Evaluation of Simplified HCV Diagnostics in HIV/HCV Co-Infected Patients in Myanmar
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Assessments
2.3. Diagnostic Testing Methodology
2.4. Study Definitions
2.5. Study Endpoints
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Treatment Initiation, Uptake, and Completion
3.3. Treatment Efficacy and Factors Associated with SVR
3.4. Virological Failure
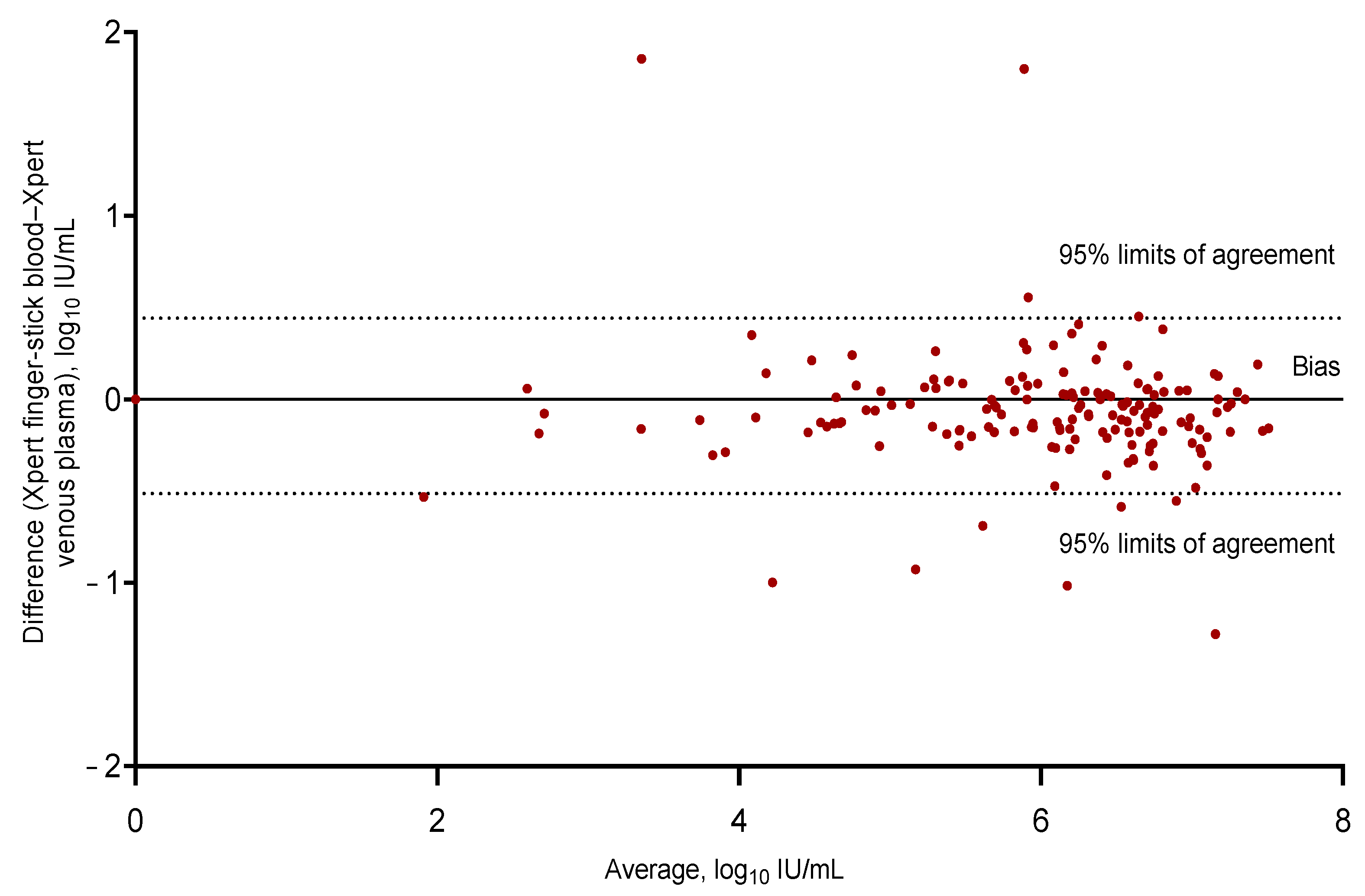
3.5. Diagnostic Performance of Fingerstick Testing
3.6. Sensitivity & Specificity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, J.S.; Young, M.; Marshall, C.; Tate-Baker, J.; Madison, M.; Sharma, S.; Silva, C.; Jones, T.; Davies, J. Minimal Compared With Standard Monitoring During Sofosbuvir-Based Hepatitis C Treatment: A Randomized Controlled Trial. Open Forum Infect. Dis. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Barber, E.; Cook, N.A.; Gomaa, A.; Harley, Y.X.; Jones, C.; Lim, A.G.; Mohamed, Z.; Nayagam, A.S.; Ndow, G.; et al. Aiming at the Global Elimination of Viral Hepatitis: Challenges Along the Care Continuum. Open Forum Infect. Dis. 2018, 5, 252. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.; Win, T.M.; Tidhar, T.; Htay, H.; Draper, B.; Aung, P.T.Z.; Xiao, Y.; Bowring, A.; Kuschel, C.; Shilton, S.; et al. Hepatitis C elimination in Myanmar: Modelling the impact, cost, cost-effectiveness and economic benefits. Lancet Reg. Health West. Pac. 2021, 10, 100129. [Google Scholar] [CrossRef] [PubMed]
- Marquez, L.K.; Chaillon, A.; Soe, K.P.; Johnson, D.C.; Zosso, J.M.; Incerti, A.; Loarec, A.; Nguyen, A.; Walker, J.G.; Mafirakureva, N.; et al. Cost and cost-effectiveness of a real-world HCV treatment program among HIV-infected individuals in Myanmar. BMJ Glob. Health 2021, 6, 4181. [Google Scholar] [CrossRef]
- Min Thaung, Y.; Chasela, C.S.; Chew, K.W.; Minior, T.; Lwin, A.A.; Sein, Y.Y.; Drame, N.; Marange, F.; van Der Horst, C.; Thwin, H.T.; et al. Treatment outcomes and costs of a simplified antiviral treatment strategy for hepatitis C among monoinfected and HIV and/or hepatitis B virus-co-infected patients in Myanmar. J. Viral Hepat. 2021, 28, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Boeke, C.E.; Adesigbin, C.; Agwuocha, C.; Anartati, A.; Aung, H.T.; Aung, K.S.; Grover, G.S.; Ngo, D.; Okamoto, E.; Ngwije, A.; et al. Initial success from a public health approach to hepatitis C testing, treatment and cure in seven countries: The road to elimination. BMJ Glob. Health 2020, 5, e003767. [Google Scholar] [CrossRef]
- Martin, N.K.; Vickerman, P.; Grebely, J.; Hellard, M.; Hutchinson, S.J.; Lima, V.D.; Foster, G.R.; Dillon, J.F.; Goldberg, D.J.; Dore, G.J.; et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology 2013, 58, 1598–1609. [Google Scholar] [CrossRef]
- Aung, K.S. Viral Hepatitis in Myanmar: Current situation and response 2016. In National Hepatitis Program; Ministry of Health and Family Welfare: New Delhi, India, 2016. [Google Scholar]
- Zaw, S.K.K.; Tun, S.T.T.; Thida, A.; Aung, T.K.; Maung, W.; Shwe, M.; Aye, M.M.; Clevenbergh, P. Prevalence of hepatitis C and B virus among patients infected with HIV: A cross-sectional analysis of a large HIV care programme in Myanmar. Trop. Dr. 2013, 43, 113–115. [Google Scholar] [CrossRef]
- Ministry of Health and Sports. Myanmar National Simplified Treatment Guidelines for Hepatitis C Infection, 2nd ed.; Ministry of Health and Sports: Naypyidaw, Myanmar, 2019. Available online: http://mohs.gov.mm/Main/content/publication/hepatitis-national-simplified-treatment-guidelines-of-viral-hepatitis-c-infection-second-edition-july-2019 (accessed on 11 March 2022).
- CT2 Study Myanmar Hepatitis C: Community-Based Testing and Treatment Summary Report. 2020. Available online: https://burnet.edu.au/system/asset/file/4513/Summary-Report_Hep_C_Myanmar_DigitalFinal.pdf (accessed on 24 January 2023).
- Catlett, B.; Hajarizadeh, B.; Cunningham, E.; Wolfson-Stofko, B.; Wheeler, A.; Khandaker-Hussain, B.; Feld, J.J.; Martró, E.; Chevaliez, S.; Pawlotsky, J.M.; et al. Diagnostic Accuracy of Assays Using Point-of-Care Testing or Dried Blood Spot Samples for the Determination of Hepatitis C Virus RNA: A Systematic Review. J. Infect. Dis. 2022, 226, 1005–1021. [Google Scholar] [CrossRef]
- Lamoury, F.M.J.; Bajis, S.; Hajarizadeh, B.; Marshall, A.D.; Martinello, M.; Ivanova, E.; Catlett, B.; Mowat, Y.; Marks, P.; Amin, J.; et al. Evaluation of the Xpert HCV Viral Load Finger-Stick Point-of-Care Assay. J. Infect. Dis. 2018, 217, 1889–1896. [Google Scholar] [CrossRef]
- Koo, V.; Tian, F.; Wong, W.W. Cost-effectiveness analysis of hepatitis C virus (HCV) point-of-care assay for HCV screening. Liver Int. 2022, 42, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Scott, N.; Nayagam, S.; Rwegasha, J.; Mbwambo, J.; Thursz, M.R.; Brown, A.S.; Hellard, M.; Lemoine, M. Cost effectiveness of simplified HCV screening-and-treatment interventions for people who inject drugs in Dar-es-Salaam, Tanzania. Int. J. Drug Policy 2022, 99, 103458. [Google Scholar] [CrossRef] [PubMed]
- Midgard, H.; Bjørnestad, R.; Egeland, M.; Dahl, E.; Finbråten, A.; Kielland, K.B.; Blindheim, M.; Dalgard, O. Peer support in small towns: A decentralized mobile Hepatitis C virus clinic for people who inject drugs. Liver Int. 2022, 42, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Tao, Y.; Fajardo, E.; Reipold, E.I.; Chou, R.; Tucker, J.D.; Easterbrook, P. Diagnostic Accuracy of Point-of-Care HCV Viral Load Assays for HCV Diagnosis: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.; Lazarus, J.V.; Ceballos, F.C.; Troya, J.; Cuevas, G.; Resino, S.; Torres-Macho, J.; Ryan, P. Differences in the hepatitis C virus cascade of care and time to initiation of therapy among vulnerable subpopulations using a mobile unit as point-of-care. Liver Int. 2022, 42, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Hyle, E.P.; Noubary, F.; Freedberg, K.A.; Wilson, D.; Bishai, W.R.; Rodriguez, W.; Bassett, I.V. Diagnostic point-of-care tests in resource-limited settings. Lancet Infect. Dis. 2014, 14, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Grebely, J.; Applegate, T.; Cunningham, P.; Feld, J.J. Hepatitis C point-of-care diagnostics: In search of a single visit diagnosis. Expert Rev. Mol. Diagn. 2017, 17, 1109–1115. [Google Scholar] [CrossRef]
- Brown, S.; Leavy, J.E.; Jancey, J. Implementation of GeneXpert for TB Testing in Low- and Middle-Income Countries: A Systematic Review. Glob. Health Sci. Pr. 2021, 9, 698–710. [Google Scholar] [CrossRef]
- Casiraghi, A.; Domenicucci, M.; Cattaneo, S.; Maggini, E.; Albertini, F.; Avanzini, S.; Marini, M.P.; Galante, C.; Guizzi, P.; Milano, G. Operational strategies of a trauma hub in early coronavirus disease 2019 pandemic. Int. Orthop. 2020, 44, 1511–1518. [Google Scholar] [CrossRef]
- Nalugwa, T.; Shete, P.B.; Nantale, M.; Farr, K.; Ojok, C.; Ochom, E.; Mugabe, F.; Joloba, M.; Dowdy, D.W.; Moore, D.A.J.; et al. Challenges with scale-up of GeneXpert MTB/RIF® in Uganda: A health systems perspective. BMC Health Serv. Res. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Euroqol Research Foundation. EQ-5D-5L User Guide. 2019. Available online: https://euroqol.org/publications/user-guides (accessed on 9 February 2021).
- Bush, K.; Kivlahan, D.R.; McDonell, M.B.; Fihn, S.D.; Bradley, K.A. The AUDIT Alcohol Consumption Questions (AUDIT-C). An Effective Brief Screening Test for Problem Drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998, 158, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO List of Prequalified In Vitro Diagnostic Products; World Health Organization: Geneva, Switzerland, 2022; Available online: https://extranet.who.int/pqweb/vitro-diagnostics/vitro-diagnostics-lists (accessed on 11 March 2022).
- Xpert® HCV Viral Load [Package Insert]; Cepheid: Solna, Sweden, 2021.
- World Health Organization. WHO Guidelines on Drawing Blood: Best Practices in Phlebotomy; World Health Organization: Geneva, Switzerland, 2010; Available online: https://www.who.int/publications/i/item/9789241599221 (accessed on 11 March 2022).
- Xpert® HCV VL Fingerstick [Package Insert]; Cepheid: Solna, Sweden, 2019.
- Rahman, M.S.; Sultana, M. Performance of Firth-and logF-type penalized methods in risk prediction for small or sparse binary data. BMC Med Res. Methodol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Alessio, L.; Onorato, L.; Sangiovanni, V.; Borrelli, F.; Manzillo, E.; Esposito, V.; Simeone, F.; Martini, S.; Capoluongo, N.; Leone, S.; et al. DAA-Based Treatment for HIV–HCV-Coinfected Patients: Analysis of Factors of Sustained Virological Response in a Real-Life Study. Antivir. Ther. 2020, 25, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, L.; Lai, A.; Calvi, E.; Ronzi, P.; Micheli, V.; Binda, F.; Ridolfo, A.; Gervasoni, C.; Galli, M.; Antinori, S.; et al. Direct-acting antivirals in hepatitis C virus (HCV)-infected and HCV/HIV-coinfected patients: Real-life safety and efficacy. HIV Med. 2017, 18, 284–291. [Google Scholar] [CrossRef]
- Patel, S.V.; Jayaweera, D.T.; Althoff, K.N.; Eron, J.J.; Radtchenko, J.; Mills, A.; Moyle, G.; Santiago, S.; Sax, P.E.; Gillman, J.; et al. Real-world efficacy of direct acting antiviral therapies in patients with HIV/HCV. PLoS ONE 2020, 15, e0228847. [Google Scholar] [CrossRef]
- Ward, K.M.; Falade-Nwulia, O.; Moon, J.; Sutcliffe, C.G.; Brinkley, S.; Haselhuhn, T.; Katz, S.; Herne, K.; Arteaga, L.; Mehta, S.H.; et al. A Randomized Controlled Trial of Cash Incentives or Peer Support to Increase HCV Treatment for Persons With HIV Who Use Drugs: The CHAMPS Study. Open Forum Infect. Dis. 2019, 6, 166. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Pineda, J.A.; Calleja, J.L.; Casado, M.; Rodríguez, M.; Turnes, J.; Amado, L.E.; Morillas, R.M.; Forns, X.; Acevedo, J.M.; et al. Efficacy of sofosbuvir and velpatasvir, with and without ribavirin, in with hepatitis C virus genotype 3 infection and cirrhosis. Gastroenterology 2018, 155, 1120–1127. [Google Scholar] [CrossRef]
- Ousley, J.; Nesbitt, R.; Kyaw, N.T.T.; Bermudez, E.; Soe, K.P.; Anicete, R.; Mon, P.E.; Ei, W.L.S.S.; Christofani, S.; Fernandez, M.; et al. Increased hepatitis C virus co-infection and injection drug use in HIV-infected fishermen in Myanmar. BMC Infect. Dis. 2018, 18, 657. [Google Scholar] [CrossRef]
- San Lin, K.; Nay Win, K.H.; Khine, T.; Lei, S.L.; Zaw, K.K.; Oo, W.M. The characteristics and trend of COVID-19 outbreak in Myanmar: Lessons from a developing country. Asia Pac. J. Public Health 2021, 33, 311–313. [Google Scholar] [CrossRef]
- Win, A. Rapid rise of COVID-19 second wave in Myanmar and implications for the Western Pacific region. Qjm: Int. J. Med. 2020, 113, 856–857. [Google Scholar] [CrossRef]
- Magro, P.; Formenti, B.; Marchese, V.; Gulletta, M.; Tomasoni, L.R.; Caligaris, S.; Castelli, F.; Matteelli, A. Impact of the SARS-CoV-2 epidemic on tuberculosis treatment outcome in Northern Italy. Eur. Respir. J. 2020, 56, 2002665. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.J.; Xu, L.; Qin, J.; Hahn, E.E.; Ngo-Metzger, Q.; Mittman, B.; Tewari, D.; Hodeib, M.; Wride, P.; Saraiya, M.; et al. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system—Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. Morb. Mortal. Wkly. Rep. 2021, 70, 109. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Accarino, E.; Camprecios, J.M.; Domínguez-Hernández, R.; Rando-Segura, A.; Riveiro-Barciela, M.; Rodríguez-Frías, F.; Barreira-Diaz, A.; Palom, A.; Casado, M.A.; Esteban-Mur, R.; et al. Impact of COVID-19 pandemic in the relink-c strategy to search and retrieve lost-to follow-up hcv patients. Hepatology 2021, 74, 548A. [Google Scholar]
- Cunningham, E.B.; Wheeler, A.; Hajarizadeh, B.; French, C.E.; Roche, R.; Marshall, A.D.; Fontaine, G.; Conway, A.; Valencia, B.M.; Bajis, S.; et al. Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 426–445. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Calzia, A.; Dublineau, A.; Rouet, F.; Nouhin, J.; Yann, S.; Pin, S.; Sun, C.; Sann, K.; Dimanche, C.; et al. Field evaluation of GeneXpert® (Cepheid) HCV performance for RNA quantification in a genotype 1 and 6 predominant patient population in Cambodia. J. Viral Hepat. 2019, 26, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Baudhuin, L.M.; Donato, L.J.; Uphoff, T.S. How novel molecular diagnostic technologies and biomarkers are revolutionizing genetic testing and patient care. Expert Rev. Mol. Diagn. 2012, 12, 25–37. [Google Scholar] [CrossRef]
- Draper, B.; Yee, W.L.; Pedrana, A.; Kyi, K.P.; Qureshi, H.; Htay, H.; Naing, W.; Thompson, A.J.; Hellard, M.; Howell, J. Reducing liver disease-related deaths in the Asia-Pacific: The important role of decentralised and non-specialist led hepatitis C treatment for cirrhotic patients. Lancet Reg. Health West. Pac. 2022, 20, 100359. [Google Scholar] [CrossRef]
- Draper, B.L.; Htay, H.; Pedrana, A.; Yee, W.L.; Howell, J.; Pyone Kyi, K.; Naing, W.; Sanda Aung, K.; Markby, J.; Easterbrook, P.; et al. Outcomes of the CT2 study: A ‘one-stop-shop’for community-based hepatitis C testing and treatment in Yangon, Myanmar. Liver Int. 2021, 41, 2578–2589. [Google Scholar] [CrossRef]
- Hengel, B.; Causer, L.; Matthews, S.; Smith, K.; Andrewartha, K.; Badman, S.; Spaeth, B.; Tangey, A.; Cunningham, P.; Saha, A.; et al. A decentralised point-of-care testing model to address inequities in the COVID-19 response. Lancet Infect. Dis. 2021, 21, e183–e190. [Google Scholar] [CrossRef]
- Opollo, V.S.; Nikuze, A.; Ben-Farhat, J.; Anyango, E.; Humwa, F.; Oyaro, B.; Wanjala, S.; Omwoyo, W.; Majiwa, M.; Akelo, V.; et al. Field evaluation of near point of care Cepheid GeneXpert HIV-1 Qual for early infant diagnosis. PLoS ONE 2018, 13, e0209778. [Google Scholar] [CrossRef]
- Myint, N.P.S.T.; Zaw, T.T.; Sain, K.; Waiyan, S.; Danta, M.; Cooper, D.; Aung, N.M.; Kyi, M.M.; Hanson, J. Sequential Helicobacter pylori eradication therapy in Myanmar; a randomized clinical trial of efficacy and tolerability. J. Gastroenterol. Hepatol. 2020, 35, 617–623. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, E.; Chan, G.; Hudoba, M.; Markin, T.; Yakimec, J.; Roland, K. Malaria screening using front-line loop-mediated isothermal amplification: Fourteen-month experience in a nonendemic regional hub-and-spoke laboratory setting. Am. J. Clin. Pathol. 2021, 155, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, Z.; Mbwambo, J.; Rwegasha, J.; Mgina, N.; Doulla, B.; Mwakale, P.; Tuaillon, E.; Chevaliez, S.; Shimakawa, Y.; Taylor-Robinson, S.D.; et al. In-field evaluation of Xpert® HCV viral load Fingerstick assay in people who inject drugs in Tanzania. Liver Int. 2020, 40, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S.; Gardiner, D.F.; Rodriguez-Torres, M.; Reddy, K.R.; Hassanein, T.; Jacobson, I.; Lawitz, E.; Lok, A.S.; Hinestrosa, F.; Thuluvath, P.J.; et al. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. New Engl. J. Med. 2014, 370, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Tun, K.T.; Mhote, N.P.P.; Htoo, S.N.; Maung, C.; Kyaw, S.W.; Oo, S.E.K.S.; Pocock, N.S. Human resources for health: Task shifting to promote basic health service delivery among internally displaced people in ethnic health program service areas in eastern Burma/Myanmar. Glob. Health Action 2014, 7, 24937. [Google Scholar] [CrossRef] [PubMed]


| Enrolment Characteristics | Total n (%) 194 |
|---|---|
| Age (median, IQR) | 39 (29–47) |
| 18–29 | 52 (27%) |
| 30–44 | 76 (39%) |
| 45–59 | 59 (30%) |
| 60+ | 7 (4%) |
| Gender | |
| Male | 140 (72%) |
| Female | 54 (28%) |
| Education | |
| Did not complete high school | 149 (77%) |
| Completed high school | 45 (23%) |
| Employment (full or part time) | |
| Not employed | 80 (41%) |
| Employed | 114 (59%) |
| Housing | |
| Own house | 101 (52%) |
| Rental house | 37 (19%) |
| Staying with family or friends | 50 (26%) |
| Other | 6 (3%) |
| Ever been in prison | |
| No | 154 (79%) |
| Yes | 40 (21%) |
| Current injecting drug use 1 | |
| No | 165 (85%) |
| Yes | 29 (15%) |
| Current tobacco smoking | |
| No | 114 (59%) |
| Yes | 80 (41%) |
| Hazardous alcohol use 2 | |
| No | 163 (84%) |
| Yes | 31 (16%) |
| CD4 | |
| Not done | 20 (10%) |
| <100 cells/μL | 26 (14%) |
| 100–200 cells/μL | 19 (9%) |
| >200 cells/μL | 129 (67%) |
| APRI score | |
| <2 | 179 (92%) |
| ≥2 | 15 (8%) |
| Antiretroviral therapy (ART) | |
| No | 29 (15%) |
| Yes | 165 (85%) |
| ITT | mITT | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n (%) | aOR | 95%CI | p Value | n (%) | aOR | 95%CI | p Value |
| Age (median, IQR) | 39 (29–49) | 40 (29;49) | ||||||
| 18–29 | 44 (29%) | reference | 37 (29%) | reference | ||||
| 30–44 | 55 (36%) | 0.43 | 0.15–1.26 | 0.12 | 42 (33%) | 0.27 | 0.03–2.42 | 0.24 |
| 45–59 | 49 (32%) | 1.01 | 0.31–3.36 | 0.97 | 43 (34%) | 0.22 | 0.01–2.83 | 0.25 |
| 60+ | 6 (4%) | 2.48 | 0.11–56.87 | 0.57 | 6 (4%) | 0.43 | 0.01–17.09 | 0.65 |
| Gender | ||||||||
| Male | 111 (72%) | reference | 91 (71%) | reference | ||||
| Female | 43 (28%) | 1.43 | 0.48–4.28 | 0.51 | 37 (29%) | 2.26 | 0.33–15.37 | 0.40 |
| Education | ||||||||
| Did not complete high school | 118 (77%) | reference | 97 (76%) | reference | ||||
| Completed high school | 36 (23%) | 1.83 | 0.65–5.17 | 0.25 | 31 (24%) | 1.94 | 0.27–13.70 | 0.50 |
| Employment (full or part time) | ||||||||
| Not employed | 59 (38%) | reference | 46 (36%) | reference | ||||
| Employed | 95 (62%) | 2.79 | 1.15–6.79 | 0.02 | 82 (64%) | 2.08 | 0.39–10.98 | 0.38 |
| Housing | ||||||||
| Own house | 78 (51%) | reference | 63 (49%) | reference | ||||
| Rental house | 32 (21%) | 1.15 | 0.38–3.50 | 0.80 | 26 (20%) | 0.32 | 0.03–3.46 | 0.35 |
| Staying with family or friends | 39 (25%) | 0.94 | 0.34–2.57 | 0.90 | 34 (27%) | 0.40 | 0.01–1.02 | 0.05 |
| Other | 5 (3%) | 6.44 | 0.25–162.92 | 0.25 | 5 (4%) | 0.18 | 0.01–6.00 | 0.34 |
| Ever been in prison | ||||||||
| No | 122 (79%) | reference | 105 (82%) | reference | ||||
| Yes | 32 (21%) | 0.47 | 0.17–1.32 | 0.15 | 23 (18%) | 1.07 | 0.14–7.72 | 0.94 |
| Current injecting drug use 1 | ||||||||
| No | 144 (93%) | reference | 110 (86%) | reference | ||||
| Yes | 10 (7%) | 0.57 | 0.12–2.69 | 0.47 | 18 (14%) | 0.18 | 0.01-3.35 | 0.25 |
| Current tobacco smoking | ||||||||
| No | 86 (56%) | reference | 76 (59%) | reference | ||||
| Yes | 68 (44%) | 0.55 | 0.16–1.31 | 0.14 | 52 (41%) | 6.90 | 0.54–88.07 | 0.13 |
| Hazardous alcohol use 2 | ||||||||
| No | 126 (82%) | reference | 106 (83%) | reference | ||||
| Yes | 28 (18%) | 0.46 | 0.16–1.31 | 0.14 | 22 (17%) | 0.75 | 0.08–6.39 | 0.79 |
| CD4 | ||||||||
| Not performed | 15 (10%) | 1.16 | 0.18–7.34 | 0.87 | 12 (9%) | 1.25 | 0.01–173.90 | 0.92 |
| <100 cells/μL | 17 (11%) | reference | 13 (10%) | reference | ||||
| 100–200 cells/μL | 13 (8%) | 1.44 | 0.19–10.80 | 0.72 | 11 (9%) | 0.61 | 0.01–72.74 | 0.84 |
| >200 cells/μL | 109 (70%) | 1.13 | 0.29–4.34 | 0.85 | 92 (72%) | 0.16 | 0.01–5.83 | 0.32 |
| APRI score | ||||||||
| <2 | 141 (92%) | reference | 121 (95%) | reference | ||||
| ≥2 | 13 (8%) | 0.26 | 0.07–0.88 | 0.03 | 7 (5%) | 5.91 | 0.12–288.71 | 0.37 |
| Antiretroviral therapy (ART) | ||||||||
| None | 22 (14%) | reference | 18 (14%) | reference | ||||
| On ART | 132 (86%) | 1.37 | 0.44–4.21 | 0.58 | 110 (86%) | 7.16 | 1.03–49.50 | 0.04 |
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Summary n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 24 | 26 | 27 | 35 | 36 | 43 | 44 | 49 | 52 | 36 (27–47) 4 |
| Gender | Male | Male | Male | Male | Male | Male | Female | Female | Male | 7/9 (78%) male |
| Education (high school) | No | Yes | No | No | Yes | No | No | No | No | 2/9 (22%) |
| Employment (full or part time) | No | No | Yes | Yes | No | No | Yes | No | Yes | 4/9 (44%) |
| Housing | Rental house | Staying with family or friends | Staying with family or friends | Staying with family or friends | Staying with family or friends | Rental house | Staying with family or friends | Staying with family or friends | Own house | 6/9 (67%) Staying with family or friends |
| Ever been in prison | Yes | No | Yes | Yes | Yes | Yes | No | Yes | No | 6/9 (67%) |
| Current injecting drug use 1 | No | Yes | Yes | No | No | Yes | No | No | No | 3/9 (33%) |
| Current tobacco smoking | No | Yes | Yes | Yes | No | Yes | No | No | No | 4/9 (44%) |
| Hazardous alcohol use 2 | No | Yes | Yes | Yes | Yes | No | No | No | No | 4/9 (44%) |
| ART | No | No | No | No | Yes | No | Yes | Yes | Yes | 4/9 (44%) |
| CD4 (cells/ul) | 216 | 222 | 420 | 276 | 342 | 306 | 221 | 383 | 529 | 306 (222–402) 4 |
| APRI | 1.33 | 0.13 | 0.43 | 0.70 | 0.81 | 0.13 | 0.38 | 0.32 | 0.33 | 0.38 (0.23–0.76) 4 |
| Screening HCV VL 3 | 6.3 | 6.4 | 4.5 | 6.4 | 6.5 | 6.9 | 6.0 | 6.7 | 5.4 | 6.4 (5.8–6.6) 4 |
| SVR12 HCV VL 3 | 4.9 | 3.6 | 2.5 | 6.2 | 5.1 | 6.4 | 5.6 | 6.6 | 5.4 | 5.4 (4.7–6.3) 4 |
| Quantifiable | Unquantifiable * | Total | |
|---|---|---|---|
| Xpert HCV VL Fingerstick Assay (Finger-stick capillary whole blood) | |||
| Detected | 163 | 1 | 164 |
| Undetected * | 1 | 134 | 135 |
| Total | 164 | 135 | 299 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyein, P.P.; Tillakeratne, S.; Phyu, S.; Yee, M.M.; Lwin, M.M.; Htike, K.L.; Aung, M.T.; Grebely, J.; Applegate, T.; Hanson, J.; et al. Evaluation of Simplified HCV Diagnostics in HIV/HCV Co-Infected Patients in Myanmar. Viruses 2023, 15, 521. https://doi.org/10.3390/v15020521
Nyein PP, Tillakeratne S, Phyu S, Yee MM, Lwin MM, Htike KL, Aung MT, Grebely J, Applegate T, Hanson J, et al. Evaluation of Simplified HCV Diagnostics in HIV/HCV Co-Infected Patients in Myanmar. Viruses. 2023; 15(2):521. https://doi.org/10.3390/v15020521
Chicago/Turabian StyleNyein, Phyo Pyae, Shane Tillakeratne, Sabai Phyu, Myint Myint Yee, Mya Mya Lwin, Kyaw Linn Htike, May Thu Aung, Jason Grebely, Tanya Applegate, Josh Hanson, and et al. 2023. "Evaluation of Simplified HCV Diagnostics in HIV/HCV Co-Infected Patients in Myanmar" Viruses 15, no. 2: 521. https://doi.org/10.3390/v15020521
APA StyleNyein, P. P., Tillakeratne, S., Phyu, S., Yee, M. M., Lwin, M. M., Htike, K. L., Aung, M. T., Grebely, J., Applegate, T., Hanson, J., Matthews, G., & Lin, K. S. (2023). Evaluation of Simplified HCV Diagnostics in HIV/HCV Co-Infected Patients in Myanmar. Viruses, 15(2), 521. https://doi.org/10.3390/v15020521






