Efficacy Validation of SARS-CoV-2-Inactivation and Viral Genome Stability in Saliva by a Guanidine Hydrochloride and Surfactant-Based Virus Lysis/Transport Buffer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Solutions
2.2. Viruses and Cells
2.3. Evaluation of the SARS-CoV-2-Inactivating Activity of Prep Buffer A
2.4. RNA Extraction and SARS-CoV-2 Gene Detection
2.5. Evaluation of Viral Genome Stability in Test Solutions
2.6. Direct Effect of Prep Buffer A on SARS-CoV-2 RNA
2.7. Statistical Analysis
3. Results
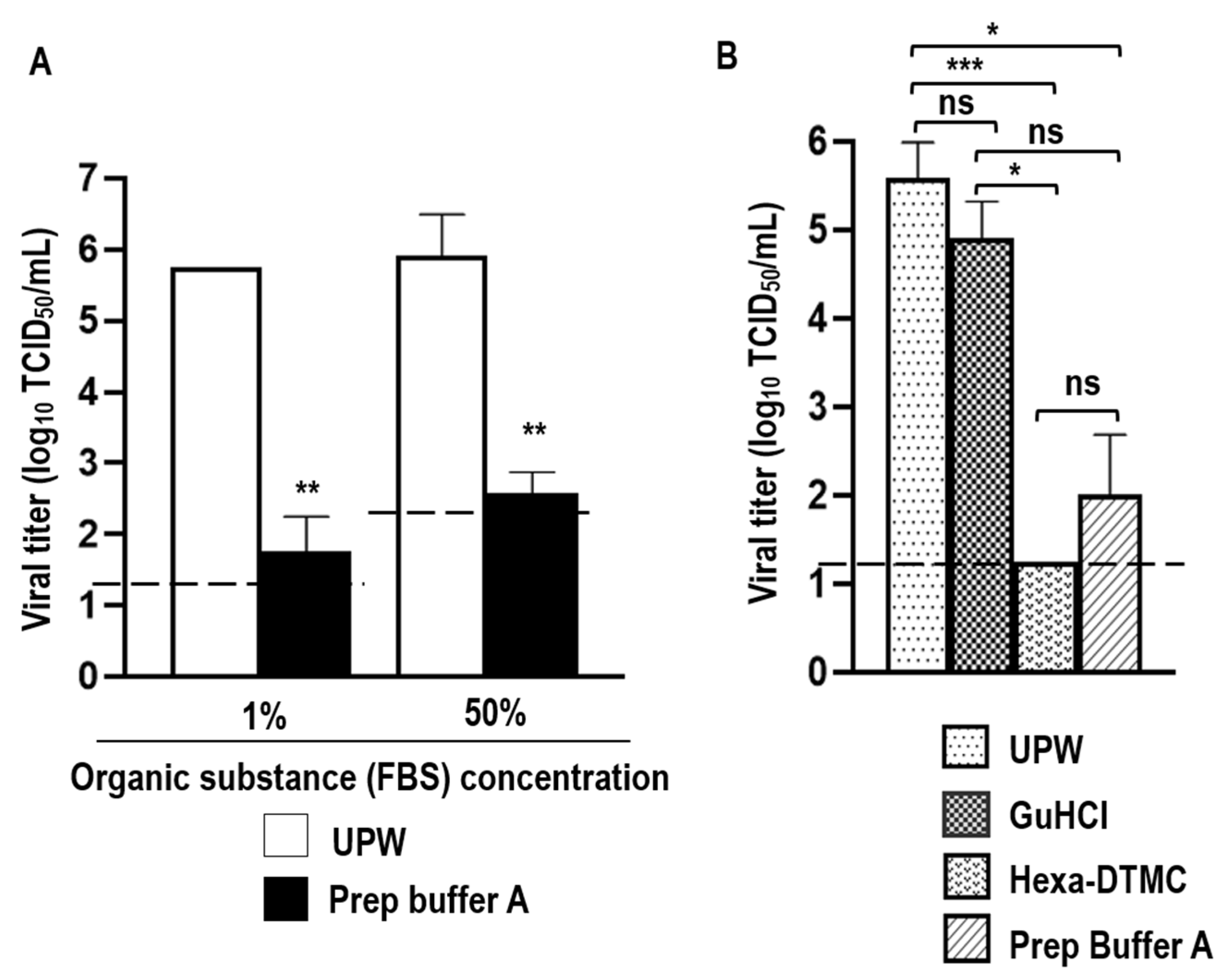
3.1. Virucidal Activity of Prep Buffer A against SARS-CoV-2
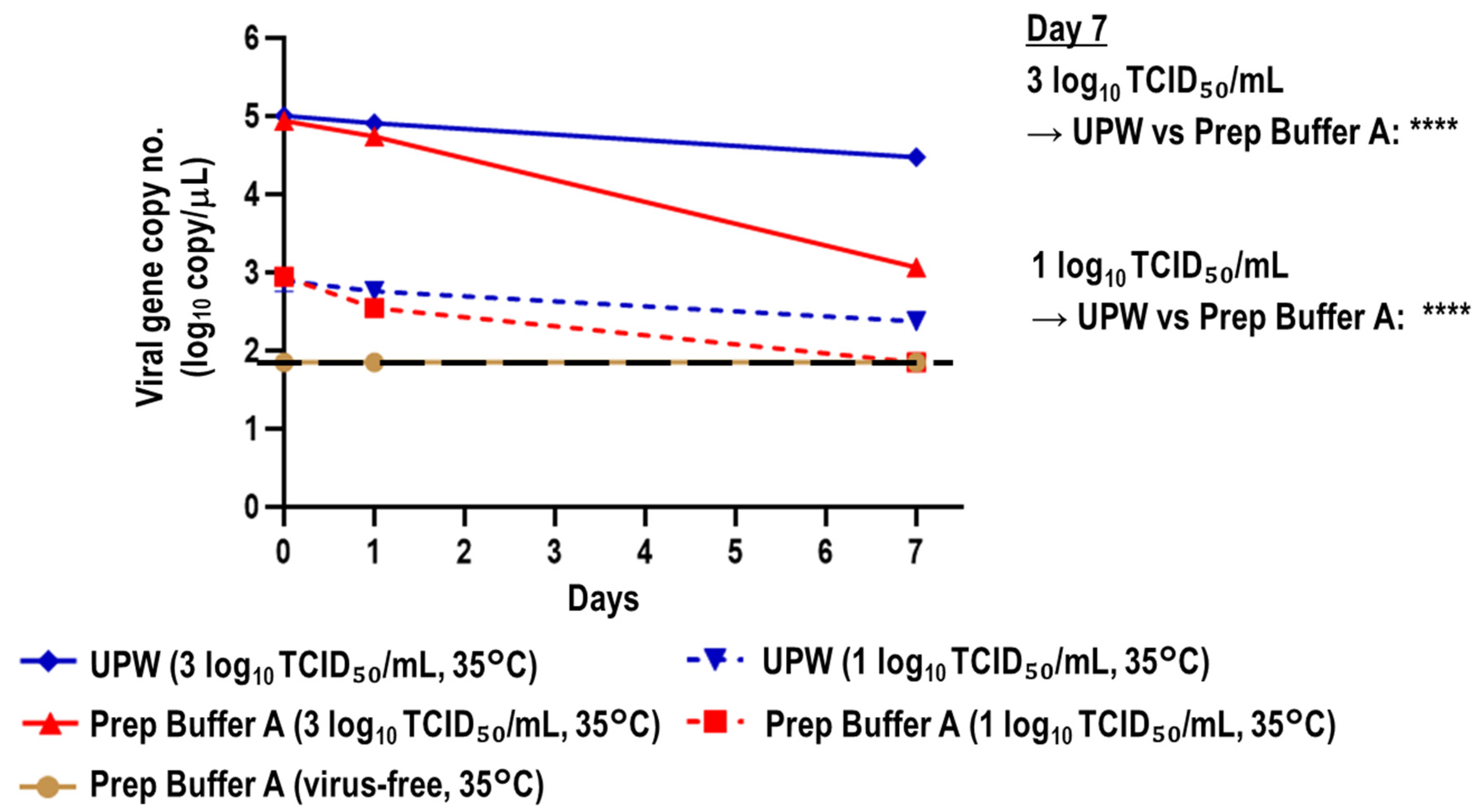
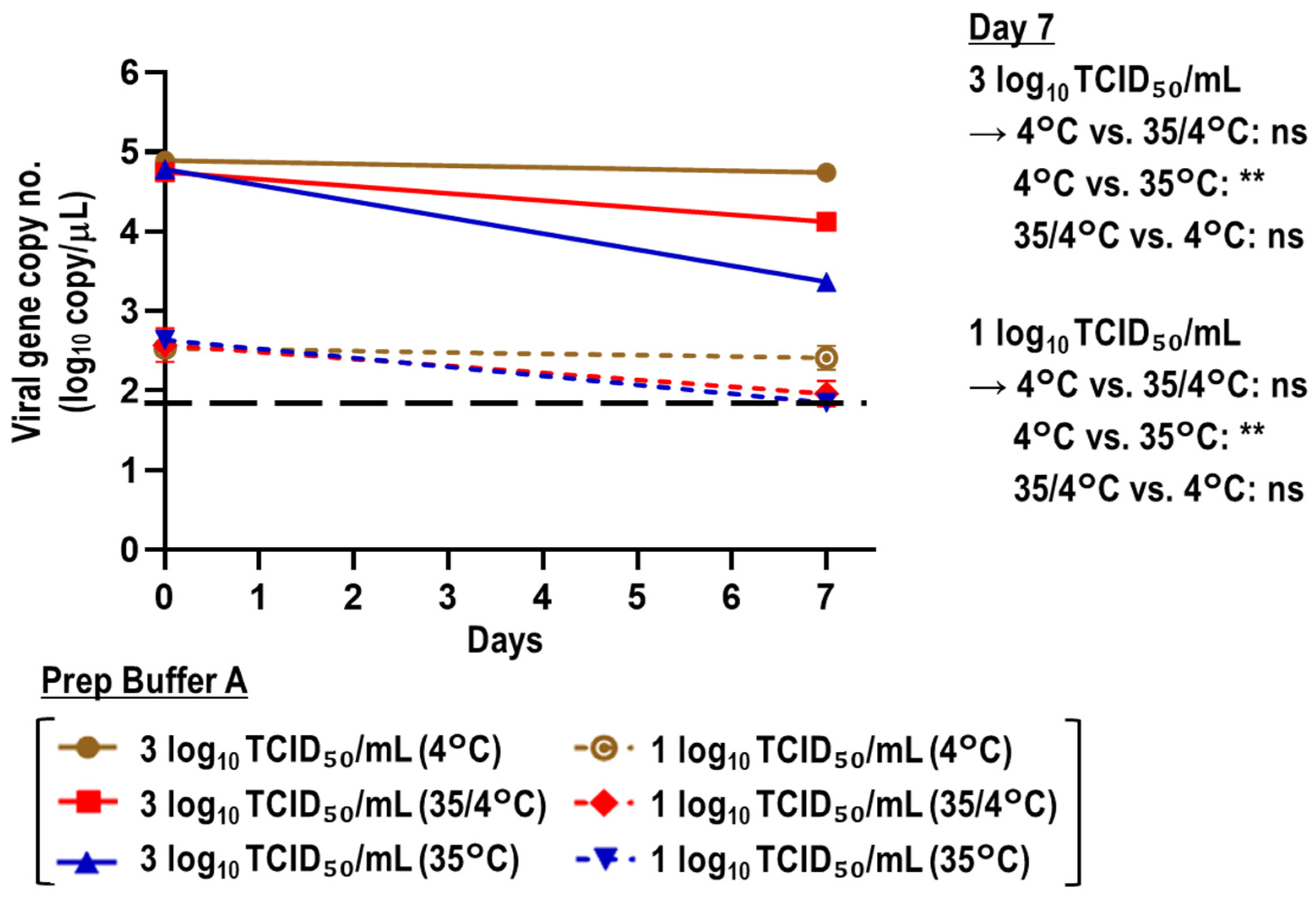
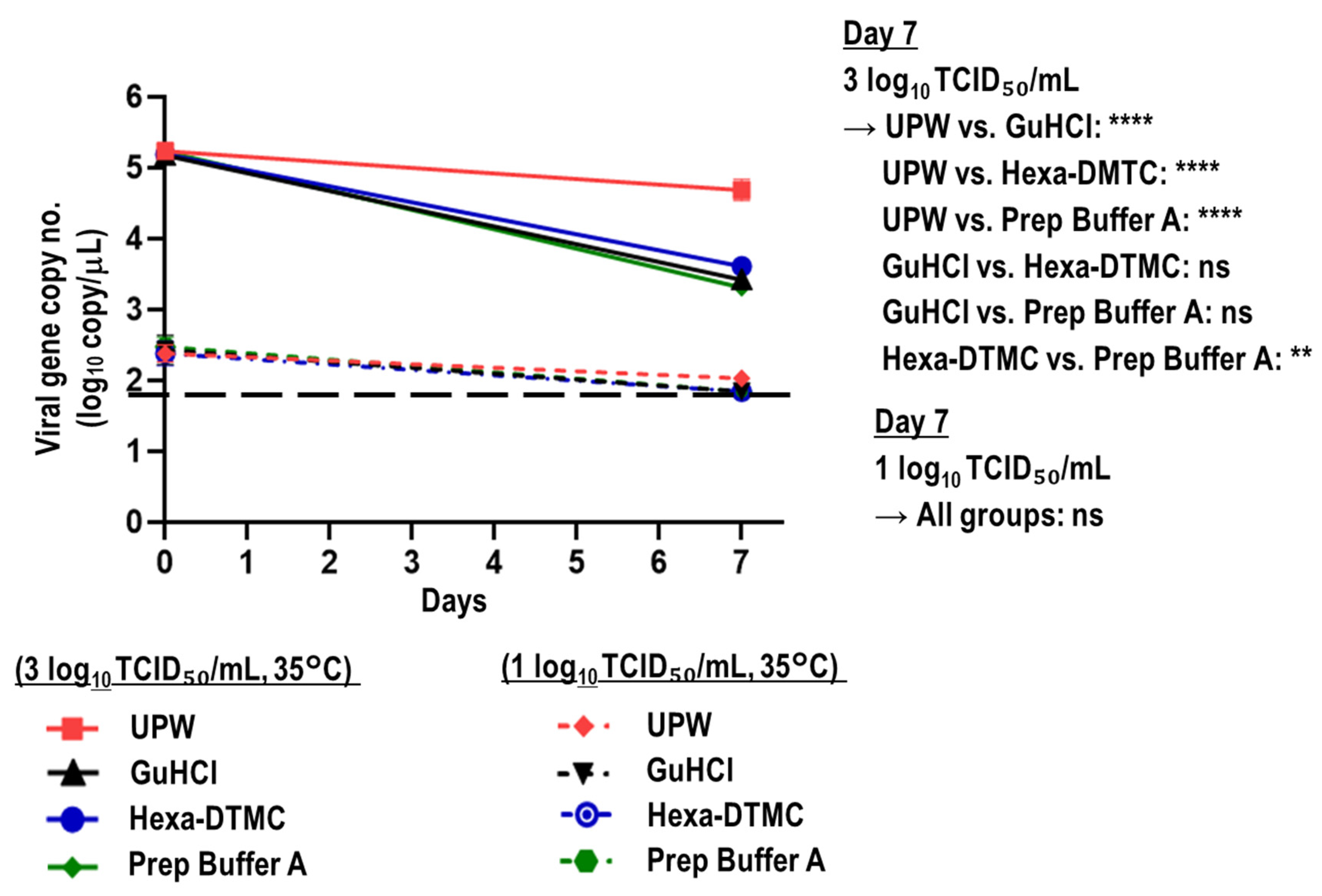
3.2. Stability of Viral RNA in SARS-CoV-2-Spiked Saliva Treated with Prep Buffer A
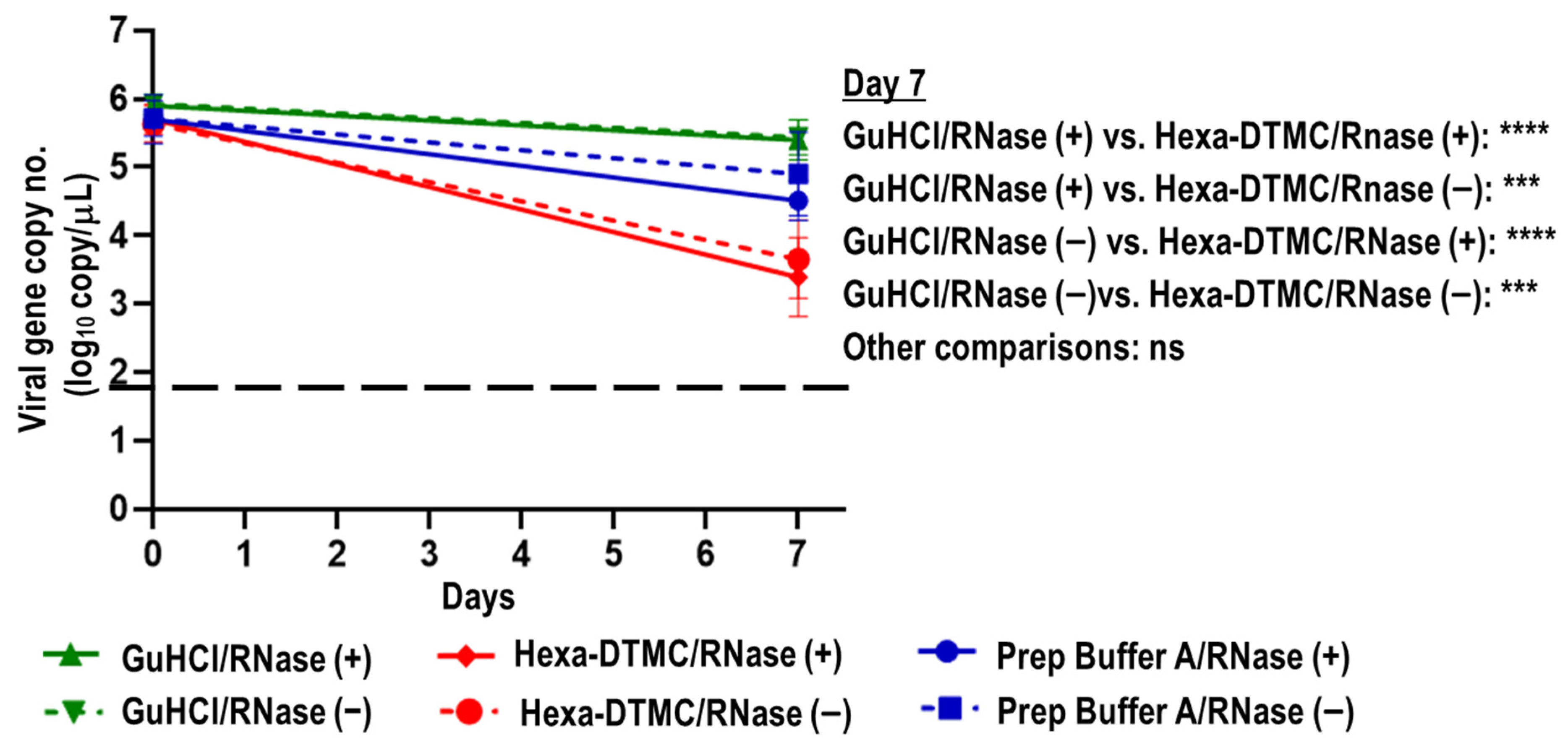
3.3. Stability of Viral RNA in SARS-CoV-2-Spiked Saliva Treated with GuHCl and Hexa-DTMC
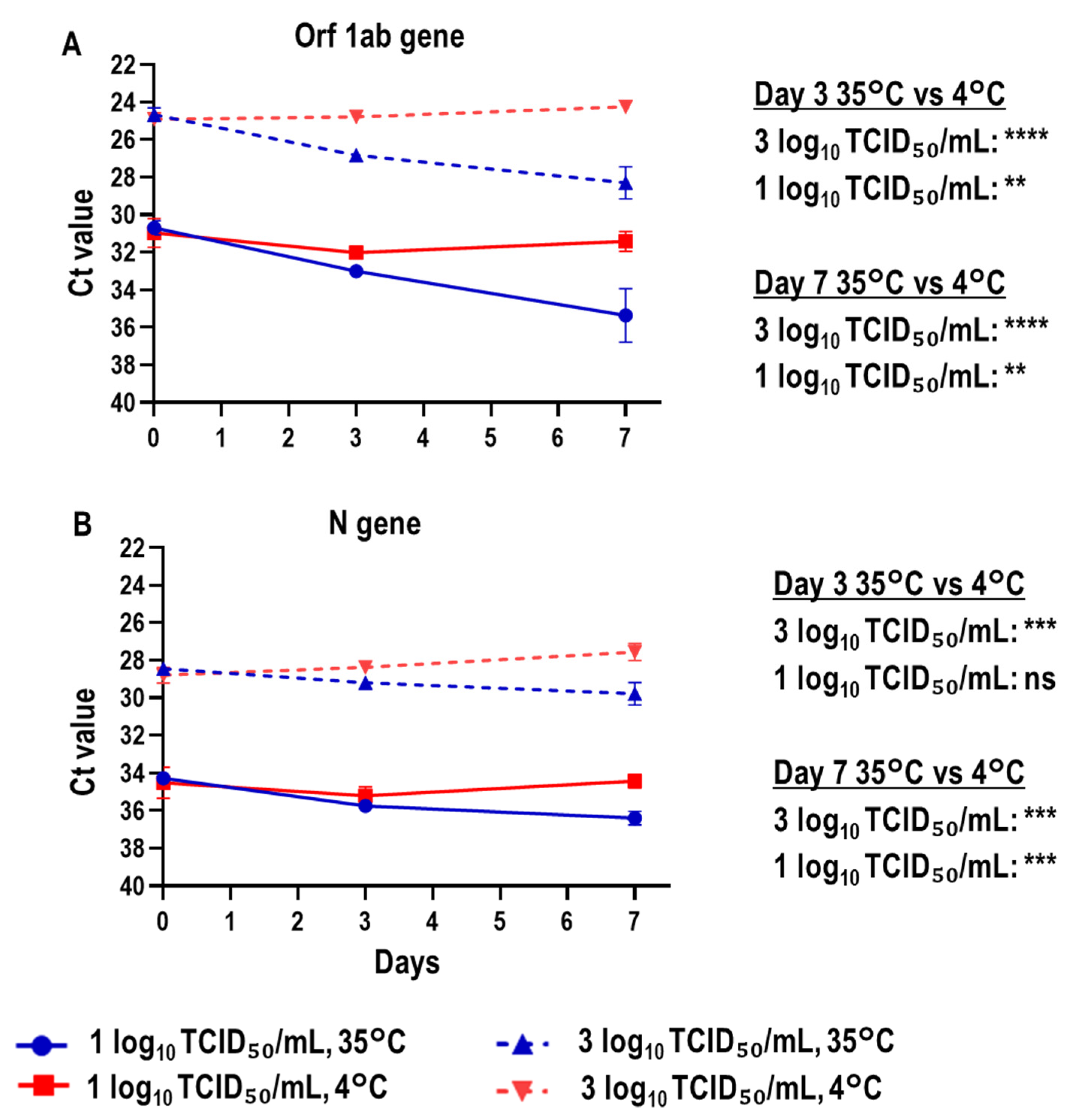
3.4. Direct Effects of Prep Buffer A and Its Individual Components on Naked SARS-CoV-2 RNA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Koopmans, M.; Go, U.; Hamer, D.H.; Petrosillo, N.; Castelli, F.; Storgaard, M.; al Khalili, S.; Simonsen, L. Comparing SARS-CoV-2 with SARS-CoV and Influenza Pandemics. Lancet Infect. Dis. 2020, 20, e238–e244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 29 January 2023).
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 Infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.; Al-Sadeq, D.W.; AL-Jighefee, H.; Younes, S.; Al-Jamal, O.; Daas, H.I.; Yassine, H.M.; Nasrallah, G.K. Challenges in Laboratory Diagnosis of the Novel Coronavirus SARS-CoV-2. Viruses 2020, 12, 582. [Google Scholar] [CrossRef] [PubMed]
- Anantharajah, A.; Helaers, R.; Defour, J.P.; Olive, N.; Kabera, F.; Croonen, L.; Deldime, F.; Vaerman, J.L.; Barbée, C.; Bodéus, M.; et al. How to Choose the Right Real-Time RT-PCR Primer Sets for the SARS-CoV-2 Genome Detection? J. Virol. Methods 2021, 295, 114197. [Google Scholar] [CrossRef]
- Opota, O.; Brouillet, R.; Greub, G.; Jaton, K. Comparison of SARS-CoV-2 RT-PCR on a High-Throughput Molecular Diagnostic Platform and the Cobas SARS-CoV-2 Test for the Diagnostic of COVID-19 on Various Clinical Samples. Pathog. Dis. 2020, 78, ftaa061. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Jernigan, D.B.; 2019-nCoV CDC Response Team. Initial Public Health Response and Interim Clinical Guidance for the 2019 Novel Coronavirus Outbreak—United States, 31 December 2019–4 February 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 140–146. [Google Scholar] [CrossRef]
- Amendola, A.; Sberna, G.; Lalle, E.; Colavita, F.; Castilletti, C.; Menchinelli, G.; Posteraro, B.; Sanguinetti, M.; Ippolito, G.; Bordi, L.; et al. Saliva Is a Valid Alternative to Nasopharyngeal Swab in Chemiluminescence-Based Assay for Detection of SARS-CoV-2 Antigen. J. Clin. Med. 2021, 10, 1471. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, J.; Xu, Y.; Chen, X. Viral Dynamics of SARS-CoV-2 in Saliva from Infected Patients. J. Infect. 2020, 81, e48–e50. [Google Scholar] [CrossRef]
- Moreira, V.M.; Mascarenhas, P.; Machado, V.; Botelho, J.; Mendes, J.J.; Taveira, N.; Almeida, M.G. Diagnosis of SARS-CoV-2 Infection by RT-PCR Using Specimens Other than Naso- And Oropharyngeal Swabs: A Systematic Review and Meta-Analysis. Diagnostics 2021, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Beyene, G.T.; Alemu, F.; Kebede, E.S.; Alemayehu, D.H.; Seyoum, T.; Tefera, D.A.; Assefa, G.; Tesfaye, A.; Habte, A.; Bedada, G.; et al. Saliva Is Superior over Nasopharyngeal Swab for Detecting SARS-CoV2 in COVID-19 Patients. Sci. Rep. 2021, 11, 22640. [Google Scholar] [CrossRef]
- Wyllie, A.L.; Fournier, J.; Casanovas-Massana, A.; Campbell, M.; Tokuyama, M.; Vijayakumar, P.; Warren, J.L.; Geng, B.; Muenker, M.C.; Moore, A.J.; et al. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Isles, N.; Chong, B.; Bond, K.; Yoga, Y.; Druce, J.; Catton, M.; Ballard, S.A.; Howden, B.P.; Williamson, D.A. Detection of SARS-CoV-2 in Saliva: Implications for Specimen Transport and Storage. J. Med. Microbiol. 2020, 70, 001285. [Google Scholar] [CrossRef] [PubMed]
- Ott, I.M.; Strine, M.S.; Watkins, A.E.; Boot, M.; Kalinich, C.C.; Harden, C.A.; Vogels, C.B.F.; Casanovas-Massana, A.; Moore, A.J.; Muenker, M.C.; et al. Simply Saliva: Stability of SARS-CoV-2 Detection Negates the Need for Expensive Collection Devices. medRxiv 2020. [Google Scholar] [CrossRef]
- Karthik, K.; Aravindh Babu, R.P.; Dhama, K.; Chitra, M.A.; Kalaiselvi, G.; Alagesan Senthilkumar, T.M.; Raj, G.D. Biosafety Concerns During the Collection, Transportation, and Processing of COVID-19 Samples for Diagnosis. Arch. Med. Res. 2020, 51, 623–630. [Google Scholar] [CrossRef]
- Dewar, R.; Baunoch, D.; Wojno, K.; Parkash, V.; Khosravi-Far, R. Viral Transportation in COVID-19 Pandemic: Inactivated Virus Transportation Should Be Implemented for Safe Transportation and Handling at Diagnostics Laboratories. Arch. Pathol. Lab. Med. 2020, 144, 916–917. [Google Scholar] [CrossRef]
- Widera, M.; Westhaus, S.; Rabenau, H.F.; Hoehl, S.; Bojkova, D.; Cinatl, J.; Ciesek, S. Evaluation of Stability and Inactivation Methods of SARS-CoV-2 in Context of Laboratory Settings. Med. Microbiol. Immunol. 2021, 210, 235–244. [Google Scholar] [CrossRef]
- Heilingloh, C.S.; Aufderhorst, U.W.; Schipper, L.; Dittmer, U.; Witzke, O.; Yang, D.; Zheng, X.; Sutter, K.; Trilling, M.; Alt, M.; et al. Susceptibility of SARS-CoV-2 to UV Irradiation. Am. J. Infect. Control 2020, 48, 1273–1275. [Google Scholar] [CrossRef]
- Jain, R.; Sarkale, P.; Mali, D.; Shete, A.; Patil, D.; Majumdar, T.; Suryawanshi, A.; Patil, S.; Mohandas, S.; Yadav, P. Inactivation of SARS-CoV-2 by Gamma Irradiation. Indian J. Med. Res. 2021, 153, 196–198. [Google Scholar]
- Leung, A.; Tran, K.; Audet, J.; Lavineway, S.; Bastien, N.; Krishnan, J. In Vitro Inactivation of SARS-CoV-2 Using Gamma Radiation. Appl. Biosaf. 2020, 25, 157–160. [Google Scholar] [CrossRef]
- Burton, J.; Love, H.; Richards, K.; Burton, C.; Summers, S.; Pitman, J.; Easterbrook, L.; Davies, K.; Spencer, P.; Killip, M.; et al. The Effect of Heat-Treatment on SARS-CoV-2 Viability and Detection. J. Virol. Methods 2021, 290, 114087. [Google Scholar] [CrossRef]
- Genoud, V.; Stortz, M.; Waisman, A.; Berardino, B.G.; Verneri, P.; Dansey, V.; Salvatori, M.; Lenicov, F.R.; Levi, V. Extraction-Free Protocol Combining Proteinase K and Heat Inactivation for Detection of SARS-CoV-2 by RT-QPCR. PLoS ONE 2021, 16, e0247792. [Google Scholar] [CrossRef]
- Pastorino, B.; Touret, F.; Gilles, M.; Luciani, L.; de Lamballerie, X.; Charrel, R.N. Evaluation of Chemical Protocols for Inactivating SARS-CoV-2 Infectious Samples. Viruses 2020, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, M.; Wolff, S.; Ludwig, S.; Schäfer, W.; Keiner, B.; Roth, N.J.; Widmer, E. Rapid SARS-CoV-2 Inactivation by Commonly Available Chemicals on Inanimate Surfaces. J. Hosp. Infect. 2020, 106, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Welch, S.R.; Davies, K.A.; Buczkowski, H.; Hettiarachchi, N.; Green, N.; Arnold, U.; Jones, M.; Hannah, M.J.; Evans, R.; Burton, C.; et al. Analysis of Inactivation of SARS-CoV-2 by Specimen Transport Media, Nucleic Acid Extraction Reagents, Detergents, and Fixatives. J.Clin. Microbiol. 2020, 58, e01713–e01720. [Google Scholar] [CrossRef] [PubMed]
- Farrell, R.E., Jr. RNA Methodologies: Laboratory Guide for Isolation and Characterization, 4th ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 165–166. [Google Scholar]
- Meingast, C.; Heldt, C.L. Arginine-Enveloped Virus Inactivation and Potential Mechanisms. Biotechnol. Prog. 2020, 36, e2931. [Google Scholar] [CrossRef] [PubMed]
- Quevedo-León, R.; Bastías-Montes, J.M.; Espinoza-Tellez, T.; Ronceros, B.; Balic, I.; Muñoz, O. Inactivation of Coronaviruses in Food Industry: The Use of Inorganic and Organic Disinfectants, Ozone, and UV Radiation. Sci. Agropecu. 2020, 11, 257–266. [Google Scholar] [CrossRef]
- Nao, N.; Sato, K.; Yamagishi, J.; Tahara, M.; Nakatsu, Y.; Seki, F.; Katoh, H.; Ohnuma, A.; Shirogane, Y.; Hayashi, M.; et al. Consensus and Variations in Cell Line Specificity among Human Metapneumovirus Strains. PLoS ONE 2019, 14, e0215822. [Google Scholar] [CrossRef]
- Takeda, Y.; Jamsransuren, D.; Matsuda, S.; Crea, R.; Ogawa, H. The Sars-Cov-2-Inactivating Activity of Hydroxytyrosol-Rich Aqueous Olive Pulp Extract (Hidrox®) and Its Use as a Virucidal Cream for Topical Application. Viruses 2021, 13, 232. [Google Scholar] [CrossRef]
- Kärber, G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Naunyn. Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Shirato, K.; Nao, N.; Katano, H.; Takayama, I.; Saito, S.; Kato, F.; Katoh, H.; Sakata, M.; Nakatsu, Y.; Mori, Y.; et al. Development of Genetic Diagnostic Methods for Detection for Novel Coronavirus 2019(NCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020, 73, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Wiraswati, H.L.; Gaffar, S.; Ekawardhani, S.; Fauziah, N.; Rinawan, F.R.; Widyatmoko, L.; Laelalugina, A.; Arimdayu, A.R.; Kusniati, T.; Andari, C.D.; et al. Evaluation and Clinical Validation of Guanidine-Based Inactivation Transport Medium for Preservation of SARS-CoV-2. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 1677621. [Google Scholar] [CrossRef] [PubMed]
- Weidner, L.; Laner-Plamberger, S.; Horner, D.; Pistorius, C.; Jurkin, J.; Karbiener, M.; Schistal, E.; Kreil, T.R.; Jungbauer, C. Sample Buffer Containing Guanidine-Hydrochloride Combines Biological Safety and RNA Preservation for SARS-CoV-2 Molecular Diagnostics. Diagnostics 2022, 12, 1186. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mazur, S.; Ork, B.L.; Postnikova, E.; Hensley, L.E.; Jahrling, P.B.; Johnson, R.; Holbrook, M.R. Inactivation and Safety Testing of Middle East Respiratory Syndrome Coronavirus. J. Virol. Methods 2015, 223, 13–18. [Google Scholar] [CrossRef]
- Burton, J.E.; Easterbrook, L.; Pitman, J.; Anderson, D.; Roddy, S.; Bailey, D.; Vipond, R.; Bruce, C.B.; Roberts, A.D. The Effect of a Non-Denaturing Detergent and a Guanidinium-Based Inactivation Agent on the Viability of Ebola Virus in Mock Clinical Serum Samples. J. Virol. Methods 2017, 250, 34–40. [Google Scholar] [CrossRef]
- Blow, J.A.; Dohm, D.J.; Negley, D.L.; Mores, C.N. Virus Inactivation by Nucleic Acid Extraction Reagents. J. Virol. Methods 2004, 119, 195–198. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008 (Updated: May 2019). Available online: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/ (accessed on 29 May 2022).
- Liu, Y.; Kumblathan, T.; Feng, W.; Pang, B.; Tao, J.; Xu, J.; Xiao, H.; Joyce, M.A.; Tyrrell, D.L.; Zhang, H.; et al. On-Site Viral Inactivation and RNA Preservation of Gargle and Saliva Samples Combined with Direct Analysis of SARS-CoV-2 RNA on Magnetic Beads. ACS Meas. Sci. Au 2022, 2, 224–232. [Google Scholar] [CrossRef]
- Scallan, M.F.; Dempsey, C.; MacSharry, J.; O’Callaghan, I.; O’Connor, P.M.; Horgan, C.P.; Durack, E.; Cotter, P.D.; Hudson, S.; Moynihan, H.A.; et al. Validation of a Lysis Buffer Containing 4 M Guanidinium Thiocyanate (GITC)/Triton X-100 for Extraction of SARS-CoV-2 RNA for COVID-19 Testing: Comparison of Formulated Lysis Buffers Containing 4 to 6 M GITC, Roche External Lysis Buffer and Qiagen RTL Lysis Buffer. bioRxiv 2020, 2020, 026435. [Google Scholar] [CrossRef]
- Banik, S.; Saibire, K.; Suryavanshi, S.; Johns, G.; Chakravorty, S.; Kwiatkowski, R.; Alland, D.; Banada, P.P. Inactivation of SARS-CoV-2 Virus in Saliva Using a Guanidium Based Transport Medium Suitable for RT-PCR Diagnostic Assays. PLoS ONE 2021, 16, e0252687. [Google Scholar] [CrossRef]
- Thom, R.E.; Eastaugh, L.S.; O’Brien, L.M.; Ulaeto, D.O.; Findlay, J.S.; Smither, S.J.; Phelps, A.L.; Stapleton, H.L.; Hamblin, K.A.; Weller, S.A. Evaluation of the SARS-CoV-2 Inactivation Efficacy Associated with Buffers from Three Kits Used on High-throughput RNA Extraction Platforms. Front. Cell. Infect. Microbiol. 2021, 11, 716436. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diagnostic Testing for SARS-CoV-2. Interim Guidance 11 September 2020. Available online: https://apps.who.int/iris/bitstream/handle/10665/334254/WHO-2019-nCoV-laboratory-2020.6-eng.pdf?sequence=1&isAllowed=y (accessed on 15 June 2022).
- Duma, Z.; Chuturgoon, A.A.; Ramsuran, V.; Edward, V.; Naidoo, P.; Mpaka-Mbatha, M.N.; Bhengu, K.N.; Nembe, N.; Pillay, R.; Singh, R.; et al. The Challenges of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Testing in Low-Middle Income Countries and Possible Cost-Effective Measures in Resource-Limited Settings. Glob. Health 2022, 18, 5. [Google Scholar] [CrossRef]
- Boers, S.A.; Mourik, B.C.; van Bussel, M.J.A.W.M.; de Brouwer, C.S.; Wessels, E.; Claas, E.C.J. Increasing Diagnostic Possibilities Using the GeneLEAD VIII Platform for Detection of SARS-CoV-2. J. Virol. Methods 2021, 298, 114291. [Google Scholar] [CrossRef] [PubMed]
- Nishibata, Y.; Koshimoto, S.; Ogaki, K.; Ishikawa, E.; Wada, K.; Yoshinari, M.; Tamura, Y.; Uozumi, R.; Masuda, S.; Tomaru, U.; et al. RNase in the Saliva Can Affect the Detection of Severe Acute Respiratory Syndrome Coronavirus 2 by Real-Time One-Step Polymerase Chain Reaction Using Saliva Samples. Pathol. Res. Pract. 2021, 220, 153381. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Malott, R.J.; Ward, L.; Kiplagat, L.; Pabbaraju, K.; Gill, K.; Berenger, B.M.; Hu, J.; Fonseca, K.; Noyce, R.S.; et al. Detection and Quantification of Infectious Severe Acute Respiratory Coronavirus-2 in Diverse Clinical and Environmental Samples. Sci. Rep. 2022, 12, 5418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komu, J.G.; Jamsransuren, D.; Matsuda, S.; Ogawa, H.; Takeda, Y. Efficacy Validation of SARS-CoV-2-Inactivation and Viral Genome Stability in Saliva by a Guanidine Hydrochloride and Surfactant-Based Virus Lysis/Transport Buffer. Viruses 2023, 15, 509. https://doi.org/10.3390/v15020509
Komu JG, Jamsransuren D, Matsuda S, Ogawa H, Takeda Y. Efficacy Validation of SARS-CoV-2-Inactivation and Viral Genome Stability in Saliva by a Guanidine Hydrochloride and Surfactant-Based Virus Lysis/Transport Buffer. Viruses. 2023; 15(2):509. https://doi.org/10.3390/v15020509
Chicago/Turabian StyleKomu, James Gitau, Dulamjav Jamsransuren, Sachiko Matsuda, Haruko Ogawa, and Yohei Takeda. 2023. "Efficacy Validation of SARS-CoV-2-Inactivation and Viral Genome Stability in Saliva by a Guanidine Hydrochloride and Surfactant-Based Virus Lysis/Transport Buffer" Viruses 15, no. 2: 509. https://doi.org/10.3390/v15020509




