen, P.; Wang, Y. Evaluation of Risk Factors for Exacerbations in Children with Adenoviral Pneumonia. Biomed. Res. Int. 2020, 2020, 4878635. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, J.N.; Li, H.; He, C.Y.; Dai, Q.S.; Li, X.L.; He, J.F.; He, H.; Li, M.B.; Jiang, L.I.; et al. An outbreak of epidemic keratoconjunctivitis caused by human adenovirus type 8 in primary school, southwest China. BMC Infect. Dis. 2019, 19, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rebelo-de-Andrade, H.; Pereira, C.; Gíria, M.; Prudêncio, E.; Brito, M.J.; Calé, E.; Taveira, N. Outbreak of acute respiratory infection among infants in Lisbon, Portugal, caused by human adenovirus serotype 3 and a new 7/3 recombinant strain. J. Clin. Microbiol. 2010, 48, 1391–1396. [Google Scholar] [CrossRef] [Green Version]
- Esparcia Rodríguez, Ó.; Gómez Martínez, A.; Martínez Nieto, M.J.; Salmerón Cifuentes, M.S.; Rodolfo Saavedra, R.; de la Cruz de Julián, I. Brote de queratoconjuntivitis epidémica por adenovirus humano serotipo 8 en una residencia de mayores [Outbreak of epidemic keratoconjunctivitis caused by human adenovirus serotype 8 in a nursing home.]. Rev. Esp. Salud Publica 2020, 94, e202009100. [Google Scholar] [PubMed]
- Glatman-Freedman, A.; Nichols, K. The effect of social determinants on immunization programs. Hum. Vaccin Immunother. 2012, 8, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyce, T.; Gudorf, A.; de Kat, C.; Muscat, M.; Butler, R.; Habersaat, K.B. Towards equity in immunisation. EuroSurveillance 2019, 24, 1800204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentine, N.B.; Koller, T.S.; Hosseinpoor, A.R. Monitoring health determinants with an equity focus: A key role in addressing social determinants, universal health coverage, and advancing the 2030 sustainable development agenda. Glob. Health Action 2016, 9, 34247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Tian, X. Vaccine development for human mastadenovirus. J. Thorac. Dis. 2018, 10 (Suppl. 19), S2280–S2294. [Google Scholar] [CrossRef] [PubMed]
- Bayati, M.; Noroozi, R.; Ghanbari-Jahromi, M.; Jalali, F.S. Inequality in the distribution of Covid-19 vaccine: A systematic review. Int. J. Equity Health 2022, 21, 122. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, V.; Keestra, S.M.; Hill, A. Global COVID-19 Vaccine Inequity: Failures in the First Year of Distribution and Potential Solutions for the Future. Front. Public Health 2022, 10, 821117. [Google Scholar] [CrossRef]
- Gray, G.C.; Goswami, P.R.; Malasig, M.D.; Hawksworth, A.W.; Trump, D.H.; Ryan, M.A.; Schnurr, D.P. Adult adenovirus infections: Loss of orphaned vaccines precipitates military respiratory disease epidemics. For the Adenovirus Surveillance Group. Clin. Infect. Dis. 2000, 31, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radin, J.M.; Hawksworth, A.W.; Blair, P.J.; Faix, D.J.; Raman, R.; Russell, K.L.; Gray, G.C. Dramatic decline of respiratory illness among US military recruits after the renewed use of adenovirus vaccines. Clin. Infect. Dis. 2014, 59, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Hoke, C.H., Jr.; Snyder, C.E., Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine 2013, 31, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Dong, J.; Wang, C.; Zhan, Y.; Zhang, H.; Wu, J.; Kong, W.; Yu, X. Characteristics of neutralizing antibodies to adenovirus capsid proteins in human and animal sera. Virology 2013, 437, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Tian, X.; Jiang, Z.; Ma, Q.; Liu, Q.; Lu, X.; Zhou, R. Human Adenovirus Serotype 3 Vector Packaged by a Rare Serotype 14 Hexon. PLoS ONE 2016, 11, e0156984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Ahmed, K.A.; Ayalew, L.E.; Popowich, S.; Kurukulasuriya, S.; Goonewardene, K.; Gunawardana, T.; Karunarathna, R.; Ojkic, D.; Tikoo, S.K.; et al. Immunogenicity and protective efficacy of virus-like particles and recombinant fiber proteins in broiler-breeder vaccination against fowl adenovirus (FAdV)-8b. Vaccine 2017, 35, 2716–2722. [Google Scholar] [CrossRef] [PubMed]
- Naesens, L.; Lenaerts, L.; Andrei, G.; Snoeck, R.; Van Beers, D.; Holy, A.; Balzarini, J.; De Clercq, E. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob. Agents Chemother. 2005, 49, 1010–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavin, P.J.; Katz, B.Z. Intravenous ribavirin treatment for severe adenovirus disease in immunocompromised children. Pediatrics 2002, 110, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morfin, F.; Dupuis-Girod, S.; Mundweiler, S.; Falcon, D.; Carrington, D.; Sedlacek, P.; Bierings, M.; Cetkovsky, P.; Kroes, A.C.; van Tol, M.J.; et al. In vitro susceptibility of adenovirus to antiviral drugs is species-dependent. Antivir. Ther. 2005, 10, 225–229. [Google Scholar] [CrossRef]
- Ganapathi, L.; Arnold, A.; Jones, S.; Patterson, A.; Graham, D.; Harper, M.; Levy, O. Use of cidofovir in pediatric patients with adenovirus infection. F1000Research 2016, 5, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.K.; Brown, B.K.; Namtu, K.C.; Berman, D.M.; Kiskaddon, A.L. Use of cidofovir with extracorporeal membrane oxygenation to treat adenovirus-associated acute respiratory distress syndrome in paediatric patients- a case series. J. Clin. Pharm. Ther. 2020, 45, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/results?term=treatment&cond=Adenovirus+Disease&Search=Apply&recrs=e&age_v=&gndr=&type=&rslt=With (accessed on 28 November 2022).
- Florescu, D.F.; Keck, M.A. Development of CMX001 (Brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev. Anti-Infect. Ther. 2014, 12, 1171–1178. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Damon, I.; Relman, D.A.; Huggins, J.; Jahrling, P.B. Smallpox research activities: U.S. interagency collaboration, 2001. Emerg. Infect. Dis. 2002, 8, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cardona, J.J.; Whited, L.K.; Chemaly, R.F. Brincidofovir: Understanding its unique profile and potential role against adenovirus and other viral infections. Future Microbiol. 2020, 15, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Painter, W.; Robertson, A.; Trost, L.C.; Godkin, S.; Lampert, B.; Painter, G. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob. Agents Chemother. 2012, 56, 2726–2734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.H.; Price, N.B.; Hartline, C.B.; Lanier, E.R.; Prichard, M.N. Selection and recombinant phenotyping of a novel CMX001 and cidofovir resistance mutation in human cytomegalovirus. Antimicrob. Agents Chemother. 2013, 57, 3321–3325. [Google Scholar] [CrossRef] [Green Version]
- Clinical Trials. Available online: https://clinicaltrials.gov/ct2/show/NCT02087306 (accessed on 28 November 2022).
- Wold, W.S.; Toth, K. New drug on the horizon for treating adenovirus. Expert Opin. Pharmacother. 2015, 16, 2095–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatsis, N.; Ertl, H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Alhashimi, M.; Elkashif, A.; Sayedahmed, E.E.; Mittal, S.K. Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases. Viruses 2021, 13, 1493. [Google Scholar] [CrossRef]
- Baden, L.R.; Stieh, D.J.; Sarnecki, M.; Walsh, S.R.; Tomaras, G.D.; Kublin, J.G.; McElrath, M.J.; Alter, G.; Ferrari, G.; Montefiori, D.; et al. Safety and immunogenicity of two heterologous HIV vaccine regimens in healthy, HIV-uninfected adults (TRAVERSE): A randomised, parallel-group, placebo-controlled, double-blind, phase 1/2a study. Lancet HIV 2020, 7, e688–e698. [Google Scholar] [CrossRef] [PubMed]
- Bullard, B.L.; Corder, B.N.; Gordon, D.N.; Pierson, T.C.; Weaver, E.A. Characterization of a Species E Adenovirus Vector as a Zika virus vaccine. Sci. Rep. 2020, 10, 3613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, Y.; Yoshii, T.; Iyori, M.; Yoshida, K.; Mizukami, H.; Fukumoto, S.; Yamamoto, D.S.; Alam, A.; Emran, T.B.; Amelia, F.; et al. Adeno-Associated Virus as an Effective Malaria Booster Vaccine Following Adenovirus Priming. Front. Immunol. 2019, 10, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Lundstrom, K. Viral Vectors for COVID-19 Vaccine Development. Viruses 2021, 13, 317. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Soraci, L.; Lattanzio, F.; Soraci, G.; Gambuzza, M.E.; Pulvirenti, C.; Cozza, A.; Corsonello, A.; Luciani, F.; Rezza, G. COVID-19 Vaccines: Current and Future Perspectives. Vaccines 2022, 10, 608. [Google Scholar] [CrossRef]
- Lundstrom, K. Application of Viral Vectors for Vaccine Development with a Special Emphasis on COVID-19. Viruses 2020, 12, 1324. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.L. Adenovirus vectors as recombinant viral vaccines. Vaccine 1995, 13, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Juillard, V.; Villefroy, P.; Godfrin, D.; Pavirani, A.; Venet, A.; Guillet, J.G. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur. J. Immunol. 1995, 25, 3467–3473. [Google Scholar] [CrossRef] [PubMed]
- Abbink, P.; Maxfield, L.F.; Ng’ang’a, D.; Borducchi, E.N.; Iampietro, M.J.; Bricault, C.A.; Teigler, J.E.; Blackmore, S.; Parenteau, L.; Wagh, K.; et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J. Virol. 2015, 89, 1512–1522. [Google Scholar] [CrossRef] [Green Version]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, Q.; Shan, C.; Yang, C.; Feng, Y.; Wu, J.; Liu, X.; Zhou, Y.; Jiang, R.; Hu, P.; et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun. 2020, 11, 4207. [Google Scholar] [CrossRef]
- Stadler, K.; Masignani, V.; Eickmann, M.; Becker, S.; Abrignani, S.; Klenk, H.D.; Rappuoli, R. SARS—Beginning to understand a new virus. Nat. Rev. Microbiol. 2003, 1, 209–218. [Google Scholar] [CrossRef]
- Du, L.; Zhao, G.; Lin, Y.; Sui, H.; Chan, C.; Ma, S.; He, Y.; Jiang, S.; Wu, C.; Yuen, K.Y.; et al. Intranasal vaccination of recombinant adeno-associated virus encoding receptor-binding domain of severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein induces strong mucosal immune responses and provides long-term protection against SARS-CoV infection. J. Immunol. 2008, 180, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Shim, B.S.; Stadler, K.; Nguyen, H.H.; Yun, C.H.; Kim, D.W.; Chang, J.; Czerkinsky, C.; Song, M.K. Sublingual immunization with recombinant adenovirus encoding SARS-CoV spike protein induces systemic and mucosal immunity without redirection of the virus to the brain. Virol. J. 2012, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Munster, V.J.; Wells, D.; Lambe, T.; Wright, D.; Fischer, R.J.; Bushmaker, T.; Saturday, G.; van Doremalen, N.; Gilbert, S.C.; de Wit, E.; et al. Protective efficacy of a novel simian adenovirus vaccine against lethal MERS-CoV challenge in a transgenic human DPP4 mouse model. NPJ Vaccines 2017, 2, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matz, K.M.; Marzi, A.; Feldmann, H. Ebola vaccine trials: Progress in vaccine safety and immunogenicity. Expert Rev. Vaccines 2019, 18, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, L.; Kremer, E.J.; Shayakhmetov, D.M. Adenovirus-based vaccines-a platform for pandemic preparedness against emerging viral pathogens. Mol. Ther. 2022, 30, 1822–1849. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681, Correction in Lancet 2021, 397, 670. [Google Scholar] [CrossRef]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582, Correction in Nature 2021, 590, E24. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111, Correction in Lancet 2021, 397, 98. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993, Correction in Lancet 2021, 396, 1978; Correction in Lancet 2021, 397, 1350. [Google Scholar] [CrossRef]
- Handel, A.; Longini, I.M., Jr.; Antia, R. What is the best control strategy for multiple infectious disease outbreaks? Proc. Biol. Sci. 2007, 274, 833–837. [Google Scholar] [CrossRef] [Green Version]
- Aledort, J.E.; Lurie, N.; Wasserman, J.; Bozzette, S.A. Non-pharmaceutical public health interventions for pandemic influenza: An evaluation of the evidence base. BMC Public Health 2007, 7, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartsch, S.M.; O’Shea, K.J.; Chin, K.L.; Strych, U.; Ferguson, M.C.; Bottazzi, M.E.; Wedlock, P.T.; Cox, S.N.; Siegmund, S.S.; Hotez, P.J.; et al. Maintaining face mask use before and after achieving different COVID-19 vaccination coverage levels: A modelling study. Lancet Public Health 2022, 7, e356–e365. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, T.; Del Mar, C.B.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Bawazeer, G.A.; van Driel, M.L.; Nair, S.; Jones, M.A.; Thorning, S.; et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. 2011, 2011, CD006207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, N.; Nettleton, S.; Buse, C.; Lewis, A.; Martin, D. The coughing body: Etiquettes, techniques, sonographies and spaces. Biosocieties 2021, 16, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Chen, Y.Q. China can prepare to end its zero-COVID policy. Nat. Med. 2022, 28, 1104–1105. [Google Scholar] [CrossRef]
- Bielecki, M.; Patel, D.; Hinkelbein, J.; Komorowski, M.; Kester, J.; Ebrahim, S.; Rodriguez-Morales, A.J.; Memish, Z.A.; Schlagenhauf, P. Air travel and COVID-19 prevention in the pandemic and peri-pandemic period: A narrative review. Travel Med. Infect. Dis. 2021, 39, 101915. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Respiratory Hygiene/Cough Etiquette in Healthcare Settings. Available online: http://www.cdc.gov/flu/professionals/infectioncontrol/resphygiene.htm (accessed on 28 November 2022).
- Beigel, J.H.; Farrar, J.; Han, A.M.; Hayden, F.G.; Hyer, R.; de Jong, M.D.; Lochindarat, S.; Nguyen, T.K.; Nguyen, T.H.; Tran, T.H.; et al. Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 2005, 353, 1374–1385, Correction in N. Engl. J. Med. 2006, 354, 884. [Google Scholar] [CrossRef] [Green Version]
- Yuen, K.Y.; Chan, P.K.; Peiris, M.; Tsang, D.N.; Que, T.L.; Shortridge, K.F.; Cheung, P.T.; To, W.K.; Ho, E.T.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef]
- Ng, E.K.; Cheng, P.K.; Ng, A.Y.; Hoang, T.L.; Lim, W.W. Influenza A H5N1 detection. Emerg. Infect. Dis. 2005, 11, 1303–1305. [Google Scholar] [CrossRef]
- Bridges, C.B.; Kuehnert, M.J.; Hall, C.B. Transmission of influenza: Implications for control in health care settings. Clin. Infect. Dis. 2003, 37, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Committee on the Development of Reusable Facemasks for Use During an Influenza Pandemic; Board on Health Sciences Policy; Institute of Medicine of the National Academies. Reusability of Facemasks during an Influenza Pandemic: Facing the Flu; The National Academies Press: Washington, WA, USA, 2006. [Google Scholar] [CrossRef]
- Heymann, A.; Chodick, G.; Reichman, B.; Kokia, E.; Laufer, J. Influence of school closure on the incidence of viral respiratory diseases among children and on health care utilization. Pediatr. Infect. Dis. J. 2004, 23, 675–677. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Impact of seasonal influenza-related school closures on families—Southeastern Kentucky, February 2008. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1405–1409. [Google Scholar]
- Perra, N. Non-pharmaceutical interventions during the COVID-19 pandemic: A review. Phys. Rep. 2021, 913, 1–52. [Google Scholar] [CrossRef]
- Glogowsky, U.; Hansen, E.; Schächtele, S. How effective are social distancing policies? Evidence on the fight against COVID-19. PLoS ONE 2021, 16, e0257363. [Google Scholar] [CrossRef] [PubMed]
- Woskie, L.R.; Hennessy, J.; Espinosa, V.; Tsai, T.C.; Vispute, S.; Jacobson, B.H.; Cattuto, C.; Gauvin, L.; Tizzoni, M.; Fabrikant, A.; et al. Early social distancing policies in Europe, changes in mobility & COVID-19 case trajectories: Insights from Spring 2020. PLoS ONE 2021, 16, e0253071. [Google Scholar] [CrossRef]
- Wellenius, G.A.; Vispute, S.; Espinosa, V.; Fabrikant, A.; Tsai, T.C.; Hennessy, J.; Dai, A.; Williams, B.; Gadepalli, K.; Boulanger, A.; et al. Impacts of social distancing policies on mobility and COVID-19 case growth in the US. Nat. Commun. 2021, 12, 3118. [Google Scholar] [CrossRef]
- Courtemanche, C.; Garuccio, J.; Le, A.; Pinkston, J.; Yelowitz, A. Strong Social Distancing Measures In The United States Reduced The COVID-19 Growth Rate. Health Aff. 2020, 39, 1237–1246. [Google Scholar] [CrossRef]
- Gostin, L. Public health strategies for pandemic influenza: Ethics and the law. JAMA 2006, 295, 1700–1704. [Google Scholar] [CrossRef] [PubMed]
- Blendon, R.J.; Benson, J.M.; DesRoches, C.M.; Raleigh, E.; Taylor-Clark, K. The public’s response to severe acute respiratory syndrome in Toronto and the United States. Clin. Infect. Dis. 2004, 38, 925–931. [Google Scholar] [CrossRef] [Green Version]
- Cobb, J.S.; Seale, M.A. Examining the effect of social distancing on the compound growth rate of COVID-19 at the county level (United States) using statistical analyses and a random forest machine learning model. Public Health 2020, 185, 27–29. [Google Scholar] [CrossRef]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: Systematic review and meta-analysis. BMJ 2021, 375, e068302, Erratum in BMJ 2021, 375, N2997. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Bulir, D.; De Prophetis, E.; Jamil, M.; Rosella, L.C.; Mertz, D.; Regehr, C.; Smieja, M. COVID-19 international border surveillance at Toronto’s Pearson Airport: A cohort study. BMJ Open 2021, 11, e05071. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.; Ampuero, M.; Garcés, C.; Gaggero, A.; García, P.; Velasquez, M.S.; Luza, R.; Alvarez, P.; Paredes, F.; Acevedo, J.; et al. Performance of SARS-CoV-2 rapid antigen test compared with real-time RT-PCR in asymptomatic individuals. Int. J. Infect. Dis. 2021, 107, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Razavi Bazaz, S.; Morshedi Rad, D.; Shimoni, O.; Jin, D.; Rawlinson, W.; Ebrahimi Warkiani, M. A Portable RT-LAMP/CRISPR Machine for Rapid COVID-19 Screening. Biosensors 2021, 11, 369. [Google Scholar] [CrossRef]
- Burns, J.; Movsisyan, A.; Stratil, J.M.; Biallas, R.L.; Coenen, M.; Emmert-Fees, K.M.; Geffert, K.; Hoffmann, S.; Horstick, O.; Laxy, M.; et al. International travel-related control measures to contain the COVID-19 pandemic: A rapid review. Cochrane Database Syst. Rev. 2021, 3, CD013717. [Google Scholar] [CrossRef]
- Ziegler, T.; Mamahit, A.; Cox, N.J. 65 years of influenza surveillance by a World Health Organization-coordinated global network. Influenza Other Respir. Viruses 2018, 12, 558–565. [Google Scholar] [CrossRef]
- Szecsenyi, J.; Uphoff, H.; Ley, S.; Brede, H.D. Influenza surveillance: Experiences from establishing a sentinel surveillance system in Germany. J. Epidemiol. Community Health 1995, 49 (Suppl. 1), 9–13. [Google Scholar] [CrossRef] [Green Version]
- Lange, W.; Schöttler, M. Real-time influenza surveillance in Germany--results of a pilot project. Med. Microbiol. Immunol. 2002, 191, 139–144. [Google Scholar] [CrossRef]
- Flahault, A.; Dias-Ferrao, V.; Chaberty, P.; Esteves, K.; Valleron, A.J.; Lavanchy, D. FluNet as a tool for global monitoring of influenza on the Web. JAMA 1998, 280, 1330–1332. [Google Scholar] [CrossRef]
- Western Pacific Region Global Influenza Surveillance and Response System. Epidemiological and virological characteristics of influenza in the Western Pacific Region of the World Health Organization, 2006–2010. PLoS ONE 2012, 7, e37568. [Google Scholar] [CrossRef]
- Adlhoch, C.; Gomes, H.C. Sustainability of surveillance systems for SARS-CoV-2. Lancet Infect. Dis. 2022, 22, 914–915. [Google Scholar] [CrossRef] [PubMed]
- Ganbaatar, U.; Liu, C. CRISPR-Based COVID-19 Testing: Toward Next-Generation Point-of-Care Diagnostics. Front. Cell Infect. Microbiol. 2021, 11, 663949. [Google Scholar] [CrossRef] [PubMed]
- Kajon, A.E.; Ison, M.G. Severe Infections with Human Adenovirus 7d in 2 Adults in Family, Illinois, USA, 2014. Emerg. Infect. Dis. 2016, 11, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, V.; Chen, J.S.; Hsu, G.J.; Chen, H.P.; Chao, H.C.; Huang, S.W.; Tsai, I.S.; Hsu, B.M. Surveillance of Adenovirus and Norovirus Contaminants in the Water and Shellfish of Major Oyster Breeding Farms and Fishing Ports in Taiwan. Pathogens 2022, 736, 316. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G. Wastewater surveillance for population-wide Covid-19: The present and future. Sci. Total Environ. 2020, 736, 139631. [Google Scholar] [CrossRef]
- La Rosa, G.; Iaconelli, M.; Mancini, P.; Bonanno Ferraro, G.; Veneri, C.; Bonadonna, L.; Lucentini, L.; Suffredini, E. First detection of SARS-CoV-2 in untreated wastewaters in Italy. Sci. Total Environ. 2020, 12, 139652. [Google Scholar] [CrossRef]
- Guajardo-Leiva, S.; Chnaiderman, J.; Gaggero, A.; Díez, B. Metagenomic Insights into the Sewage RNA Virosphere of a Large City. Viruses 2020, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Aw, T.G.; Howe, A.; Rose, J.B. Metagenomic approaches for direct and cell culture evaluation of the virological quality of wastewater. J. Virol. Methods 2014, 210, 15–21. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; et al. Avian influenza overview June–September 2022. EFSA J. 2022, 20, e07597. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Zheng, P.P.; Zhang, T.F.; Wen, G.Y.; Shao, H.B.; Luo, Q.P. Fowl adenovirus serotype 4: Epidemiology, pathogenesis, diagnostic detection, and vaccine strategies. Poult. Sci. 2017, 96, 2630–2640. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yao, K.C.; Liu, Y.Y.; You, G.J.; Li, S.Y.; Liu, P.; Zhao, Q.; Wen Rui Wu, Y.P.; Huang, X.B.; Cao, S.J.; et al. Isolation and molecular characterization of prevalent Fowl adenovirus strains in southwestern China during 2015–2016 for the development of a control strategy. Emerg. Microbes. Infect. 2017, 6, e103. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.K.; Ngo, T.T.; Tran, P.M.; Pham, T.T.T.; Vu, H.T.T.; Nguyen, N.T.H.; Thwaites, G.; Virtala, A.K.; Vapalahti, O.; Baker, S.; et al. Respiratory viruses in individuals with a high frequency of animal exposure in southern and highland Vietnam. J. Med. Virol. 2020, 92, 971–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, G.C.; Abdelgadir, A. While We Endure This Pandemic, What New Respiratory Virus Threats Are We Missing? Open Forum Infect. Dis. 2021, 8, ofab078. [Google Scholar] [CrossRef] [PubMed]
- Enríquez, A.; Sáenz, C. Primeras Lecciones y Desafíos de la Pandemia de COVID-19 Para los Países del SICA; Serie Estudios y Perspectivas-Sede Subregional de la CEPAL en México, N° 189 (LC/TS.2021/38; LC/MEX/TS.2021/5), Ciudad de México; Comisión Económica para América Latina y el Caribe (CEPAL): Santiago de Chile, Chile, 2021. [Google Scholar]
- Murthy, S.; Leligdowicz, A.; Adhikari, N.K. Intensive care unit capacity in low-income countries: A systematic review. PLoS ONE 2015, 10, e0116949. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, F.A.; Deo, N.; Surani, S.; Maynard, W.; Gibbs, M.L.; Kashyap, R. Critical care practices in the world: Results of the global intensive care unit need assessment survey 2020. World J. Crit. Care Med. 2022, 11, 169–177. [Google Scholar] [CrossRef]
- Hinton, R.; Armstrong, C.; Asri, E.; Baesel, K.; Barnett, S.; Blauvelt, C.; Buang, S.N.B.; Bury, L.; Das, J.K.; Franz-Vasdeki, J.; et al. Specific considerations for research on the effectiveness of multisectoral collaboration: Methods and lessons from 12 country case studies. Glob. Health 2021, 17, 18. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saint-Pierre Contreras, G.; Muñoz Gomez, G.; Silva Ojeda, F. En búsqueda de otros virus respiratorios durante la pandemia COVID-19 [In search of other respiratory viruses during the COVID-19 pandemic]. Rev. Clin. Esp. 2021, 221, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Buda, S.; Biere, B.; Reiche, J.; Schlosser, F.; Duwe, S.; Wedde, M.; von Kleist, M.; Mielke, M.; Wolff, T.; et al. Trends in respiratory virus circulation following COVID-19-targeted nonpharmaceutical interventions in Germany, January–September 2020: Analysis of national surveillance data. Lancet Reg. Health Eur. 2021, 6, 100112. [Google Scholar] [CrossRef]
- Qiu, Z.; Cao, Z.; Zou, M.; Tang, K.; Zhang, C.; Tang, J.; Zeng, J.; Wang, Y.; Sun, Q.; Wang, D.; et al. The effectiveness of governmental nonpharmaceutical interventions against COVID-19 at controlling seasonal influenza transmission: An ecological study. BMC Infect. Dis. 2022, 22, 331. [Google Scholar] [CrossRef]
- Zhong, R.; Yi, F.; Xiang, F.; Qiu, Y.F.; Zhu, L.; Zou, Y.H.; Wang, W.; Zhang, Q. Hepatitis of unknown etiology in children: Current evidence and association. World J. Clin. Cases 2022, 10, 12837–12843. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.C.; Deep, A. Characterization of a Hepatitis Outbreak in Children, 2021 to 2022. JAMA Netw. Open 2022, 5, e223709. [Google Scholar] [CrossRef]
- Gutierrez Sanchez, L.H.; Shiau, H.; Baker, J.M.; Saaybi, S.; Buchfellner, M.; Britt, W.; Sanchez, V.; Potter, J.L.; Ingram, L.A.; Kelly, D.; et al. A Case Series of Children with Acute Hepatitis and Human Adenovirus Infection. N. Engl. J. Med. 2022, 387, 620–630. [Google Scholar] [CrossRef]
- Brodin, P.; Arditi, M. Severe acute hepatitis in children: Investigate SARS-CoV-2 superantigens. Lancet Gastroenterol. Hepatol. 2022, 7, 594–595. [Google Scholar] [CrossRef] [PubMed]
- Kelgeri, C.; Couper, M.; Gupte, G.L.; Brant, A.; Patel, M.; Johansen, L.; Valamparampil, J.; Ong, E.; Hartog, H.; Perera, M.T.P.R.; et al. Clinical Spectrum of Children with Acute Hepatitis of Unknown Cause. N. Engl. J. Med. 2022, 387, 611–619. [Google Scholar] [CrossRef]
- Cantor, A.; Miller, J.; Zachariah, P.; DaSilva, B.; Margolis, K.; Martinez, M. Acute Hepatitis Is a Prominent Presentation of the Multisystem Inflammatory Syndrome in Children: A Single-Center Report. Hepatology 2020, 72, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. Investigation into Acute Hepatitis of Unknown Aetiology in Children in England: Technical Briefing 1. 2022. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1071198/acute-hepatitis-technical-briefing-1_4_.pdf (accessed on 17 June 2022).
- Gong, K.; Xu, X.; Yao, J.; Ye, S.; Yu, X.; Tu, H.; Lan, Y.; Fan, Y.C.; Shi, Y. Acute hepatitis of unknown origin in children: A combination of factors. Front. Pharmacol. 2022, 13, 1056385. [Google Scholar] [CrossRef]
- Collis, P.B.; Dudding, B.A.; Winter, P.E.; Russell, P.K.; Buescher, E.L. Adenovirus vaccines in military recruit populations: A cost-benefit analysis. J. Infect. Dis. 1973, 128, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Singh, N.; Vemula, S.V.; Couëtil, L.; Katz, J.M.; Donis, R.; Sambhara, S.; Mittal, S.K. Impact of Preexisting Adenovirus Vector Immunity on Immunogenicity and Protection Conferred with an Adenovirus-Based H5N1 Influenza Vaccine. PLoS ONE 2012, 7, e33428. [Google Scholar] [CrossRef] [Green Version]
- Ondondo, B.O. The influence of delivery vectors on HIV vaccine efficacy. Front. Microbiol. 2014, 5, 439. [Google Scholar] [CrossRef] [Green Version]
- Li, J.X.; Hou, L.H.; Meng, F.Y.; Wu, S.P.; Hu, Y.M.; Liang, Q.; Chu, K.; Zhang, Z.; Xu, J.J.; Tang, R.; et al. Immunity duration of a recombinant adenovirus type-5 vector-based Ebola vaccine and a homologous prime-boost immunisation in healthy adults in China: Final report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Glob. Health 2017, 5, e324–e334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F.C.; Hou, L.H.; Li, J.X.; Wu, S.P.; Liu, P.; Zhang, G.R.; Hu, Y.M.; Meng, F.Y.; Xu, J.J.; Tang, R.; et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: Preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 2015, 385, 2272–2279. [Google Scholar] [CrossRef]
- Ji, T.; Li, L.; Li, W.; Zheng, X.; Ye, X.; Chen, H.; Zhou, Q.; Jia, H.; Chen, B.; Lin, Z.; et al. Emergence and characterization of a putative novel human adenovirus recombinant HAdV-C104 causing pneumonia in Southern China. Virus Evol. 2021, 7, veab018. [Google Scholar] [CrossRef] [PubMed]
- Beatty, M.S.; Curiel, D.T. Chapter two—Adenovirus strategies for tissue-specific targeting. Adv. Cancer Res. 2012, 115, 39–67. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.K.; Dmitriev, I.P.; Kashentseva, E.A.; Raes, G.; Li, L.; Kim, S.W.; Lu, Z.H.; Arbeit, J.M.; Fleming, T.P.; Kaliberov, S.A.; et al. Curiel, D.T.; Gillanders, W.E. Development of an adenovirus vector vaccine platform for targeting dendritic cells. Cancer Gene Ther. 2018, 25, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Food and Drugs Administration. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-limits-use-janssen-covid-19-vaccine-certain-individuals#:~:text=Today%2C%20the%20U.S.%20Food%20and,who%20elect%20to%20receive%20the (accessed on 30 November 2022).
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene Ther. 2013, 13, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021, 22, 10522. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Migueles, S.A.; Huang, J.; Bolkhovitinov, L.; Stuccio, S.; Griesman, T.; Pullano, A.A.; Kang, B.H.; Ishida, E.; Zimmerman, M.; et al. A replication-competent adenovirus-vectored influenza vaccine induces durable systemic and mucosal immunity. J. Clin. Investig. 2021, 131, e140794. [Google Scholar] [CrossRef]
- Petro-Turnquist, E.M.; Bullard, B.L.; Pekarek, M.J.; Weaver, E.A. Adenoviral-Vectored Centralized Consensus Hemagglutinin Vaccine Provides Broad Protection against H2 Influenza a Virus. Vaccines 2022, 10, 926. [Google Scholar] [CrossRef]
- Gu, J.; Su, Q.Q.; Zuo, T.T.; Chen, Y.B. Adenovirus diseases: A systematic review and meta-analysis of 228 case reports. Infection 2021, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, J.; Liu, C.; Duan, Y.; Zhu, Y.; Xu, B.; Xie, Z. Case-control study of the epidemiological and clinical features of human adenovirus 55 and human adenovirus 7 infection in children with acute lower respiratory tract infections in Beijing, China, 2008–2013. BMC Infect. Dis. 2018, 18, 634. [Google Scholar] [CrossRef]
- Mao, N.; Zhu, Z.; Rivailler, P.; Yang, J.; Li, Q.; Han, G.; Yin, J.; Yu, D.; Sun, L.; Jiang, H.; et al. Multiple divergent Human mastadenovirus C co-circulating in mainland of China. Infect. Genet. Evol. 2019, 76, 104035. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, S.; Zhang, L.; Wu, H.; Tian, X.; Li, X.; Xu, D.; Dai, J.; Gu, S.; Liu, Q.; et al. Analysis of severe human adenovirus infection outbreak in Guangdong Province, southern China in 2019. Virol. Sin. 2022, 37, 331–340. [Google Scholar] [CrossRef]
- Mollentze, N.; Babayan, S.A.; Streicker, D.G. Identifying and prioritizing potential human-infecting viruses from their genome sequences. PLoS Biol. 2021, 19, e3001390. [Google Scholar] [CrossRef]
- Griswold, D.P.; Gempeler, A.; Kolias, A.; Hutchinson, P.J.; Rubiano, A.M. Personal protective equipment for reducing the risk of COVID-19 infection among health care workers involved in emergency trauma surgery during the pandemic: An umbrella review. J. Trauma Acute Care Surg. 2021, 90, e72–e80. [Google Scholar] [CrossRef]
| Oncogenic Potential | |||||||
|---|---|---|---|---|---|---|---|
| Species | Hemagglutination Groups | Types | Tumors in Animals | Transformation in Cell Culture | % GC | Associated Disease | Severe or Deadly Diseases (Reported) |
| HAdV-A | IV (little or none) | 12, 18, 31 | High | Positive | 46–47 | Cryptic enteric infection | Unknown |
| HAdV-B | I (Complete for monkey erythrocyte) | 3, 7, 11, 14, 16, 21, 34, 35, 50 | Moderate | Positive | 49–51 | Conjunctivitis, Acute respiratory disease, Hemorrhagic cystitis, Central nervous system | Type 3 and 7 |
| HAdV-C | II (Partial for rat erythrocytes) | 1, 2, 5, 6 | Low or none | Positive | 55 | Endemic infection, Respiratory symptoms | Type 5 |
| HAdV-D | III (Complete for rat erythrocytes) | 8, 9, 10, 13, 15, 17, 19, 20, 22–30, 32, 33, 36–39, 42–49, 51, 53, 54 | Low or none (Mammary tumors) | Positive | 55–57 | Keratoconjunctivitis in immunocompromised and AIDS patients | Unknown |
| HAdV-E | III | 4 | Low or none | Positive | 58 | Conjunctivitis, Acute respiratory disease | Unknown |
| HAdV-F | III | 40, 41 | Unknown | Negative | 51 | Infantile diarrhea | Unknown |
| HAdV-G | Unknown | 52 | Unknown | Unknown | 55 | Gastroenteritis | Unknown |
| Protein | E1A 29.1 kDa | E1B 20 kDa | E2B DNA Polymerase | L1 pIIIa | L2 Penton Base | L3 Hexon | E2A DBP | L4 pVIII | E3 11.7 kDa | L5 Fiber | E4 ORF 6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HAdV-B35 | 95.8 | 98.3 | 93.2 | 99.5 | 98.6 | 94.4 | 62.9 | 99.1 | 99.1 | 62.1 | 97.7 |
| HAdV-B34 | 97.7 | 99.4 | 98.7 | 99.3 | 95.3 | 91.3 | 99.4 | 99.1 | 98.1 | 62.2 | 97.7 |
| HAdV-B14 | 97.7 | 100.0 | 99.7 | 99.8 | 99.5 | 92.2 | 99.8 | 99.6 | 99.1 | 99.1 | 99.3 |
| HAdV-B11 | 96.6 | 98.3 | 93.0 | 99.3 | 98.4 | 98.4 | 99.2 | 99.1 | 98.1 | 92.3 | 98.0 |
| HAdV-B7 | 79.4 | 87.8 | 89.9 | 92.7 | 85.3 | 86.6 | 82.6 | 94.3 | 90.6 | 91.1 | 96.7 |
| HAdV-B3 | 79.0 | 87.2 | 89.9 | 93.0 | 85.5 | 86.3 | 83.8 | 94.3 | 89.6 | 56.7 | 97.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saint-Pierre Contreras, G.; Conei Valencia, D.; Lizama, L.; Vargas Zuñiga, D.; Avendaño Carvajal, L.F.; Ampuero Llanos, S. An Old Acquaintance: Could Adenoviruses Be Our Next Pandemic Threat? Viruses 2023, 15, 330. https://doi.org/10.3390/v15020330
Saint-Pierre Contreras G, Conei Valencia D, Lizama L, Vargas Zuñiga D, Avendaño Carvajal LF, Ampuero Llanos S. An Old Acquaintance: Could Adenoviruses Be Our Next Pandemic Threat? Viruses. 2023; 15(2):330. https://doi.org/10.3390/v15020330
Chicago/Turabian StyleSaint-Pierre Contreras, Gustavo, Daniel Conei Valencia, Luis Lizama, Daniela Vargas Zuñiga, Luis Fidel Avendaño Carvajal, and Sandra Ampuero Llanos. 2023. "An Old Acquaintance: Could Adenoviruses Be Our Next Pandemic Threat?" Viruses 15, no. 2: 330. https://doi.org/10.3390/v15020330
APA StyleSaint-Pierre Contreras, G., Conei Valencia, D., Lizama, L., Vargas Zuñiga, D., Avendaño Carvajal, L. F., & Ampuero Llanos, S. (2023). An Old Acquaintance: Could Adenoviruses Be Our Next Pandemic Threat? Viruses, 15(2), 330. https://doi.org/10.3390/v15020330





