Experimental Yellow Fever in the Squirrel Monkey (Saimiri spp.): Hematological, Biochemical, and Immunological Findings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus
2.2. Experimental Infection
2.3. Collection of Biological Samples
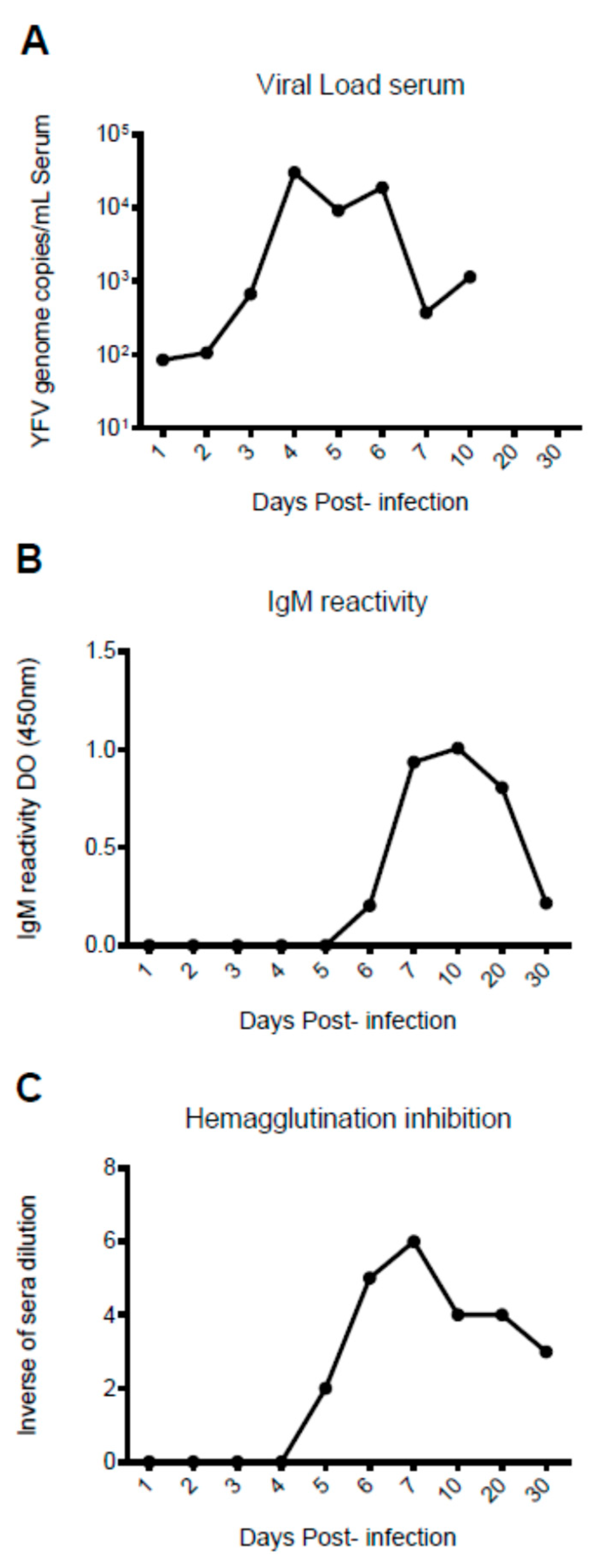
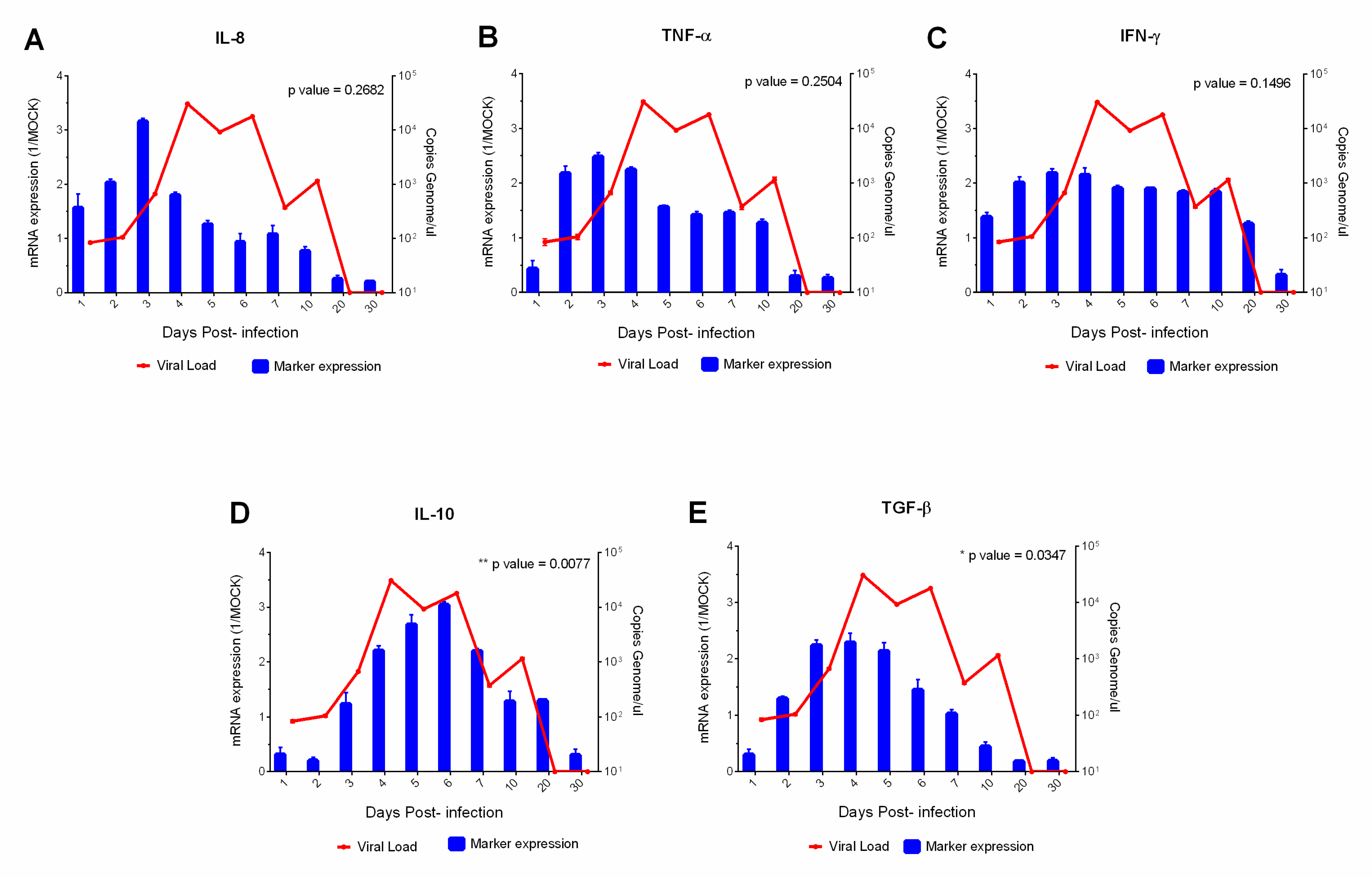
2.4. YFV Viral Load and Cytokines
2.5. Serology
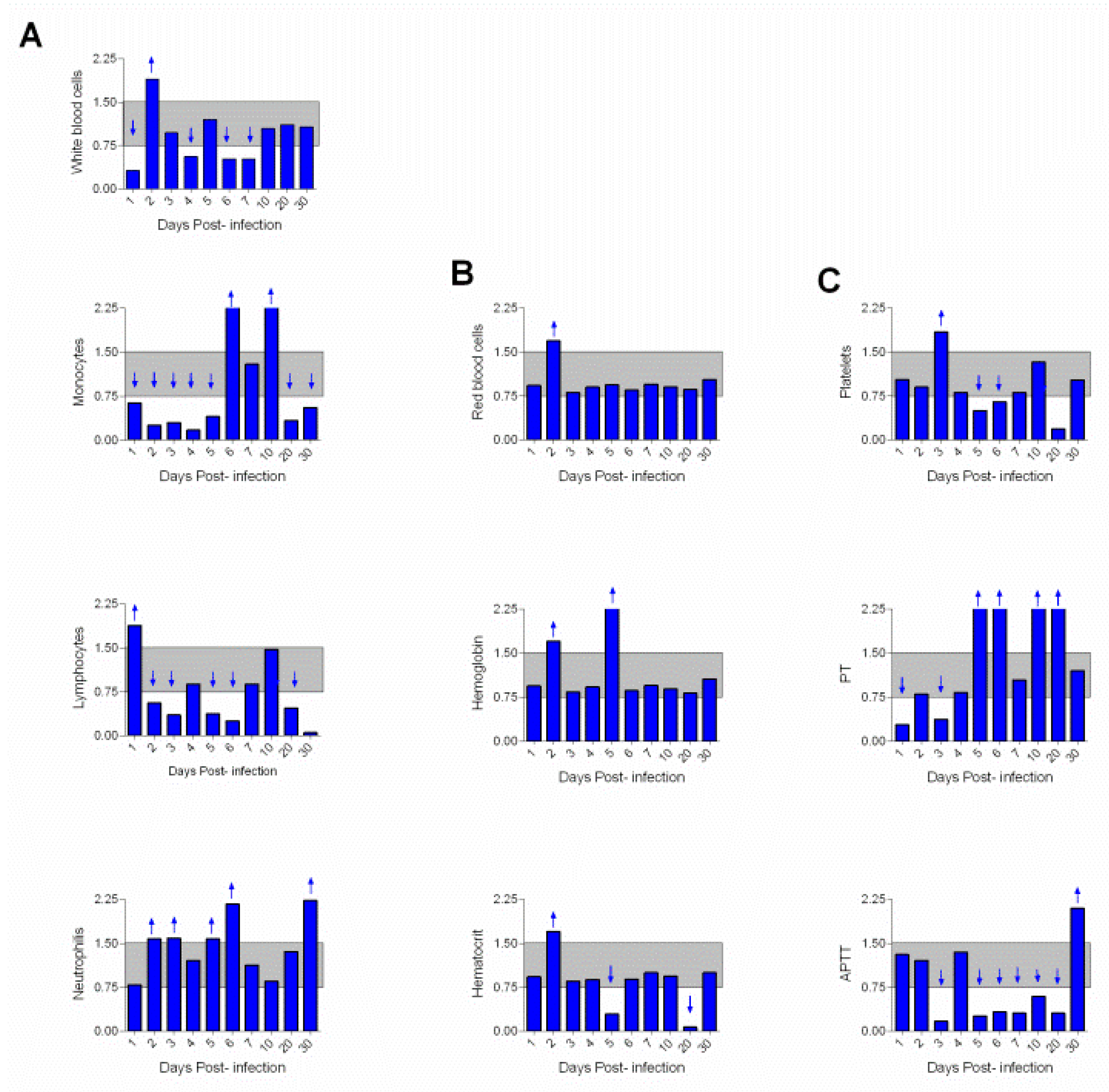
2.6. Hemogram and Coagulogram
2.7. Biochemistry
2.8. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.C.; Jesus, G.J.; Aguiar, R.S.; Iani, F.C.M.; Xavier, J.; Quick, J.; Du Plessis, L.; Dellicour, S.; et al. Genomic and Epidemiological Monitoring of Yellow Fever Virus Transmission Potential. Science 2018, 361, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, B.; Hirsch, A.; Goulart, V.D.L.R.; Passos, L.; Teixeira, C.P.; James, P.; Young, R.J. Good neighbours: Distribution of black-tufted marmoset (Callithrix penicillata) in an urban environment. Wildl. Res. 2015, 42, 579–589. [Google Scholar] [CrossRef]
- Lima, C.D.; Madec, Y.; Bersot, M.I.; Campos, S.S.; Motta, M.A.; Santos, F.B.; Vazeille, M.; Vasconcelos, P.F.D.C.; Lourenço-De-Oliveira, R.; Failloux, A.-B. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017, 7, 4848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igarashi, A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya Viruses. J. Gen. Virol. 1978, 40, 531–544. [Google Scholar] [CrossRef]
- Gubler, D.J.; Kuno, G.; Sather, G.E. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am. J. Trop. Med. Hyg. 1984, 33, 158–165. [Google Scholar] [CrossRef]
- Clarke, D.H.; Casals, J. Techniques for hemagglutination and hemagglutination- inhibition with arthropod-borne viruses. Am. J. Trop. Med. Hyg. 1958, 7, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Shope, R.E. The use of a microhemagglutination-inhibition test to follow antibody response after arthropod-borne virus infection in a community of forest animals. Ann. Microbiol. 1963, 11, 167–171. [Google Scholar]
- Altmann, J. Observacional study of behavior: Sampling Methods. Behavior 1974, 49, 227–267. [Google Scholar] [CrossRef] [Green Version]
- Conselho Nacional de Controle de Experimentação Animal (CONCEA). Resolução Normativa n0 07, de 30 de abril de 2015. Determina o uso de Primatas não -Humanos para uso científico. Diário Oficial da União 17 maio 2012. Available online: http://www.icmbio.gov.br/cepsul/images/stories/legislacao/Instrucao_normativa/2015/in_ibama_07_2015_institui_categorias_uso_manejo_fauna_silvestre_cativeiro.pdf (accessed on 12 April 2018).
- Domingo, C.; Patel, P.; Yillah, J.; Weidmann, M.; Méndez, J.A.; Nakouné, E.R.; Niedrig, M. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J. Clin. Microbiol. 2012, 50, 4054–4060. [Google Scholar] [CrossRef] [Green Version]
- Rozen, S.; Skaletsky, H.J. Primer3 on the WWW for general users and for biologist programmers. In Bioinformatics Methods and Protocols: Methods in Molecular Biology SK, 1st ed.; Misener, S., Ed.; Humana Press: Totowa, NJ, USA, 2000; pp. 365–386. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Martin, D.A.; Muth, D.A.; Brown, T.; Johnson, A.J.; Karabatsos, N.; Roehrig, J.T. Standardization of Immunoglobulin M Capture Enzyme-Linked Immunosorbent Assays for Routine Diagnosis of Arboviral Infections. J. Clin. Microbiol. 2000, 38, 1823–1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, M.S.; Castro, P.H.; Silva, G.A.; Casseb, S.M.; Dias, A.G., Jr.; Rodrigues, S.G.; da Silva Azevedo, R.D.S.; Silva, M.F.C.E.; Zauli, D.A.G.; Araújo, M.S.S.; et al. Callithrix penicillata: A feasible experimental model for dengue virus infection. Immunol. Lett. 2014, 158, 126–133. [Google Scholar] [CrossRef]
- Stokes, A.; Bauer, J.H.; Hudson, N.P. Transmission of yellow fever to Macacus rhesus, preliminary note. JAMA 1928, 90, 253–254. [Google Scholar] [CrossRef]
- Davis, N.C.; Shannon, R.C. Studies on South American yellow fever; transmission of virus Brazilian monkey; preliminary observations. J. Exp. Med. 1929, 50, 81–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, N.C. Susceptibility of capuchin (Cebus) monkey to yellow fever virus. Am. J. Med. Hyg. 1930, 11, 321–334. [Google Scholar] [CrossRef]
- Monath, T.P.; Barret, A.D. Pathogenesis and pathophysiology of yellow fever. Adv. Virus. Res. 2003, 60, 343–395. [Google Scholar] [PubMed]
- Ferreira, M.S.; Júnior, P.S.B.; Cerqueira, V.D.; Rivero, G.R.C.; Júnior, C.A.O.; Castro, P.H.G.; Silva, G.A.D.; Silva, W.B.D.; Imbeloni, A.A.; Sousa, J.R.; et al. Experimental yellow fever virus infection in the squirrel monkey (Saimiri spp.) I: Gross anatomical and histopathological findings in organs at necropsy. Mem. Inst. Oswaldo. Cruz. 2020, 115, e190501. [Google Scholar] [CrossRef]
- Monath, T.P.; Brinker, K.R.; Chandler, F.W.; Kemp, G.E.; Cropp, C.B. Pathophysiologic correlations in a rhesus monkey model of yellow fever: With special observations on the acute necrosis of B cell areas of lymphoid tissues. Am. J. Trop. Med. Hyg. 1981, 30, 431–443. [Google Scholar] [CrossRef]
- Engelmann, F.; Josset, L.; Girke, T.; Park, B.; Barron, A.; Dewane, J.; Hammarlund, E.; Lewis, A.; Axthelm, M.K.; Slifka, M.K.; et al. Pathophysiologic and Transcriptomic Analyses of Viscerotropic Yellow Fever in a rhesus Macaque Model. PLoS Negl. Trop. Dis. 2014, 8, e3295. [Google Scholar] [CrossRef] [Green Version]
- Gubler, D.J.; Sather, G. Laboratory diagnosis of dengue and dengue hemorrhagic fever. In Proceedings of the International Symposium on Yellow Fever and Dengue, Rio de Janeiro, Brasil; 1988; pp. 291–322. [Google Scholar]
- Almeida, M.A.B.; Cardoso, J.C.; Santos, E.; Fonseca, D.F.; Cruz, L.L.; Faraco, F.J.C.; Bercini, M.A.; Vettorello, K.C.; Porto, M.A.; Mohrdieck, R.; et al. Surveillance for Yellow Fever Virus in Non-Human Primates in Southern Brazil, 2001–2011: A Tool for Prioritizing Human Populations for Vaccination. PLoS Negl. Trop. Dis. 2014, 8, e2741. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, P.F.C. Febre amarela: Reflexões sobre a doença, as perspectivas para o século XXI e o risco da reurbanização. Rev. Bras. Epidemiol. 2002, 5, 244–258. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, P.F.C. Febre amarela. Ver. Soc. Bras. Med. Trop. 2003, 36, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Tuboi, S.H.; Costa, Z.G.A.; Vasconcelos, P.F.C.; Hatch, D. Clinical and epidemiological characteristics of yellow fever in Brazil: Analysis of reported cases 1998–2002. Trans. R Soc. Trop. Med. Hyg. 2007, 101, 169–175. [Google Scholar] [CrossRef]
- Monath, T.P. Yellow fever: An update. Lancet Infect. Dis. 2001, 1, 11–20. [Google Scholar] [CrossRef]
- Simmons, C.D.; Farrar, J.J.; Nguyen, V.V.; Wills, B. DM Dengue. N. Engl. J. Med. 2012, 15, 135–147. [Google Scholar]
- Quaresma, J.A.C.; Barros, V.L.R.S.; Pagliari, C.; Fernandes, E.R.; Guedes, F.; Takakura, C.F.H.; Andrade, H.F., Jr.; Vasconcelos, P.F.C.; Duarte, M.I.S. Revisiting the liver in human yellow fevr: Virus-induced apoptosis in hepatocytes associated with TGFβ, TNFα, and NK cells activity. Virology 2006, 345, 22–30. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.S.; Martins, L.C.; de Melo, K.F.L.; da Silva, W.B.; Imbeloni, A.A.; Muniz, J.A.P.C.; de Oliveira, C.F.; Freitas, M.N.O.; dos Santos, É.B.; Chagas, L.L.; et al. Experimental Yellow Fever in the Squirrel Monkey (Saimiri spp.): Hematological, Biochemical, and Immunological Findings. Viruses 2023, 15, 613. https://doi.org/10.3390/v15030613
Ferreira MS, Martins LC, de Melo KFL, da Silva WB, Imbeloni AA, Muniz JAPC, de Oliveira CF, Freitas MNO, dos Santos ÉB, Chagas LL, et al. Experimental Yellow Fever in the Squirrel Monkey (Saimiri spp.): Hematological, Biochemical, and Immunological Findings. Viruses. 2023; 15(3):613. https://doi.org/10.3390/v15030613
Chicago/Turabian StyleFerreira, Milene S., Lívia C. Martins, Karla F. L. de Melo, Wellington B. da Silva, Aline A. Imbeloni, José Augusto P. C. Muniz, Camille F. de Oliveira, Maria Nazaré O. Freitas, Éder B. dos Santos, Liliane L. Chagas, and et al. 2023. "Experimental Yellow Fever in the Squirrel Monkey (Saimiri spp.): Hematological, Biochemical, and Immunological Findings" Viruses 15, no. 3: 613. https://doi.org/10.3390/v15030613





