Humoral SARS-CoV-2 Immune Response in COVID-19 Recovered Vaccinated and Unvaccinated Individuals Related to Post-COVID-Syndrome
Abstract
:1. Introduction
2. Materials and Methods
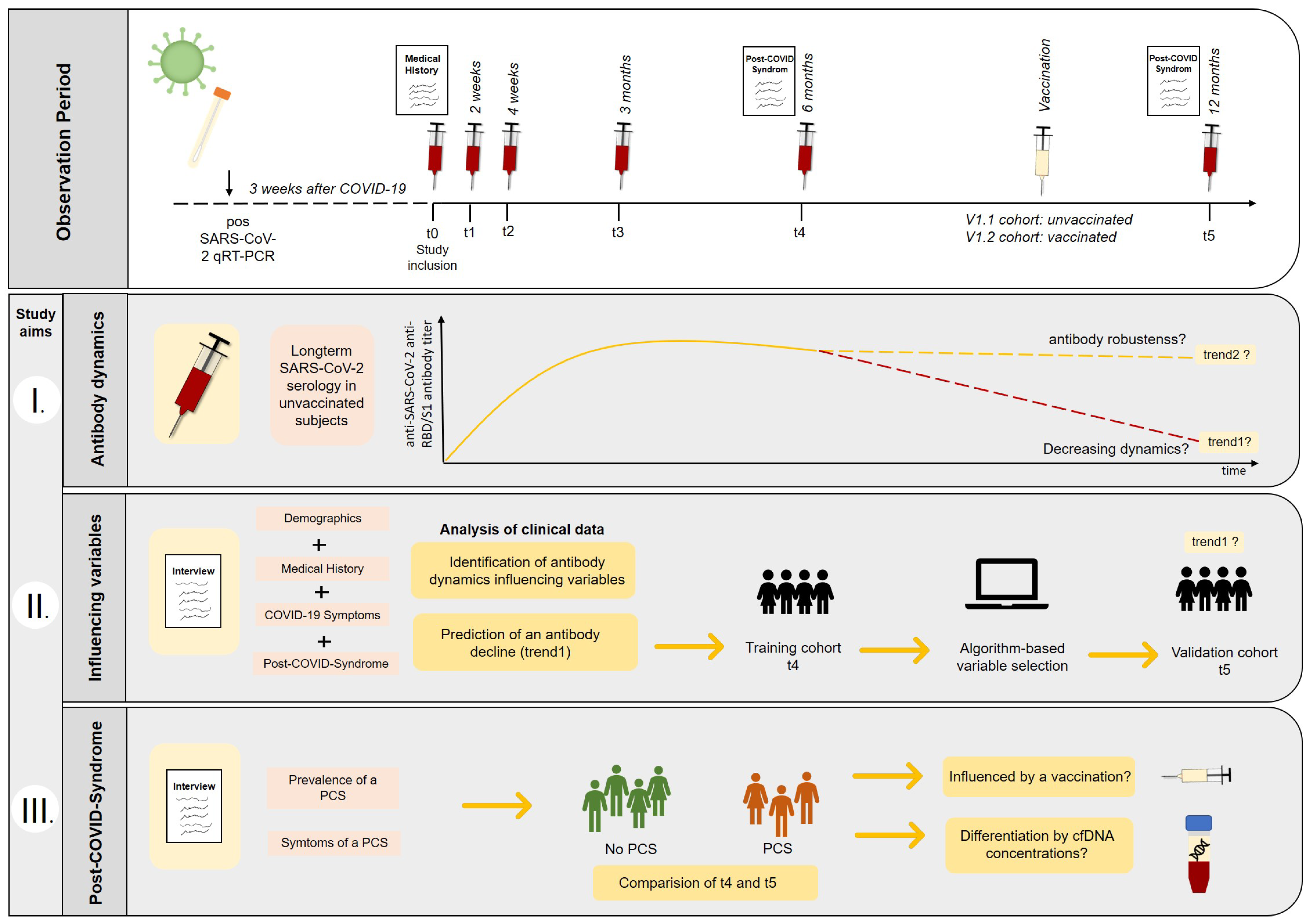
2.1. Participant Recruitment and Sample Collection
2.2. Long-Term Appointments and Definition of Cohorts
2.3. Anti-SARS-CoV-2 Antibody Detection and HLA Typing
2.4. Analysis of cfDNA
2.5. General Data Analysis
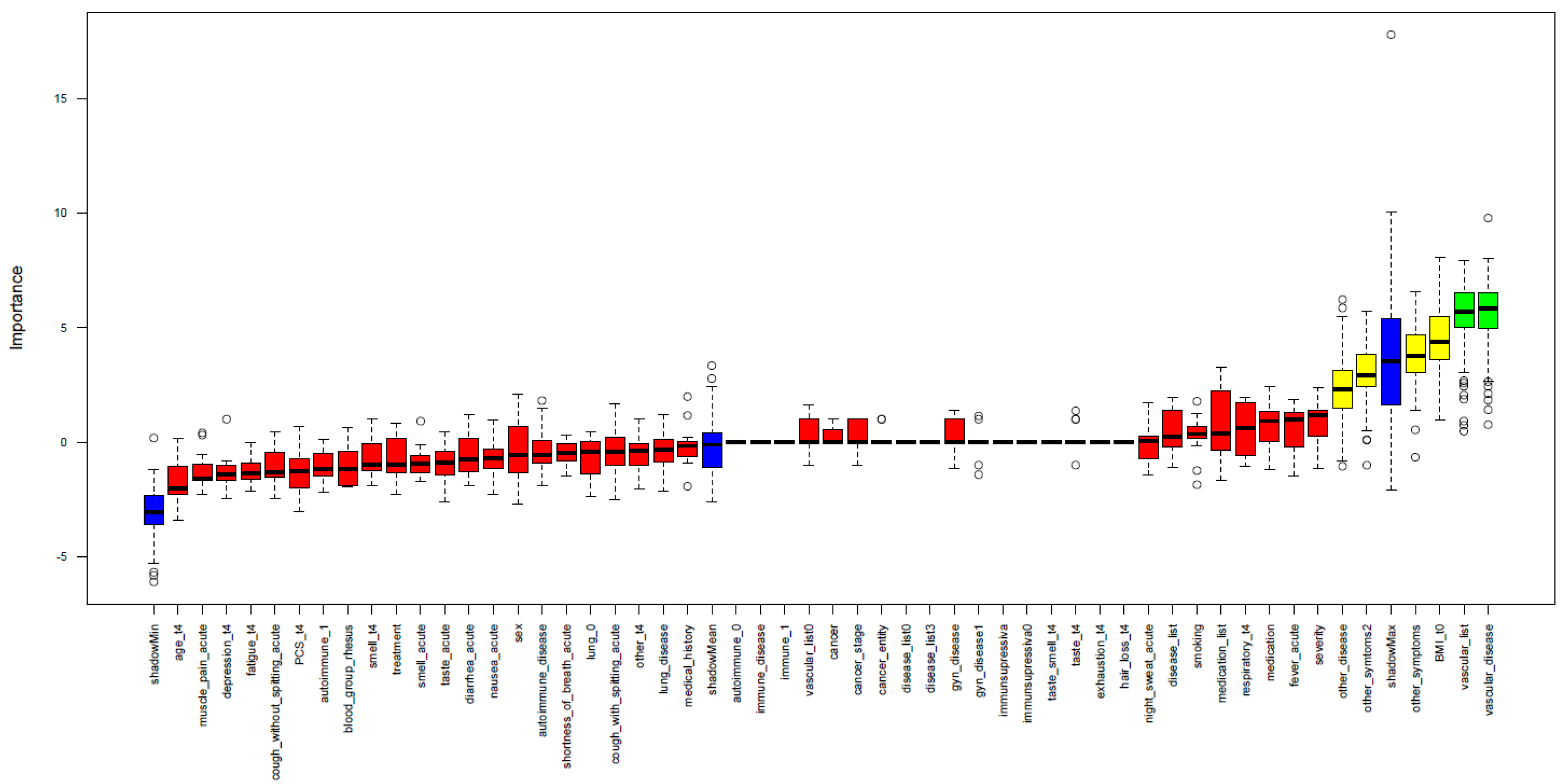
2.6. Performance of Feature Selection
3. Results
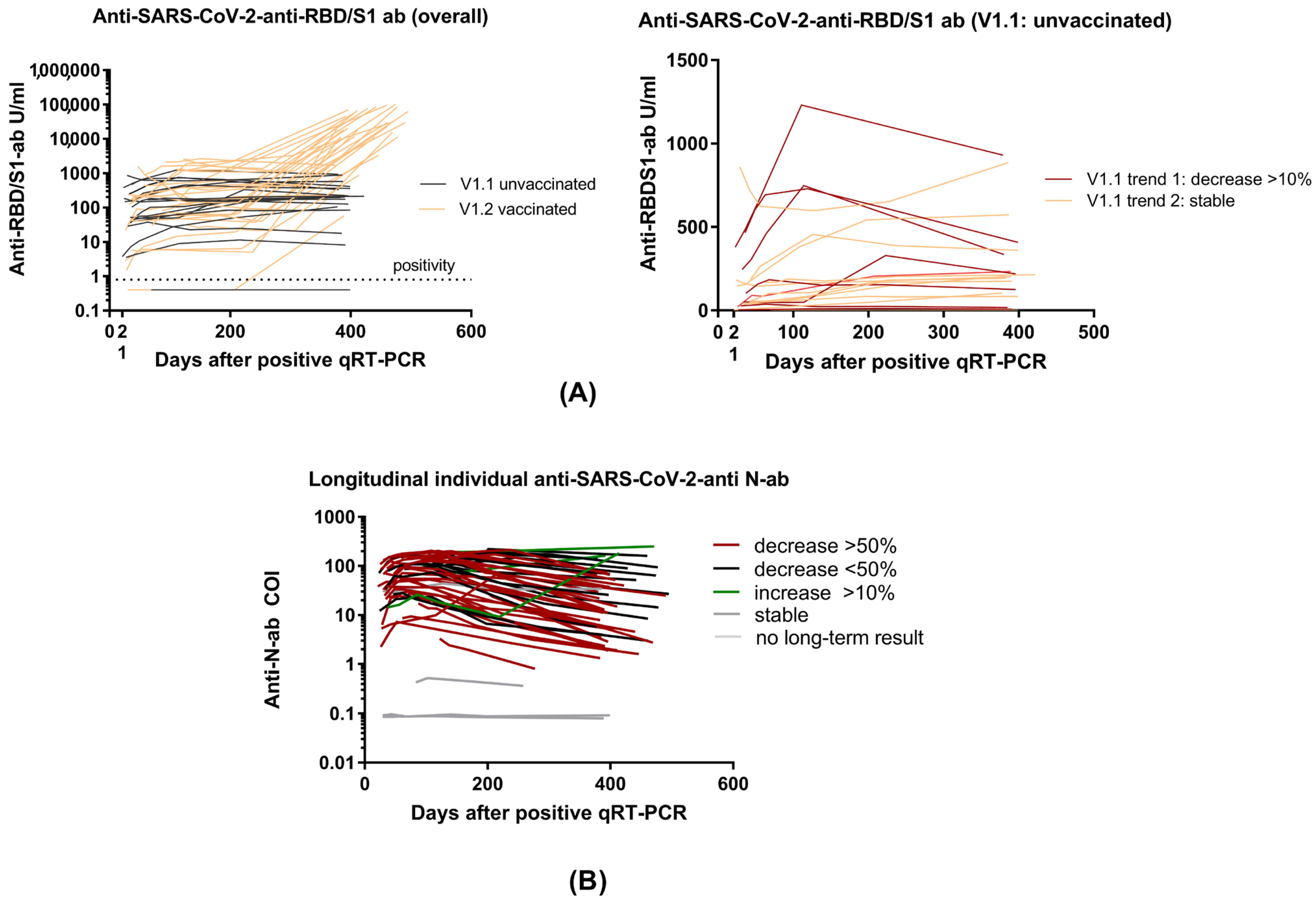
3.1. Demographics and Overall Antibody Trends
3.2. Anti-RBD/S1 Antibody Dynamics in Unvaccinated (V1.1) and Vaccinated Population (V1.2)
3.3. Clinical Prediction of Anti-RBD/S1 Antibody Decrease
3.4. Identification of Risk Factors or Suitable Biomarker for PCS Occurring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahn, D.-G.; Shin, H.-J.; Kim, M.-H.; Lee, S.; Kim, H.-S.; Myoung, J.; Kim, B.-T.; Kim, S.-J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- Figueiredo-Campos, P.; Blankenhaus, B.; Mota, C.; Gomes, A.; Serrano, M.; Ariotti, S.; Costa, C.; Nunes-Cabaço, H.; Mendes, A.M.; Gaspar, P.; et al. Seroprevalence of anti-SARS-CoV-2 antibodies in COVID-19 patients and healthy volunteers up to 6 months post disease onset. Eur. J. Immunol. 2020, 50, 2025–2040. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, J.; Shang, J.; Wang, Y.; Wan, J.; Yan, Y.; Liu, W.; Zhang, H.-P.; Wang, J.; Wang, X.; et al. Clinical value of laboratory indicators for predicting disease progression and death in patients with COVID-19: A retrospective cohort study. BMJ Open 2021, 11, e043790. [Google Scholar] [CrossRef]
- Huckriede, J.; Anderberg, S.B.; Morales, A.; de Vries, F.; Hultström, M.; Bergqvist, A.; Ortiz-Pérez, J.T.; Sels, J.W.; Wichapong, K.; Lipcsey, M.; et al. Evolution of NETosis markers and DAMPs have prognostic value in critically ill COVID-19 patients. Sci. Rep. 2021, 11, 15701. [Google Scholar] [CrossRef]
- Moreno-Pérez, O.; Merino, E.; Leon-Ramirez, J.-M.; Andres, M.; Ramos, J.M.; Arenas-Jiménez, J.; Asensio, S.; Sanchez, R.; Ruiz-Torregrosa, P.; Galan, I.; et al. Post-acute COVID-19 syndrome. Incidence and risk factors: A Mediterranean cohort study. J. Infect. 2021, 82, 378–383. [Google Scholar] [CrossRef]
- Pereira, C.; Harris, B.H.L.; Di Giovannantonio, M.; Rosadas, C.; Short, C.-E.; Quinlan, R.; Sureda-Vives, M.; Fernandez, N.; Day-Weber, I.; Khan, M.; et al. The Association Between Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Post–COVID-19 Syndrome in Healthcare Workers. J. Infect. Dis. 2021, 223, 1671–1676. [Google Scholar] [CrossRef]
- Ceravolo, M.G.; Arienti, C.; de Sire, A.; Andrenelli, E.; Negrini, F.; Lazzarini, S.G.; Patrini, M.; Negrini, S.; International Multiprofessional Steering Committee of Cochrane. Rehabilitation REH-COVER action Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Eur. J. Phys. Rehabil. Med. 2020, 56, 642–651. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Gerhards, C.; Thiaucourt, M.; Kittel, M.; Becker, C.; Ast, V.; Hetjens, M.; Neumaier, M.; Haselmann, V. Longitudinal assessment of anti-SARS-CoV-2 antibody dynamics and clinical features following convalescence from a COVID-19 infection. Int. J. Infect. Dis. 2021, 107, 221–227. [Google Scholar] [CrossRef]
- Krajewski, R.; Gołębiowska, J.; Makuch, S.; Mazur, G.; Agrawal, S. Update on serologic testing in COVID–19. Clin. Chim. Acta 2020, 510, 746–750. [Google Scholar] [CrossRef]
- Long, Q.-X.; Liu, B.-Z.; Deng, H.-J.; Wu, G.-C.; Deng, K.; Chen, Y.-K.; Liao, P.; Qiu, J.-F.; Lin, Y.; Cai, X.-F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Norddahl, G.L.; Melsted, P.; Gunnarsdottir, K.; Holm, H.; Eythorsson, E.; Arnthorsson, A.O.; Helgason, D.; Bjarnadottir, K.; Ingvarsson, R.F.; et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020, 383, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yu, X.; Gao, C.; Zhang, L.; Zhai, H.; Hu, Y.; Liu, E.; Wang, Q.; Gao, Y.; Wei, D.; et al. Characterization of antibody responses to SARS-CoV-2 in convalescent COVID-19 patients. J. Med. Virol. 2021, 93, 2227–2233. [Google Scholar] [CrossRef]
- Wang, K.; Long, Q.-X.; Deng, H.-J.; Hu, J.; Gao, Q.-Z.; Zhang, G.-J.; He, C.-L.; Huang, L.-Y.; Hu, J.-L.; Chen, J.; et al. Longitudinal Dynamics of the Neutralizing Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin. Infect. Dis. 2021, 73, e531–e539. [Google Scholar] [CrossRef]
- Yao, X.-Y.; Liu, W.; Li, Z.-Y.; Xiong, H.-L.; Su, Y.-Y.; Li, T.-D.; Zhang, S.-Y.; Zhang, X.-J.; Bi, Z.-F.; Deng, C.-X.; et al. Neutralizing and Binding Antibody Kinetics of COVID-19 Patients during Hospital and Convalescent Phases. medRxiv 2020. [Google Scholar]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Mucosal versus systemic antibody responses to SARS-CoV-2 antigens in COVID-19 patients. Allergy Immunol. 2020. [Google Scholar]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; de Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef]
- Suthar, M.S.; Zimmerman, M.G.; Kauffman, R.C.; Mantus, G.; Linderman, S.L.; Hudson, W.H.; Vanderheiden, A.; Nyhoff, L.; Davis, C.W.; Adekunle, O.; et al. Rapid Generation of Neutralizing Antibody Responses in COVID-19 Patients. Cell Rep. Med. 2020, 1, 100040. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C. Impfempfehlungen der Ständigen Impfkommission (STIKO): Impfungen bei Immundefizienz und Impfung gegen COVID-19. Hautarzt 2021, 72, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; Alwan, N.A.; Walker, A.S. Trajectory of long covid symptoms after covid-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef]
- Wijeratne, S.; Butt, A.; Burns, S.; Sherwood, K.; Boyd, O.; Swaminathan, R. Cell-Free Plasma DNA as a Prognostic Marker in Intensive Treatment Unit Patients. Ann. N. Y. Acad. Sci. 2004, 1022, 232–238. [Google Scholar] [CrossRef]
- Ahmed, A.; Soliman, R.; Samir, S. Cell Free DNA and Procalcitonin as Early Markers of Complications in ICU Patients with Multiple Trauma and Major Surgery. Clin. Lab. 2016, 62, 2395–2404. [Google Scholar] [CrossRef]
- Forsblom, E.; Aittoniemi, J.; Ruotsalainen, E.; Helmijoki, V.; Huttunen, R.; Jylhävä, J.; Hurme, M.; Järvinen, A. High Cell-Free DNA Predicts Fatal Outcome among Staphylococcus aureus Bacteraemia Patients with Intensive Care Unit Treatment. PLoS ONE 2014, 9, e87741. [Google Scholar] [CrossRef]
- Dwivedi, D.J.; Toltl, L.J.; Swystun, L.L.; Pogue, J.; Liaw, K.-L.; Weitz, J.I.; Cook, D.J.; Fox-Robichaud, A.E.; Liaw, P.C.; the Canadian Critical Care Translational Biology Group. Prognostic utility and characterization of cell-free DNA in patients with severe sepsis. Crit. Care 2012, 16, R151. [Google Scholar] [CrossRef]
- Hammad, R.; Eldosoky, M.A.E.R.; Fouad, S.H.; Elgendy, A.; Tawfeik, A.M.; Alboraie, M.; Abdelmaksoud, M.F. Circulating cell-free DNA, peripheral lymphocyte subsets alterations and neutrophil lymphocyte ratio in assessment of COVID-19 severity. Innate Immun. 2021, 27, 240–250. [Google Scholar] [CrossRef]
- Degenhardt, F.; Seifert, S.; Szymczak, S. Evaluation of variable selection methods for random forests and omics data sets. Brief. Bioinform. 2019, 20, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, A.; Larkman, J.; Xu, Y.; Cardoso, V.R.; Gkoutos, G.V. A random forest based biomarker discovery and power analysis framework for diagnostics research. BMC Med. Genom. 2020, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Kayaaslan, B.; Eser, F.; Kalem, A.K.; Kaya, G.; Kaplan, B.; Kacar, D.; Hasanoglu, I.; Coskun, B.; Guner, R. Post-COVID syndrome: A single-center questionnaire study on 1007 participants recovered from COVID-19. J. Med. Virol. 2021, 93, 6566–6574. [Google Scholar] [CrossRef]
- Pei, L.; Wang, X.; Guo, B.; Guo, H.; Yu, Y. Do air pollutants as well as meteorological factors impact Corona Virus Disease 2019 (COVID-19)? Evidence from China based on the geographical perspective. Environ. Sci. Pollut. Res. 2021, 28, 35584–35596. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.-X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of immune responses to SARS-CoV-2 in mild-moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef]
- Ali, A.M.; Ali, K.M.; Fatah, M.H.; Tawfeeq, H.M.; Rostam, H.M. SARS-CoV-2 reinfection in patients negative for immunoglobulin G following recovery from COVID-19. New Microbes New Infect. 2021, 43, 100926. [Google Scholar] [CrossRef]
- Borgogna, C.; De Andrea, M.; Griffante, G.; Lai, A.; Bergna, A.; Galli, M.; Zehender, G.; Castello, L.; Ravanini, P.; Cattrini, C.; et al. SARS-CoV-2 reinfection in a cancer patient with a defective neutralizing humoral response. J. Med. Virol. 2021, 93, 6444–6446. [Google Scholar] [CrossRef]
- Glöckner, S.; Hornung, F.; Baier, M.; Weis, S.; Pletz, M.W.; Deinhardt-Emmer, S.; Löffler, B.; the CoNAN Study Group. Robust Neutralizing Antibody Levels Detected after Either SARS-CoV-2 Vaccination or One Year after Infection. Viruses 2021, 13, 2003. [Google Scholar] [CrossRef]
- Zeng, F.; Wu, M.; Wang, J.; Li, J.; Hu, G.; Wang, L. Over 1-year duration and age difference of SARS-CoV-2 antibodies in convalescent COVID-19 patients. J. Med. Virol. 2021, 93, 6506–6511. [Google Scholar] [CrossRef]
- Fischer, J.C.; Schmidt, A.G.; Bölke, E.; Uhrberg, M.; Keitel, V.; Feldt, T.; Jensen, B.; Häussinger, D.; Adams, O.; Schneider, E.M.; et al. Association of HLA genotypes, AB0 blood type and chemokine receptor 5 mutant CD195 with the clinical course of COVID-19. Eur. J. Med. Res. 2021, 26, 107. [Google Scholar] [CrossRef]
- Amoroso, A.; Magistroni, P.; Vespasiano, F.; Bella, A.; Bellino, S.; Puoti, F.; Alizzi, S.; Vaisitti, T.; Boros, S.; Grossi, P.A.; et al. HLA and AB0 Polymorphisms May Influence SARS-CoV-2 Infection and COVID-19 Severity. Transplantation 2021, 105, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Riestra-Ayora, J.; Yanes-Diaz, J.; Esteban-Sanchez, J.; Vaduva, C.; Molina-Quiros, C.; Larran-Jimenez, A.; Martin-Sanz, E. Long-term follow-up of olfactory and gustatory dysfunction in COVID-19: 6 months case–control study of health workers. Eur. Arch. Otorhinolaryngol. 2021, 278, 4831–4837. [Google Scholar] [CrossRef] [PubMed]
- Hugon, J.; Msika, E.-F.; Queneau, M.; Farid, K.; Paquet, C. Long COVID: Cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 2022, 269, 44–46. [Google Scholar] [CrossRef] [PubMed]
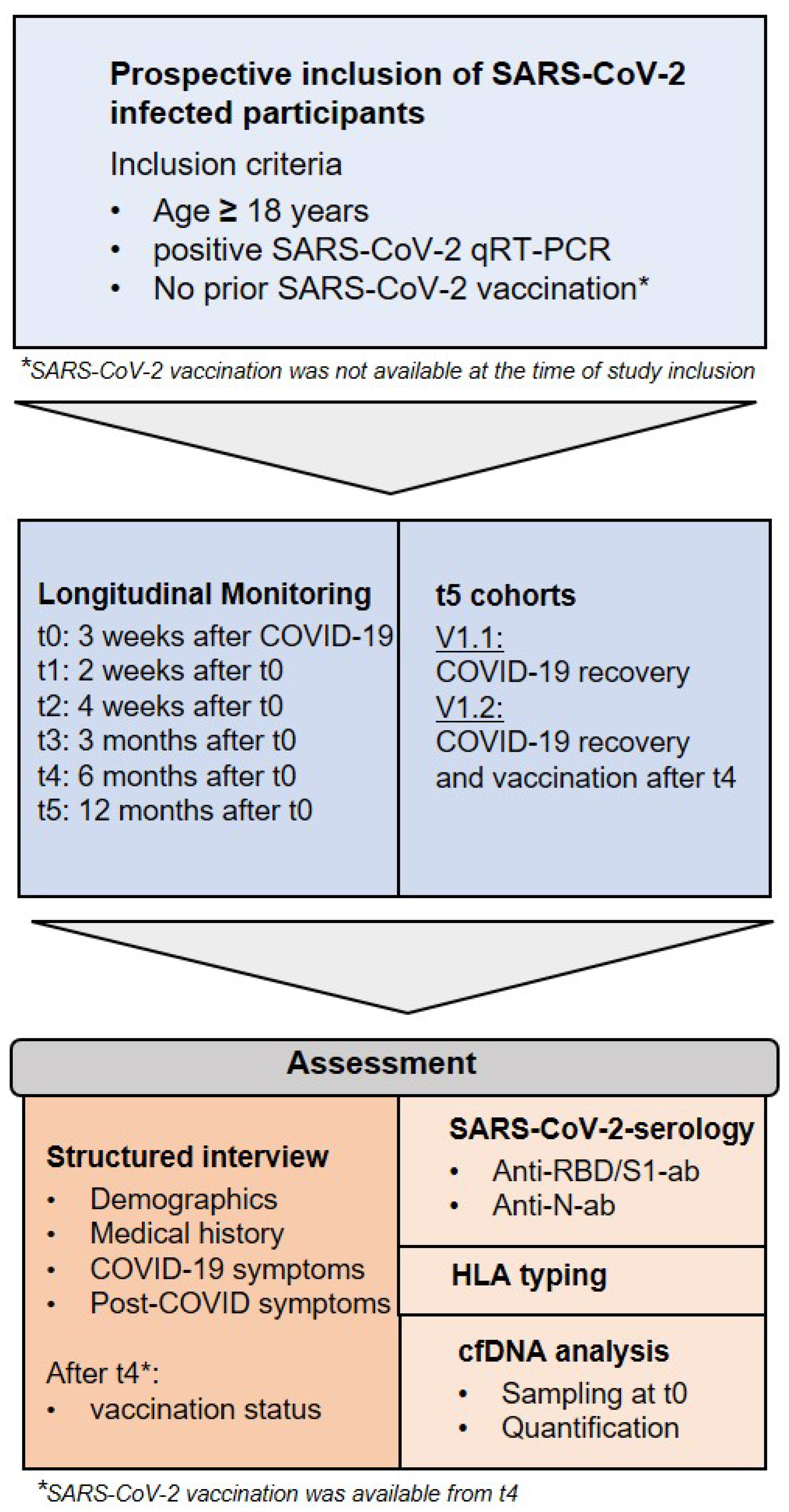


| Cohort | Description |
|---|---|
| t0 | Study inclusion (at least 3 weeks after COVID-19, no prior SARS-CoV-2 vaccination) |
| t1 | 2 weeks after t0 |
| t2 | 4 weeks after t0 |
| t3 | 3 months after t0 |
| t4 | 6 months after t0 |
| t5 | 12 months after t0 (some subjects were vaccinated before t5) |
| V1 | Recovered SARS-CoV-2-infected unvaccinated participants from t0–t4 |
| V1.1 | Recovered SARS-CoV-2-infected unvaccinated participants at t5 |
| V1.2 | Recovered SARS-CoV-2-infected and vaccinated participants at t5 |
| Training | Data from V1.2. at t4 |
| Validation | Identical with V1.1 |
| Trend 1 | Declining anti-S/RBD1-ab in unvaccinated subjects (≥10% to previous value) |
| Trend 2 | Stable anti-S/RBD1-ab in unvaccinated subjects |
| T4 (n = 49) | t5 (n = 49) | p-Value | |
|---|---|---|---|
| Age (median [IQR]) | 51.00 [32.50, 58.25] | 53.12 [35.48, 60.02] | 0.462 |
| Gender f/m (%) | 31/18 (63.3/36.7) | 33/17 (65.3/34.7) | 1 |
| Smoking (%) | 3 (8.3) | 4 (6.2) | 1 |
| BMI t0 (mean (SD)) | 25.50 (4.61) | 25.82 (4.77) | 0.74 |
| Treatment (%) | |||
| Ambulant | 42 (87.5) | 42 (87.5) | |
| Hospitalization: normal | 4 (8.3) | 4 (8.3) | |
| Hospitalization: ICU | 2 (4.2) | 2 (4.2) | |
| COVID-19 symptoms | |||
| Fever (%) | 25 (52.1) | 25 (52.1) | 1 |
| Anosmia COVID-19 (%) | 37 (77.1) | 35 (72.9) | 0.814 |
| Ageusia COVID-19 (%) | 39 (81.2) | 38 (79.2) | 1 |
| Anosmia and Ageusia (%) | 40 (83.3) | 39 (81.2) | 1 |
| Days after qRT-PCR (mean (SD)) | 243.81 (44.49) | 414.18 (35.03) | <0.001 |
| Vaccination before the appointment (%) | 0 (0.0) | 30 (61.2) | <0.001 |
| Preexisting medical history | |||
| Autoimmune (%) | 8 (16.7) | 8 (16.7) | 1 |
| Immune (%) | 1 (2.1) | 1 (2.1) | 1 |
| Vascular (%) | 9 (18.8) | 12 (25.0) | 0.622 |
| Diabetes (%) | 1 (2.1) | 1 (2.1) | 1 |
| Cancer (%) | 1 (2.1) | 1 (2.1) | 1 |
| Lung (%) | 4 (12.5) | 8 (16.7) | 0.773 |
| Other diseases (%) | 14 (29.2) | 13 (27.1) | 1 |
| Medication (%) | 24 (50.0) | 27 (56.2) | 0.683 |
| Recovered, Unvaccinated V1.1 (n = 19) | Recovered, Vaccinated V1.2 (n = 29) | p-Value | |
|---|---|---|---|
| Anti-RBD/S1 antibodies | |||
| Titer U/mL (median [IQR]) | 204.30 [115.30, 357.95] | 20,331.00 [11,497.00, 62,414.00] | <0.001 |
| Comparison previous t % (median [IQR]) | 103.00 [73.48, 109.69] | 13,574.76 [5798.89, 29,051.89] | <0.001 |
| Dynamics (%) | <0.001 | ||
| Stable | 7 (36.8) | 0 (0.0) | |
| Increase | 5 (26.3) | 28 (96.6) | |
| Decrease | 7 (36.8) | 1 (3.4) | |
| PCS t4 (%) | 9 (47.4) | 14 (48.3) | 1 |
| PCS t5 (%) | 8 (42.1) | 11 (37.9) | 1 |
| 6 Months (t4) (n = 49) | 12 Months (t5) (n = 49) | p-Value | |
|---|---|---|---|
| Anti-SARS-CoV-2-anti-RBD/S1 antibodies | |||
| Titer U/mL (median [IQR]) | 172.10 [48.99, 506.20] | 3519.00 [218.80, 29,354.00] | <0.001 |
| Comparison previous t % (median [IQR]) | 106.85 [96.16, 134.37] | 4077.99 [105.10, 14,520.00] | <0.001 |
| Dynamics (%) | 0.020 | ||
| Stable | 15 (30.6) | 7 (14.3) | |
| Increase | 20 (40.8) | 34 (69.4) | |
| Decrease | 14 (28.6) | 8 (16.3) | |
| Dynamics unvaccinated cohort V1.1 (%) | 0.558 | ||
| Stable | 15 (30.6) | 7 (36.8) | |
| Increase | 20 (40.8) | 5 (26.3) | |
| Decrease | 14 (28.6) | 7 (36.8) | |
| Anti-SARS-CoV-2 anti-N-antibodies | |||
| Titer COI (median [IQR]) | 32.82 [8.33, 94.69] | 13.78 [4.01, 51.89] | 0.109 |
| Dynamics (%) | 0.735 | ||
| Stable [negatives] | 5 [1] (10.2) | 4 [3] (8.2) | |
| Increase | 5 (10.2) | 3 (6.1) | |
| Decrease | 39 (79.6) | 42 (85.7) |
| t4 (n = 49) | t5 (n = 49) | p-Value | |
|---|---|---|---|
| PCS (%) | 23 (46.9) | 19 (38.6) | 0.541 |
| PCS Dynamics | |||
| Regression | Na | 9 (18.4) | |
| Persistence | Na | 14 (28.6) | |
| Initial manifestation | Na | 5 (10.2) | |
| Perpetual asymptomatic | Na | 21 (42.9) | |
| PCS Characteristics | |||
| Anosmia and ageusia (%) | 4 (17.4) | 1 (5.3) | 0.356 |
| Anosmia | 5 (21.7) | 4 (21.1) | 1 |
| Ageusia | 4 (17.4) | 2 (10.5) | 0.673 |
| Fatigue | 6 (26.1) | 5 (23.3) | 1 |
| Exhaustion | 3 (13.0) | 6 (31.6) | 0.257 |
| Depression | 2 (8.7) | 1 (5.3) | 0.582 |
| Loss of hair | 2 (8.7) | 2 (10.5) | 1 |
| Respiratory symptoms | 3 (13.0) | 4 (21.1) | 0.682 |
| Cognitive impairments | 1 (4.3) | 8 (42.1) | 0.006 |
| Other symptoms | 3 (13.0) | 1 (5.3) | 0.614 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerhards, C.; Kittel, M.; Ast, V.; Bugert, P.; Froelich, M.F.; Hetjens, M.; Haselmann, V.; Neumaier, M.; Thiaucourt, M. Humoral SARS-CoV-2 Immune Response in COVID-19 Recovered Vaccinated and Unvaccinated Individuals Related to Post-COVID-Syndrome. Viruses 2023, 15, 454. https://doi.org/10.3390/v15020454
Gerhards C, Kittel M, Ast V, Bugert P, Froelich MF, Hetjens M, Haselmann V, Neumaier M, Thiaucourt M. Humoral SARS-CoV-2 Immune Response in COVID-19 Recovered Vaccinated and Unvaccinated Individuals Related to Post-COVID-Syndrome. Viruses. 2023; 15(2):454. https://doi.org/10.3390/v15020454
Chicago/Turabian StyleGerhards, Catharina, Maximilian Kittel, Volker Ast, Peter Bugert, Matthias F. Froelich, Michael Hetjens, Verena Haselmann, Michael Neumaier, and Margot Thiaucourt. 2023. "Humoral SARS-CoV-2 Immune Response in COVID-19 Recovered Vaccinated and Unvaccinated Individuals Related to Post-COVID-Syndrome" Viruses 15, no. 2: 454. https://doi.org/10.3390/v15020454





