Novel Tetrahydroisoquinoline-Based Heterocyclic Compounds Efficiently Inhibit SARS-CoV-2 Infection In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Chemical Synthesis of Novel Heterocyclic Compounds
2.2.1. Synthesis of tert-butyl rel-4-(((3R,4S)-3-(1H-indol-3-yl)-1-oxo-2-propyl-1,2,3,4-tetrahydroisoquinolin-4-yl)methyl)piperazine-1-carboxylate (trans-1)
2.2.2. Synthesis of rel-(3R,4S)-3-(1H-indol-3-yl)-4-(piperazin-1-ylmethyl)-2-propyl-3,4-dihydroisoquinolin-1(2H)-one (trans-2)
2.3. Antiviral Assessment
2.4. Cytotoxicity of Tested Compounds
2.5. Time of the Drug Addition Assay
2.6. Immunofluorescence Assay
2.7. Western Blot Analysis
3. Results
3.1. Synthesis of Novel Tetrahydroisoquinoline-Based Heterocyclic Compounds
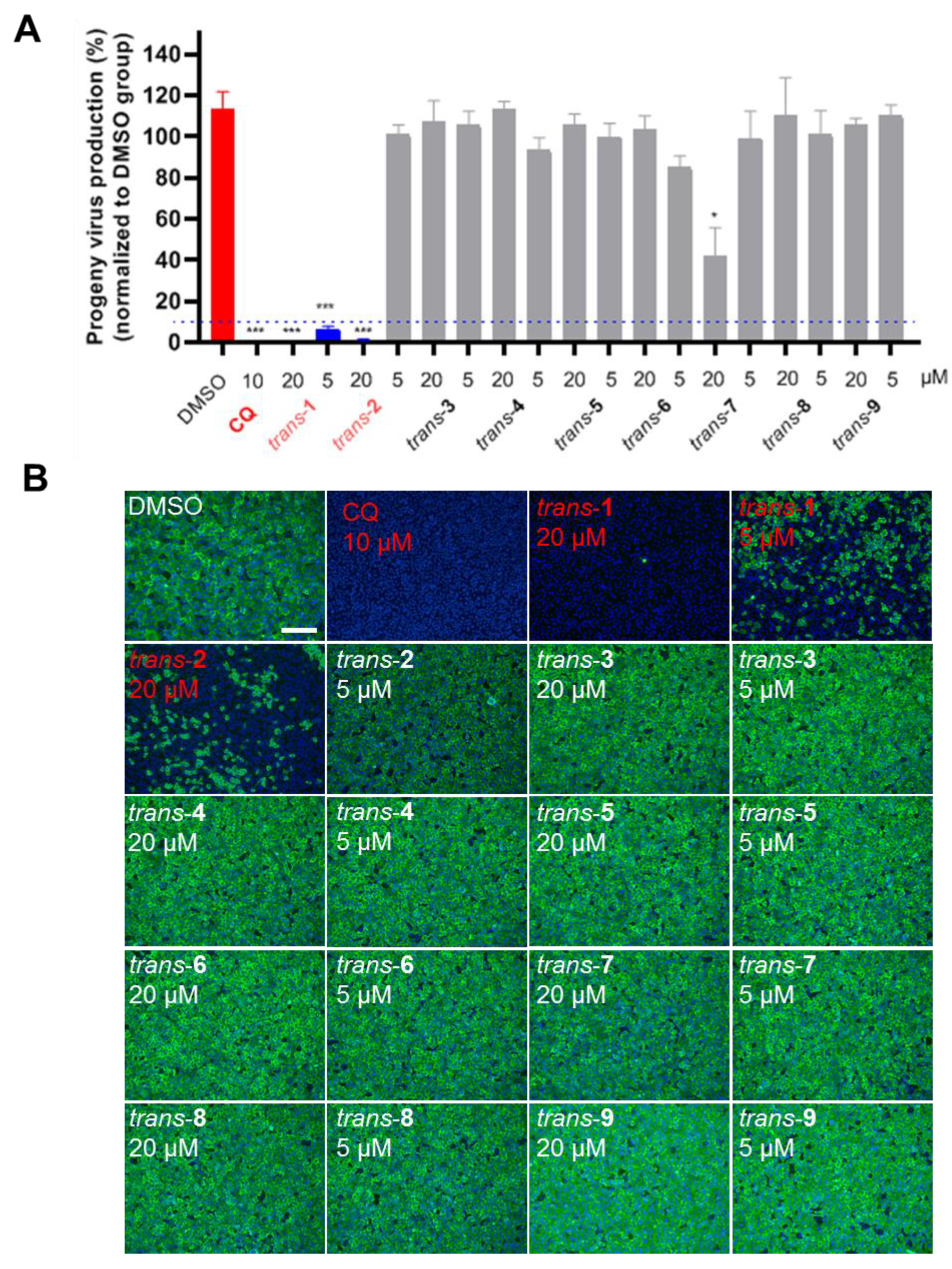
3.2. Anti-SARS-CoV-2 Activity of Novel Heterocyclic Compounds
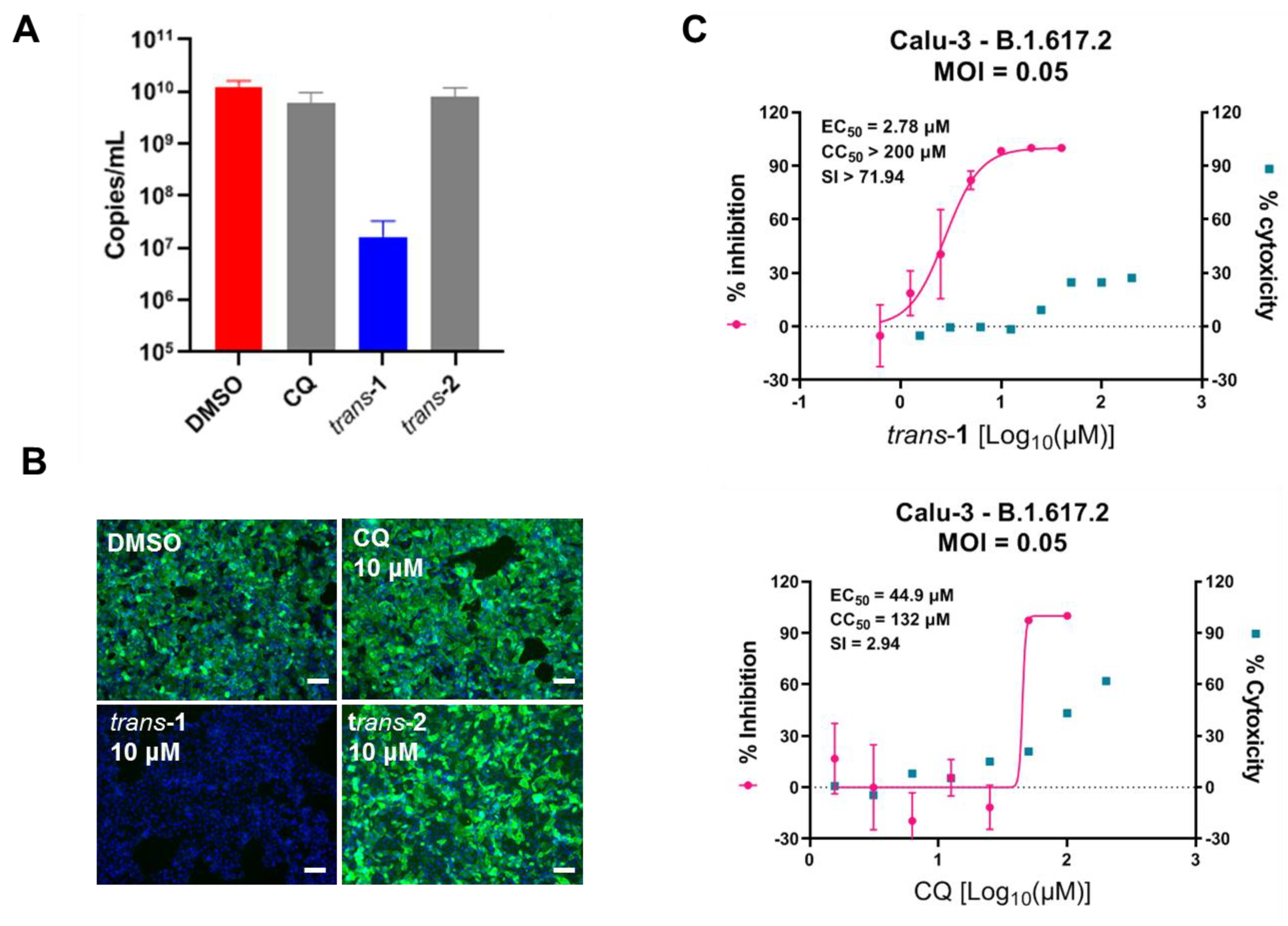
3.3. Compound Trans-1 Efficiently Inhibits SARS-CoV-2 Replication in Human Lung Cells
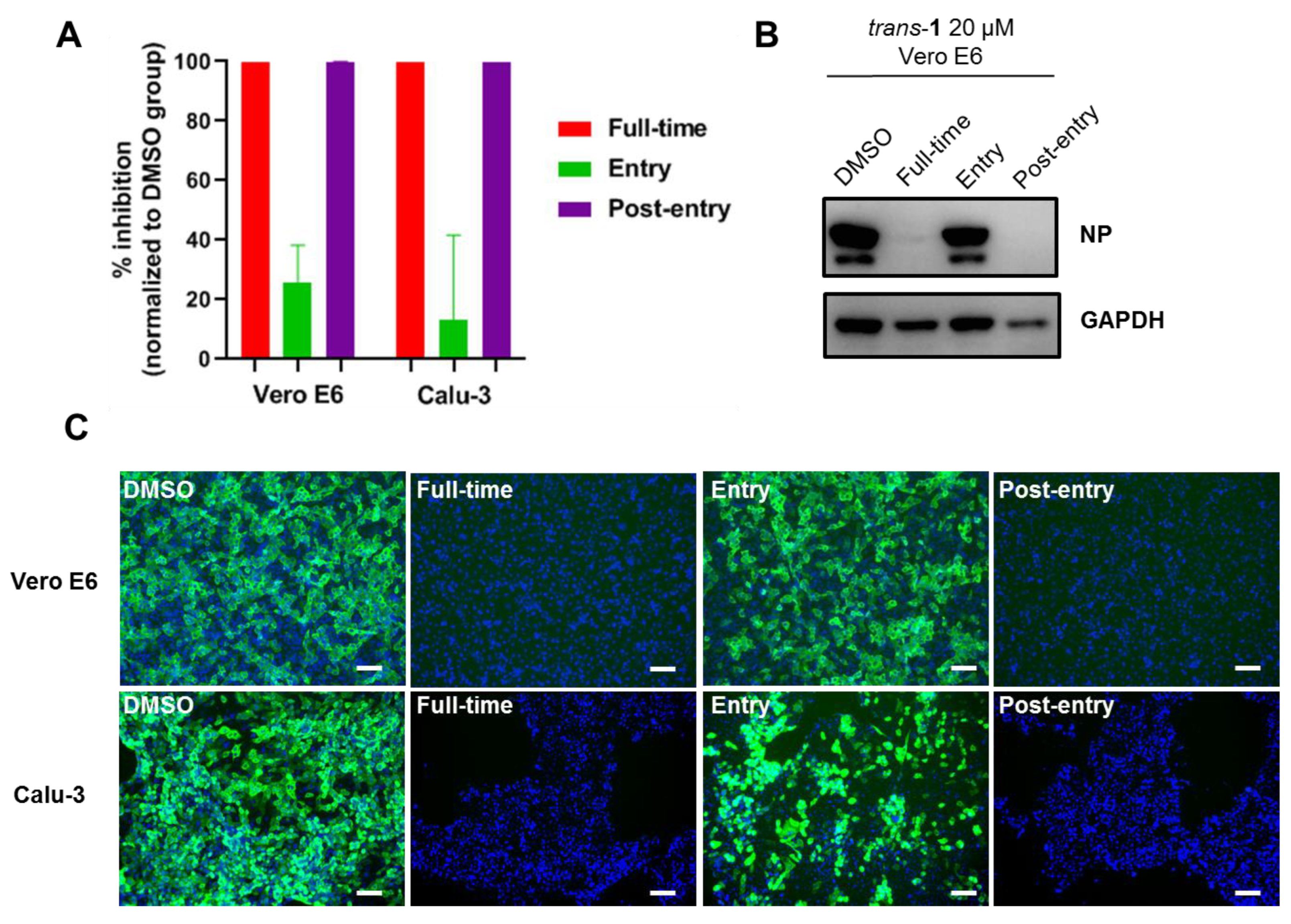
3.4. Mode of Action of Trans-1
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID-19 Weekly Epidemiological Update. 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---4-january-2023 (accessed on 4 January 2023).
- Zhou, P.; Shi, Z.L. SARS-CoV-2 spillover events. Science 2021, 371, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.L.; Sit, T.H.C.; Brackman, C.J.; Chuk, S.S.Y.; Gu, H.; Tam, K.W.S.; Law, P.Y.T.; Leung, G.M.; Peiris, M.; Poon, L.L.M. Transmission of SARS-CoV-2 delta variant (AY.127) from pet hamsters to humans, leading to onward human-to-human transmission: A case study. Lancet 2022, 399, 1070–1078. [Google Scholar] [CrossRef] [PubMed]
- Holshue, M.L.; DeBolt, C.; Lindquist, S.; Lofy, K.H.; Wiesman, J.; Bruce, H.; Spitters, C.; Ericson, K.; Wilkerson, S.; Tural, A.; et al. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martin-Quiros, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Ebenezer, O.; Jordaan, M.A.; Carena, G.; Bono, T.; Shapi, M.; Tuszynski, J.A. An Overview of the Biological Evaluation of Selected Nitrogen-Containing Heterocycle Medicinal Chemistry Compounds. Int. J. Mol. Sci. 2022, 23, 8117. [Google Scholar] [CrossRef]
- Chin, L.W.; Cheng, Y.W.; Lin, S.S.; Lai, Y.Y.; Lin, L.Y.; Chou, M.Y.; Chou, M.C.; Yang, C.C. Anti-herpes simplex virus effects of berberine from Coptidis rhizoma, a major component of a Chinese herbal medicine, Ching-Wei-San. Arch. Virol. 2010, 155, 1933–1941. [Google Scholar] [CrossRef]
- Varghese, F.S.; Kaukinen, P.; Glasker, S.; Bespalov, M.; Hanski, L.; Wennerberg, K.; Kummerer, B.M.; Ahola, T. Discovery of berberine, abamectin and ivermectin as antivirals against chikungunya and other alphaviruses. Antivir. Res. 2016, 126, 117–124. [Google Scholar] [CrossRef]
- Hung, T.C.; Jassey, A.; Liu, C.H.; Lin, C.J.; Lin, C.C.; Wong, S.H.; Wang, J.Y.; Yen, M.H.; Lin, L.T. Berberine inhibits hepatitis C virus entry by targeting the viral E2 glycoprotein. Phytomedicine Int. J. Phytother. Phytopharm. 2019, 53, 62–69. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Luo, C.; Liu, H.; Xu, W.; Chen, G.; Liew, O.W.; Zhu, W.; Puah, C.M.; Shen, X.; et al. Binding interaction of quercetin-3-beta-galactoside and its synthetic derivatives with SARS-CoV 3CL(pro): Structure-activity relationship studies reveal salient pharmacophore features. Bioorganic Med. Chem. 2006, 14, 8295–8306. [Google Scholar] [CrossRef]
- Kim, D.E.; Min, J.S.; Jang, M.S.; Lee, J.Y.; Shin, Y.S.; Song, J.H.; Kim, H.R.; Kim, S.; Jin, Y.H.; Kwon, S. Natural Bis-Benzylisoquinoline Alkaloids-Tetrandrine, Fangchinoline, and Cepharanthine, Inhibit Human Coronavirus OC43 Infection of MRC-5 Human Lung Cells. Biomolecules 2019, 9, 696. [Google Scholar] [CrossRef]
- Schmeller, T.; Latz-Bruning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Zou, G.; Fan, J.; Yuan, Z. Identification of palmatine as an inhibitor of West Nile virus. Arch. Virol. 2010, 155, 1325–1329. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Lu, J.W.; Huang, Y.L.; Lai, Z.Z. Palmatine inhibits Zika virus infection by disrupting virus binding, entry, and stability. Biochem. Biophys. Res. Commun. 2019, 518, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Croaker, A.; King, G.J.; Pyne, J.H.; Anoopkumar-Dukie, S.; Liu, L. Sanguinaria canadensis: Traditional Medicine, Phytochemical Composition, Biological Activities and Current Uses. Int. J. Mol. Sci. 2016, 17, 1414. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Liu, J.; Cao, R.Y.; Xu, M.Y.; Wang, X.; Zhang, H.Y.; Hu, H.R.; Li, Y.F.; Hu, Z.H.; Zhong, W.; Wang, M.L. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Floyd, D.M.; Stein, P.; Wang, Z.; Liu, J.; Castro, S.; Clark, J.A.; Connelly, M.; Zhu, F.; Holbrook, G.; Matheny, A.; et al. Hit-to-Lead Studies for the Antimalarial Tetrahydroisoquinolone Carboxanilides. J. Med. Chem. 2016, 59, 7950–7962. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Burdzhiev, N.T.; Baramov, T.I.; Stanoeva, E.R.; Yanev, S.G.; Stoyanova, T.D.; Dimitrova, D.H.; Kostadinova, K.A. Synthesis of novel trans-4-(phthalimidomethyl)- and 4-(imidazol-1-ylmethyl)-3-indolyl-tetrahydroisoquinolinones as possible aromatase inhibitors. Chem. Pap. 2019, 73, 1263–1277. [Google Scholar] [CrossRef]
- Kandinska, M.; Burdzhiev, N.; Cheshmedzhieva, D.; Ilieva, S.; Grozdanov, P.; Vilhelmova-Ilieva, N.; Nikolova, N.; Lozanova, V.; Nikolova, I. Synthesis of Novel 1-Oxo-2,3,4-trisubstituted Tetrahydroisoquinoline Derivatives, Bearing Other Heterocyclic Moieties and Comparative Preliminary Study of Anti-coronavirus Activity of Selected Compounds. Molecules 2023, 28, 1495–1518. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Mosbauer, K.; Hofmann-Winkler, H.; Kaul, A.; Kleine-Weber, H.; Kruger, N.; Gassen, N.C.; Muller, M.A.; Drosten, C.; Pohlmann, S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature 2020, 585, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Shaquiquzzaman, M.; Verma, G.; Marella, A.; Akhter, M.; Akhtar, W.; Khan, M.F.; Tasneem, S.; Alam, M.M. Piperazine scaffold: A remarkable tool in generation of diverse pharmacological agents. Eur. J. Med. Chem. 2015, 102, 487–529. [Google Scholar] [CrossRef]
- Kumari, S.; Mishra, C.B.; Idrees, D.; Prakash, A.; Yadav, R.; Hassan, M.I.; Tiwari, M. Design, synthesis, in silico and biological evaluation of novel 2-(4-(4-substituted piperazin-1-yl)benzylidene)hydrazine carboxamides. Mol. Divers. 2017, 21, 163–174. [Google Scholar] [CrossRef]
- Cao, R.; Hu, H.; Li, Y.; Wang, X.; Xu, M.; Liu, J.; Zhang, H.; Yan, Y.; Zhao, L.; Li, W.; et al. Anti-SARS-CoV-2 Potential of Artemisinins In Vitro. ACS Infect. Dis. 2020, 6, 2524–2531. [Google Scholar] [CrossRef]
- Rolain, J.M.; Colson, P.; Raoult, D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 2007, 30, 297–308. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Pohlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784. [Google Scholar] [CrossRef]
- Boulware, D.R.; Pullen, M.F.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.; Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M.; et al. A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for COVID-19. N. Engl. J. Med. 2020, 383, 517–525. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Burdzhiev, N.T.; Hu, H.; Li, Y.; Li, J.; Lozanova, V.V.; Kandinska, M.I.; Wang, M. Novel Tetrahydroisoquinoline-Based Heterocyclic Compounds Efficiently Inhibit SARS-CoV-2 Infection In Vitro. Viruses 2023, 15, 502. https://doi.org/10.3390/v15020502
Wang X, Burdzhiev NT, Hu H, Li Y, Li J, Lozanova VV, Kandinska MI, Wang M. Novel Tetrahydroisoquinoline-Based Heterocyclic Compounds Efficiently Inhibit SARS-CoV-2 Infection In Vitro. Viruses. 2023; 15(2):502. https://doi.org/10.3390/v15020502
Chicago/Turabian StyleWang, Xi, Nikola T. Burdzhiev, Hengrui Hu, Yufeng Li, Jiang Li, Vesela V. Lozanova, Meglena I. Kandinska, and Manli Wang. 2023. "Novel Tetrahydroisoquinoline-Based Heterocyclic Compounds Efficiently Inhibit SARS-CoV-2 Infection In Vitro" Viruses 15, no. 2: 502. https://doi.org/10.3390/v15020502





