Occurrence and Molecular Characterization of Cryptosporidium Infection in HIV/Aids Patients in Algeria
Abstract
1. Introduction
2. Materials and Methods
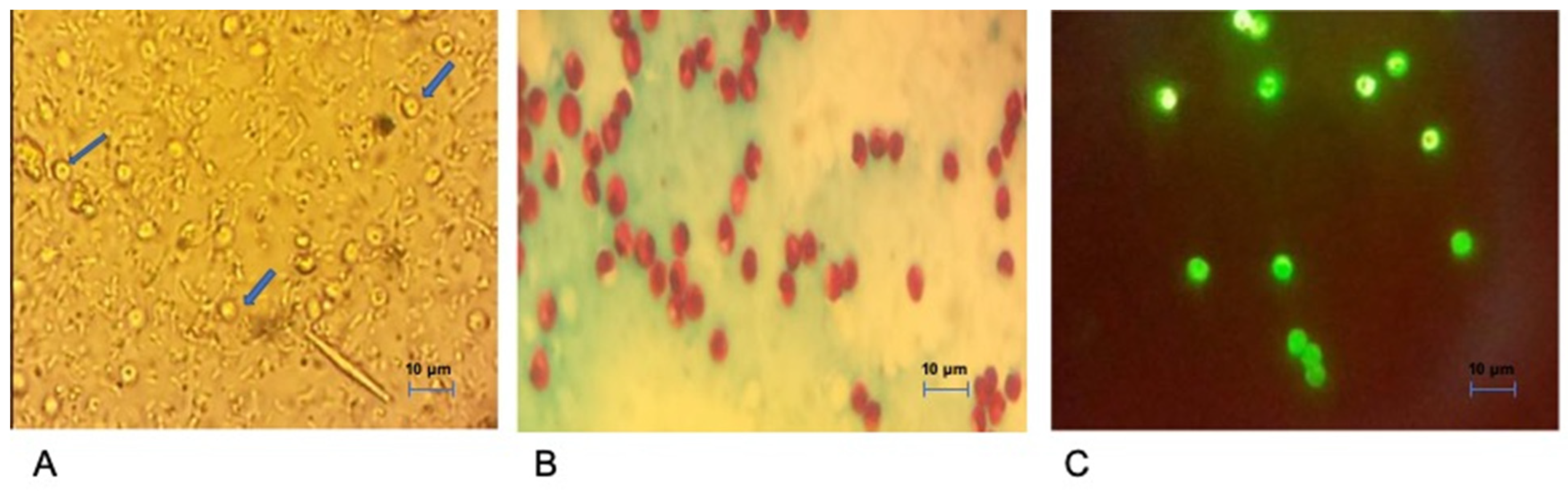
2.1. Patients, Faeces Sampling and Microscopy
2.2. Molecular Characterisation of Cryptosporidium spp.
2.2.1. Identification and Characterisation of Cryptosporidium Species
2.2.2. Gp60 Sequence Amplification
2.2.3. DNA Sequence Analysis
2.3. Consent and Ethical Approval
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients
3.2. Cryptosporidium Species and gp60 Genotypes Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Platts-Mills, J.A.; Juma, J.; Kabir, F.; Nkeze, J.; Okoi, C.; Operario, D.J.; Uddin, J.; Ahmed, S.; Alonso, P.L.; et al. Use of Quantitative Molecular Diagnostic Methods to Identify Causes of Diarrhoea in Children: A Reanalysis of the GEMS Case-Control Study. Lancet 2016, 388, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, L.; Surawicz, C.M. Infectious Causes of Chronic Diarrhoea. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 563–571. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, R.D.; DuPont, H.L. Etiology and Pharmacologic Management of Noninfectious Diarrhea in HIV-Infected Individuals in the Highly Active Antiretroviral Therapy Era. Clin. Infect. Dis. 2012, 55, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.A.; Healey, P.; Gordon, M.A. Review Article: The Aetiology, Investigation and Management of Diarrhoea in the HIV-Positive Patient: Review: Diarrhoea in HIV-Positive Patients. Aliment. Pharmacol. Ther. 2011, 34, 587–603. [Google Scholar] [CrossRef]
- Mwachari, C.; Batchelor, B.I.F.; Paul, J.; Waiyaki, P.G.; Gilks, C.F. Chronic Diarrhoea among HIV-Infected Adult Patients in Nairobi, Kenya. J. Infect. 1998, 37, 48–53. [Google Scholar] [CrossRef]
- Mwachari, C.W.; Meier, A.S.; Muyodi, J.; Gatei, W.; Waiyaki, P.; Cohen, C.R. Chronic Diarrhoea in HIV-1-Infected Adults in Nairobi, Kenya: Evaluation of Risk Factors and the WHO Treatment Algorithm. AIDS 2003, 17, 2124–2126. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Safarpour, H.; Xiao, L.; Zarean, M.; Hatam-Nahavandi, K.; Barac, A.; Picot, S.; Rahimi, M.T.; Rubino, S.; Mahami-Oskouei, M.; et al. Cryptosporidiosis in HIV-Positive Patients and Related Risk Factors: A Systematic Review and Meta-Analysis. Parasite 2020, 27, 27. [Google Scholar] [CrossRef]
- Aldeyarbi, H.M.; Abu El-Ezz, N.M.T.; Karanis, P. Cryptosporidium and Cryptosporidiosis: The African Perspective. Environ. Sci. Pollut. Res. Int. 2016, 23, 13811–13821. [Google Scholar] [CrossRef]
- Wang, R.; Li, J.; Chen, Y.; Zhang, L.; Xiao, L. Widespread Occurrence of Cryptosporidium Infections in Patients with HIV/AIDS: Epidemiology, Clinical Feature, Diagnosis, and Therapy. Acta Trop. 2018, 187, 257–263. [Google Scholar] [CrossRef]
- Tali, A.; Addebbous, A.; Asmama, S.; Chabaa, L.; Zougaghi, L. Respiratory Cryptosporidiosis in Two Patients with HIV Infection in a Tertiary Care Hospital in Morocco. Ann. Biol. Clin. 2011, 69, 605–608. [Google Scholar] [CrossRef]
- Botero-Garcés, J.; Villegas-Arbeláez, E.; Giraldo, S.; Urán-Velásquez, J.; Arias-Agudelo, L.; Alzate-Ángell, J.C.; García-Montoya, G.M.; Galván-Díaz, A.L. Prevalence of Intestinal Parasites in a Cohort of HIVinfected Patients from Antioquia, Colombia. Biomédica 2021, 41, 153–164. [Google Scholar] [CrossRef]
- Laatamna, A.E.; Wagnerová, P.; Sak, B.; Květoňová, D.; Xiao, L.; Rost, M.; McEvoy, J.; Saadi, A.R.; Aissi, M.; Kváč, M. Microsporidia and Cryptosporidium in Horses and Donkeys in Algeria: Detection of a Novel Cryptosporidium Hominis Subtype Family (Ik) in a Horse. Vet. Parasitol. 2015, 208, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, D.; Khelef, D.; Goucem, R.; Adjou, K.T.; Adamu, H.; Zhang, H.; Xiao, L. Common Occurrence of Zoonotic Pathogen Cryptosporidium Meleagridis in Broiler Chickens and Turkeys in Algeria. Vet. Parasitol. 2013, 196, 334–340. [Google Scholar] [CrossRef]
- Benhouda, D.; Hakem, A.; Sannella, A.R.; Benhouda, A.; Cacciò, S.M. First Molecular Investigation of Cryptosporidium spp. in Young Calves in Algeria. Parasite 2017, 24, 15. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Sharma, P.; Sharma, A.; Malla, N. Evaluation of Ziehl-Neelsen Staining, Auramine Phenol Staining, Antigen Detection Enzyme Linked Immunosorbent Assay and Polymerase Chain Reaction, for the Diagnosis of Intestinal Cryptosporidiosis. Trop. Parasitol. 2012, 2, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Whipp, M.J.; Haydon, S.R.; Gasser, R.B. Cryptosporidium Cuniculus—New Records in Human and Kangaroo in Australia. Parasites Vectors 2014, 7, 492. [Google Scholar] [CrossRef] [PubMed]
- Glaberman, S.; Moore, J.E.; Lowery, C.J.; Chalmers, R.M.; Sulaiman, I.; Elwin, K.; Rooney, P.J.; Millar, B.C.; Dooley, J.S.G.; Lal, A.A.; et al. Three Drinking-Water-Associated Cryptosporidiosis Outbreaks, Northern Ireland. Emerg. Infect. Dis. 2002, 8, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, I.M.; Hira, P.R.; Zhou, L.; Al-Ali, F.M.; Al-Shelahi, F.A.; Shweiki, H.M.; Iqbal, J.; Khalid, N.; Xiao, L. Unique Endemicity of Cryptosporidiosis in Children in Kuwait. J. Clin. Microbiol. 2005, 43, 2805–2809. [Google Scholar] [CrossRef] [PubMed]
- Framm, S.R.; Soave, R. Agents of Diarrhea. Med. Clin. N. Am. 1997, 81, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Shimelis, T.; Tassachew, Y.; Lambiyo, T. Cryptosporidium and Other Intestinal Parasitic Infections among HIV Patients in Southern Ethiopia: Significance of Improved HIV-Related Care. Parasites Vectors 2016, 9, 270. [Google Scholar] [CrossRef]
- Silva-Freitas, M.L.; Cota, G.F.; Machado-de-Assis, T.S.; Giacoia-Gripp, C.; Rabello, A.; Da-Cruz, A.M.; Santos-Oliveira, J.R. Immune Activation and Bacterial Translocation: A Link between Impaired Immune Recovery and Frequent Visceral Leishmaniasis Relapses in HIV-Infected Patients. PLoS ONE 2016, 11, e0167512. [Google Scholar] [CrossRef] [PubMed]
- Casado, J.L.; Abad-Fernández, M.; Moreno, S.; Pérez-Elías, M.J.; Moreno, A.; Bernardino, J.I.; Vallejo, A. Visceral Leishmaniasis as an Independent Cause of High Immune Activation, T-Cell Senescence, and Lack of Immune Recovery in Virologically Suppressed HIV-1-Coinfected Patients. HIV Med. 2015, 16, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.M.; Awad-El-Kariem, F.M.; Franzen, C.; Ellis, D.S.; Müller, A.; Counihan, H.M.; Hayes, P.J.; Gazzard, B.G. Eradication of Cryptosporidia and Microsporidia Following Successful Antiretroviral Therapy. JAIDS J. Acquir. Immune Defic. Syndr. 2000, 25, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Mele, R.; Gomez Morales, M.A.; Tosini, F.; Pozio, E. Indinavir Reduces Cryptosporidium Parvum Infection in Both in Vitro and in Vivo Models. Int. J. Parasitol. 2003, 33, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Essid, R.; Menotti, J.; Hanen, C.; Aoun, K.; Bouratbine, A. Genetic Diversity of Cryptosporidium Isolates from Human Populations in an Urban Area of Northern Tunisia. Infect. Genet. Evol. 2018, 58, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Adamu, H.; Petros, B.; Zhang, G.; Kassa, H.; Amer, S.; Ye, J.; Feng, Y.; Xiao, L. Distribution and Clinical Manifestations of Cryptosporidium Species and Subtypes in HIV/AIDS Patients in Ethiopia. PLoS Negl. Trop. Dis. 2014, 8, e2831. [Google Scholar] [CrossRef]
- Zulu, I.; Kelly, P.; Njobvu, L.; Sianongo, S.; Kaonga, K.; McDonald, V.; Farthing, M.; Pollok, R. Nitazoxanide for Persistent Diarrhoea in Zambian Acquired Immune Deficiency Syndrome Patients: A Randomized-Controlled Trial. Aliment. Pharmacol. Ther. 2005, 21, 757–763. [Google Scholar] [CrossRef]
- Amadi, B.; Mwiya, M.; Sianongo, S.; Payne, L.; Watuka, A.; Katubulushi, M.; Kelly, P. High Dose Prolonged Treatment with Nitazoxanide Is Not Effective for Cryptosporidiosis in HIV Positive Zambian Children: A Randomised Controlled Trial. BMC Infect. Dis. 2009, 9, 195. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Barchiesi, F.; Ancarani, F.; Scalise, G. Activity of Nitazoxanide Alone and in Combination with Azithromycin and Rifabutin against Cryptosporidium Parvum in Cell Culture. J. Antimicrob. Chemother. 2000, 45, 453–456. [Google Scholar] [CrossRef]
- Werneck-Silva, A.L.; Prado, I.B. Dyspepsia in HIV-Infected Patients under Highly Active Antiretroviral Therapy. J. Gastroenterol. Hepatol 2007, 22, 1712–1716. [Google Scholar] [CrossRef]
- Akinbo, F.O.; Okaka, C.E.; Omoregie, R. Prevalence of Intestinal Parasitic Infections among HIV Patients in Benin City, Nigeria. Libyan J. Med. 2010, 5, 5506. [Google Scholar] [CrossRef] [PubMed]
- Ayinmode, A.B.; Zhang, H.; Dada-Adegbola, H.O.; Xiao, L. Cryptosporidium Hominis Subtypes and Enterocytozoon Bieneusi Genotypes in HIV-Infected Persons in Ibadan, Nigeria. Zoonoses Public Health 2014, 61, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Harp, J.A.; Harmsen, A.G. Requirements for CD4+ Cells and Gamma Interferon in Resolution of Established Cryptosporidium Parvum Infection in Mice. Infect. Immun. 1993, 61, 3928–3932. [Google Scholar] [CrossRef]
- Maggi, P.; Larocca, A.M.; Quarto, M.; Serio, G.; Brandonisio, O.; Angarano, G.; Pastore, G. Effect of Antiretroviral Therapy on Cryptosporidiosis and Microsporidiosis in Patients Infected with Human Immunodeficiency Virus Type 1. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Nannini, E.C.; Okhuysen, P.C. HIV1 and the Gut in the Era of Highly Active Antiretroviral Therapy. Curr. Gastroenterol. Rep. 2002, 4, 392–398. [Google Scholar] [CrossRef]
- Feng, Y.; Torres, E.; Li, N.; Wang, L.; Bowman, D.; Xiao, L. Population Genetic Characterisation of Dominant Cryptosporidium Parvum Subtype IIaA15G2R1. Int. J. Parasitol. 2013, 43, 1141–1147. [Google Scholar] [CrossRef]
- Rahmouni, I.; Essid, R.; Aoun, K.; Bouratbine, A. Glycoprotein 60 Diversity in Cryptosporidium Parvum Causing Human and Cattle Cryptosporidiosis in the Rural Region of Northern Tunisia. Am. J. Trop. Med. Hyg. 2014, 90, 346–350. [Google Scholar] [CrossRef]
- Quílez, J.; Vergara-Castiblanco, C.; Monteagudo, L.; del Cacho, E.; Sánchez-Acedo, C. Host Association of Cryptosporidium Parvum Populations Infecting Domestic Ruminants in Spain. Appl. Environ. Microbiol. 2013, 79, 5363–5371. [Google Scholar] [CrossRef]
- Alves, M.; Xiao, L.; Antunes, F.; Matos, O. Distribution of Cryptosporidium Subtypes in Humans and Domestic and Wild Ruminants in Portugal. Parasitol. Res. 2006, 99, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Sahraoui, L.; Thomas, M.; Chevillot, A.; Mammeri, M.; Polack, B.; Vallée, I.; Follet, J.; Ain-Baaziz, H.; Adjou, K.T. Molecular Characterization of Zoonotic Cryptosporidium spp. and Giardia Duodenalis Pathogens in Algerian Sheep. Vet. Parasitol. Reg. Stud. Rep. 2019, 16, 100280. [Google Scholar] [CrossRef]
- Quílez, J.; Torres, E.; Chalmers, R.M.; Hadfield, S.J.; Del Cacho, E.; Sánchez-Acedo, C. Cryptosporidium Genotypes and Subtypes in Lambs and Goat Kids in Spain. Appl. Environ. Microbiol. 2008, 74, 6026–6031. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, H.; Zhao, X.; Zhang, L.; Zhang, G.; Guo, M.; Liu, L.; Feng, Y.; Xiao, L. Zoonotic Cryptosporidium Species and Enterocytozoon Bieneusi Genotypes in HIV-Positive Patients on Antiretroviral Therapy. J. Clin. Microbiol. 2013, 51, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Addiss, D.G.; Pond, R.S.; Remshak, M.; Juranek, D.D.; Stokes, S.; Davis, J.P. Reduction of Risk of Watery Diarrhea with Point-of-Use Water Filters during a Massive Outbreak of Waterborne Cryptosporidium Infection in Milwaukee, Wisconsin, 1993. Am. J. Trop. Med. Hyg. 1996, 54, 549–553. [Google Scholar] [CrossRef]
- Menu, E.; Mosnier, E.; Cotrel, A.; Favennec, L.; Razakandrainibe, R.; Valot, S.; Blanchet, D.; Dalle, F.; Costa, D.; Gaillet, M.; et al. Cryptosporidiosis Outbreak in Amazonia, French Guiana, 2018. PLoS Negl. Trop. Dis. 2022, 16, e0010068. [Google Scholar] [CrossRef]
- Watier-Grillot, S.; Costa, D.; Petit, C.; Razakandrainibe, R.; Larréché, S.; Tong, C.; Demont, G.; Billetorte, D.; Mouly, D.; Fontan, D.; et al. Cryptosporidiosis Outbreaks Linked to the Public Water Supply in a Military Camp, France. PLoS Negl. Trop. Dis. 2022, 16, e0010776. [Google Scholar] [CrossRef] [PubMed]
- Quilez, J.; Sanchez-Acedo, C.; Avendaño, C.; del Cacho, E.; Lopez-Bernad, F. Efficacy of Two Peroxygen-Based Disinfectants for Inactivation of Cryptosporidium Parvum Oocysts. Appl. Environ. Microbiol. 2005, 71, 2479–2483. [Google Scholar] [CrossRef] [PubMed]
- Aragón, T.J.; Novotny, S.; Enanoria, W.; Vugia, D.J.; Khalakdina, A.; Katz, M.H. Endemic Cryptosporidiosis and Exposure to Municipal Tap Water in Persons with Acquired Immunodeficiency Syndrome (AIDS): A Case-Control Study. BMC Public Health 2003, 3, 2. [Google Scholar] [CrossRef]
- Widmer, G.; Sullivan, S. Genomics and Population Biology of Cryptosporidium Species. Parasite Immunol. 2012, 34, 61–71. [Google Scholar] [CrossRef]
- Izadi, S.; Mohaghegh, M.A.; Ghayour-Najafabadi, Z.; Azami, M.; Mirzaei, F.; Namdar, F.; Mohebali, M.; Leshan Wannigama, D.; Hejazi, S.-H. Frequency and Molecular Identification of Cryptosporidium Species among Immunocompromised Patients Referred to Hospitals, Central Iran, 2015–2016. Iran J. Parasitol. 2020, 15, 31–39. [Google Scholar]
- Merino, F.J.; Köster, P.C.; Fuentes, I.; Carmena, D. Imported Cryptosporidiosis Caused by Cryptosporidium Hominis IbA13G3 in Spain. The Relevance of Molecular-Based Surveillance. Enferm. Infecc. Microbiol. Clin. 2019, 37, 552–554. [Google Scholar] [CrossRef]
- Cama, V.A.; Ross, J.M.; Crawford, S.; Kawai, V.; Chavez-Valdez, R.; Vargas, D.; Vivar, A.; Ticona, E.; Navincopa, M.; Williamson, J.; et al. Differences in Clinical Manifestations among Cryptosporidium Species and Subtypes in HIV-Infected Persons. J. Infect. Dis. 2007, 196, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Ayinmode, A.B.; Fagbemi, B.O.; Xiao, L. Molecular Characterization of Cryptosporidium in Children in Oyo State, Nigeria: Implications for Infection Sources. Parasitol. Res. 2012, 110, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Gatei, W.; Wamae, C.N.; Mbae, C.; Waruru, A.; Mulinge, E.; Waithera, T.; Gatika, S.M.; Kamwati, S.K.; Revathi, G.; Hart, C.A. Cryptosporidiosis: Prevalence, Genotype Analysis, and Symptoms Associated with Infections in Children in Kenya. Am. J. Trop. Med. Hyg. 2006, 75, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Cama, V.; Gilman, R.H.; Vivar, A.; Ticona, E.; Ortega, Y.; Bern, C.; Xiao, L. Mixed Cryptosporidium Infections and HIV. Emerg. Infect. Dis. 2006, 12, 1025–1028. [Google Scholar] [CrossRef] [PubMed]

| Cryptosporidiosis | ||||
|---|---|---|---|---|
| Positive | Negative | Total | p-Value | |
| CD4 count > 200 cells/mm3 | 2 | 202 | 204 | - |
| CD4 count [50–200] cells/mm3 | 10 | 85 | 95 | <0.001 |
| CD4 count < 50 cells/mm3 | 18 | 30 | 48 | <0.001 |
| CD4 count not available | 3 | - | 3 | |
| Total | 33 | 317 | 350 | |
| Sex | Age (years) | Contact with Animals | Water Consumption | Diarrhoea | CD4 (cells/mm3) | Cryptosporidium Species | Subtypes |
|---|---|---|---|---|---|---|---|
| F | 54 | Cats | Tap water | Chronic | 26 | C. parvum | IIaA15G2R1 |
| M | 64 | Sheep and cattle | Well water | Chronic | 45 | C. parvum | IIdA19G1 |
| F | 30 | No | Tap water | Chronic | 20 | C. parvum | IIdA19G1 |
| M | 25 | No | Well water | Chronic | 57 | C. felis | |
| M | 17 | Cats and pigeons | Tap water | Chronic | No sampling | C.felis | |
| M | 41 | No | Well water | Chronic | 1 | C. parvum | IIdA19G1 |
| M | 48 | No | Tap water | Chronic | 172 | C. parvum | IIdA16G1 |
| F | 82 | No response | Tap water | Intermittent | 512 | C. parvum | IIaA21G1R1 |
| F | NR | No | Not specified | Chronic | No sampling | C. parvum | IIdA19G1 |
| M | 60 | No response | Tap water | Chronic | 55 | C. parvum | IIaA20G1R1 |
| F | 44 | No response | Not specified | Chronic | 16 | C. parvum | IIdA16G1 |
| F | 58 | No response | Tap water | Chronic | 40 | C. parvum | IIaA16G2R1 |
| M | 38 | No response | Tap water | Chronic | 178 | C. parvum | IIaA15G2R1 |
| M | 41 | Sheep and cattle (Sheep breeder) | Well water | Chronic | 92 | C. parvum | IIdA16G1 |
| M | NR | No response | Not specified | Chronic | 207 | C. parvum | IIaA15G2R1 |
| M | 66 | Living in rural areas of farmed livestock | Tap water | Intermittent | No sampling | C.hominis | IaA14 |
| F | 37 | No | Bottled water | Chronic | 9 | C.hominis | IbA10G2 |
| F | 30 | No | Tap water | Chronic | 50 | C. parvum | IIaA14G2R1 |
| F | 28 | No | Tap water | Chronic | 109 | C.hominis | IbA13G3 |
| F | NR | No | Tap water | Chronic | 47 | C.hominis | IbA13G3 |
| M | 7 | No | Tap water | Chronic | 7 | C.hominis | IaA22R2 |
| M | 42 | Sheep and cattle (Sheep breeder) | Well water | Chronic | 53 | C. parvum | IIdA16G1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semmani, M.; Costa, D.; Achour, N.; Cherchar, M.; Ziane, H.; Mouhajir, A.; Villier, V.; Adjmi Hamoudi, H.; Favennec, L.; Razakandrainibe, R. Occurrence and Molecular Characterization of Cryptosporidium Infection in HIV/Aids Patients in Algeria. Viruses 2023, 15, 362. https://doi.org/10.3390/v15020362
Semmani M, Costa D, Achour N, Cherchar M, Ziane H, Mouhajir A, Villier V, Adjmi Hamoudi H, Favennec L, Razakandrainibe R. Occurrence and Molecular Characterization of Cryptosporidium Infection in HIV/Aids Patients in Algeria. Viruses. 2023; 15(2):362. https://doi.org/10.3390/v15020362
Chicago/Turabian StyleSemmani, Malika, Damien Costa, Nassima Achour, Meriem Cherchar, Hanifa Ziane, Abdelmounaim Mouhajir, Venceslas Villier, Haiet Adjmi Hamoudi, Loic Favennec, and Romy Razakandrainibe. 2023. "Occurrence and Molecular Characterization of Cryptosporidium Infection in HIV/Aids Patients in Algeria" Viruses 15, no. 2: 362. https://doi.org/10.3390/v15020362
APA StyleSemmani, M., Costa, D., Achour, N., Cherchar, M., Ziane, H., Mouhajir, A., Villier, V., Adjmi Hamoudi, H., Favennec, L., & Razakandrainibe, R. (2023). Occurrence and Molecular Characterization of Cryptosporidium Infection in HIV/Aids Patients in Algeria. Viruses, 15(2), 362. https://doi.org/10.3390/v15020362







