Bacteriolytic Potential of Enterococcus Phage iF6 Isolated from “Sextaphag®” Therapeutic Phage Cocktail and Properties of Its Endolysins, Gp82 and Gp84
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Phage Isolation, Purification and Propagation
2.3. Transmission Electron Microscopy
2.4. Host Range
2.5. Thermal and pH Stability of Bacteriophage
2.6. Killing Assay and the Effect of Ca2+ and Mg2+
2.7. Phage Adsorption Assay
2.8. One-Step Growth Curve
2.9. Genome Sequencing, Assembly and Sequence Analysis
2.10. Comparative Genomics
2.11. Determination of DNA Packaging Strategy
2.12. Cloning of Endolysin Genes and Enzyme Purification
2.13. Endolysin Activity Turbidimetry Assays
2.14. Endolysins Minimal Active Concentration
2.15. pH Optimum for Bacteriolytic Activity and Thermal Stability of Endolysins
2.16. Lytic Activity Spectrum of Endolysins
2.17. Statistical Analysis
2.18. Accession Number
3. Results
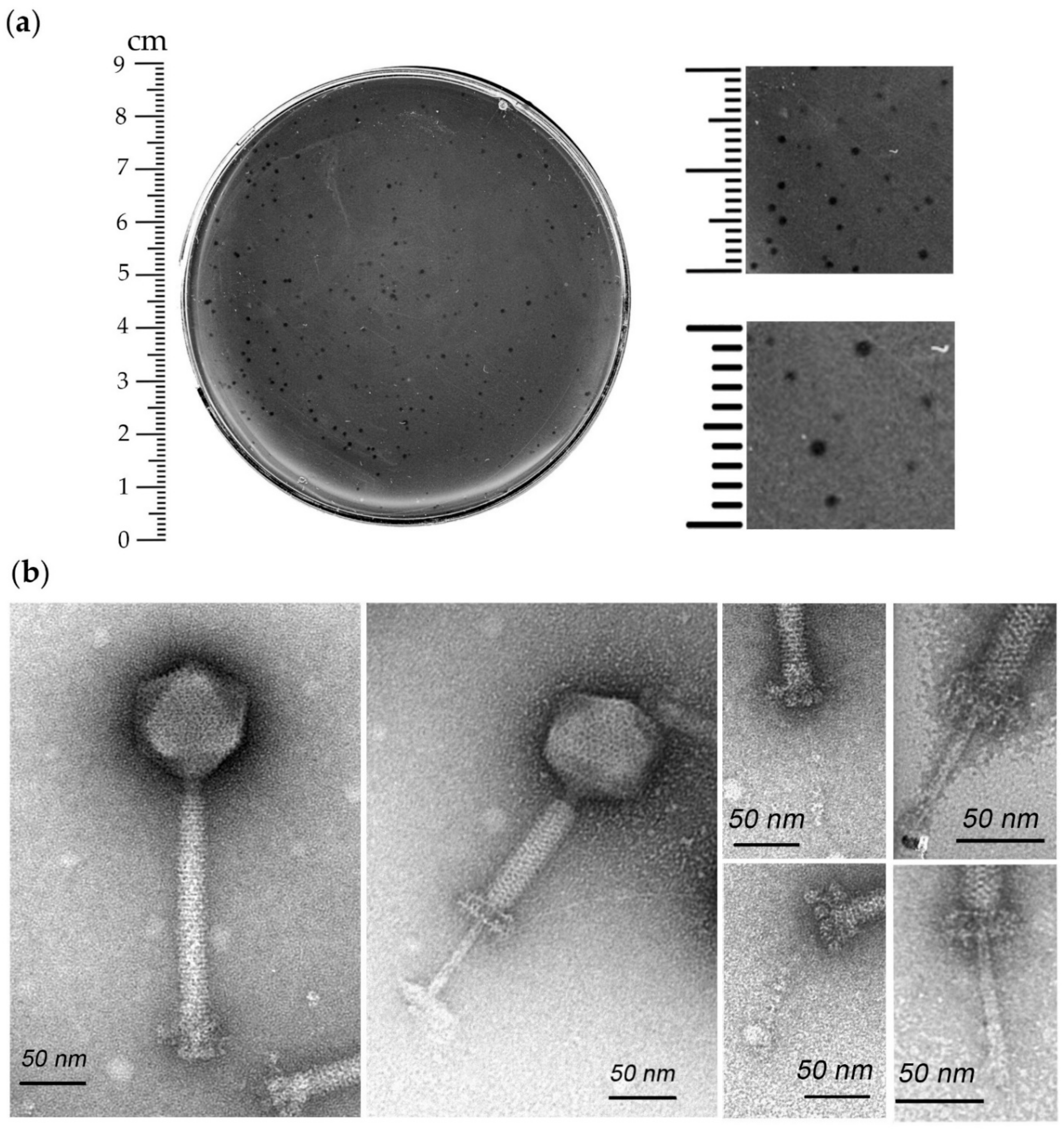
3.1. Isolation, Host Range and Morphological Characterization of iF6
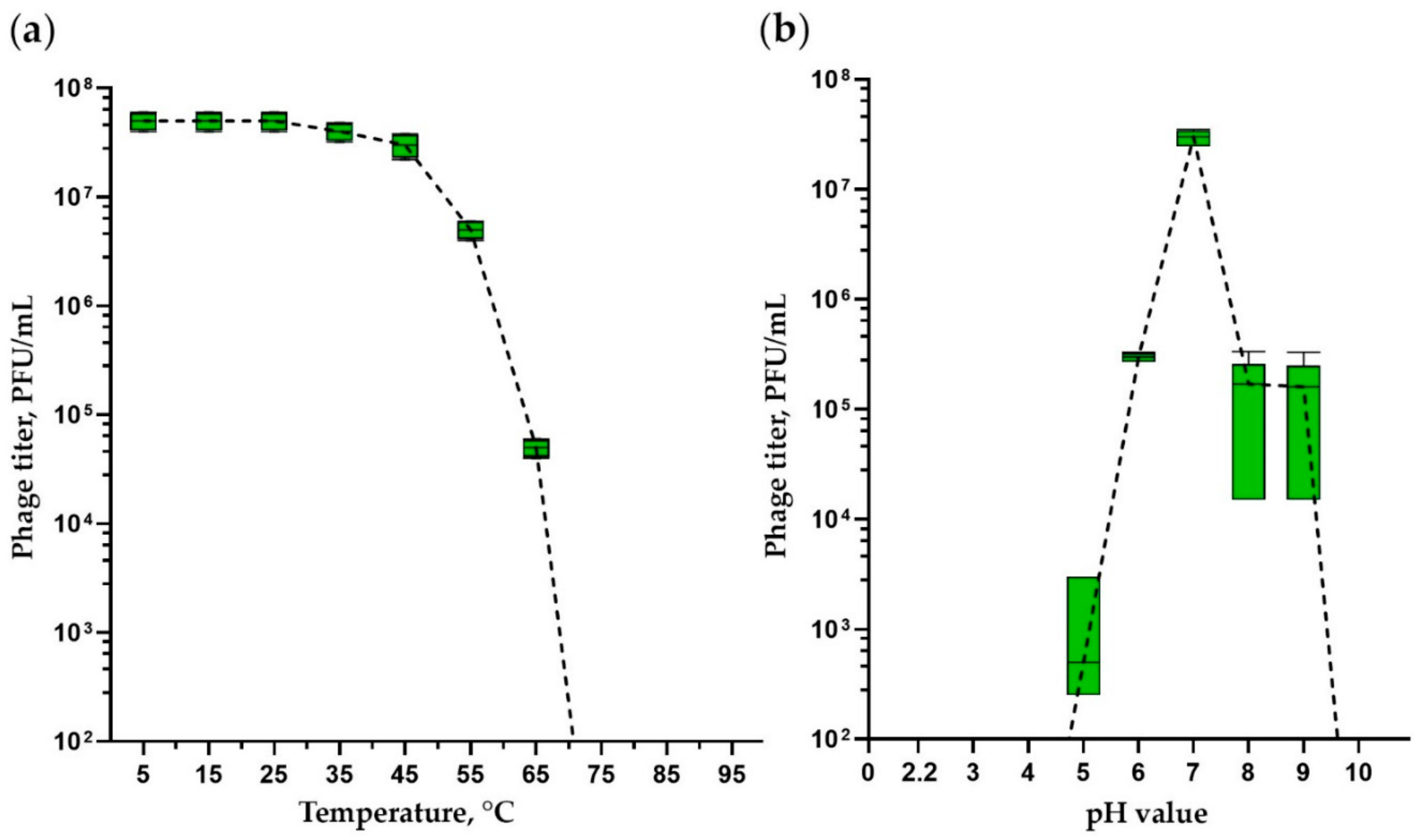
3.2. Thermal and pH Stability Tests
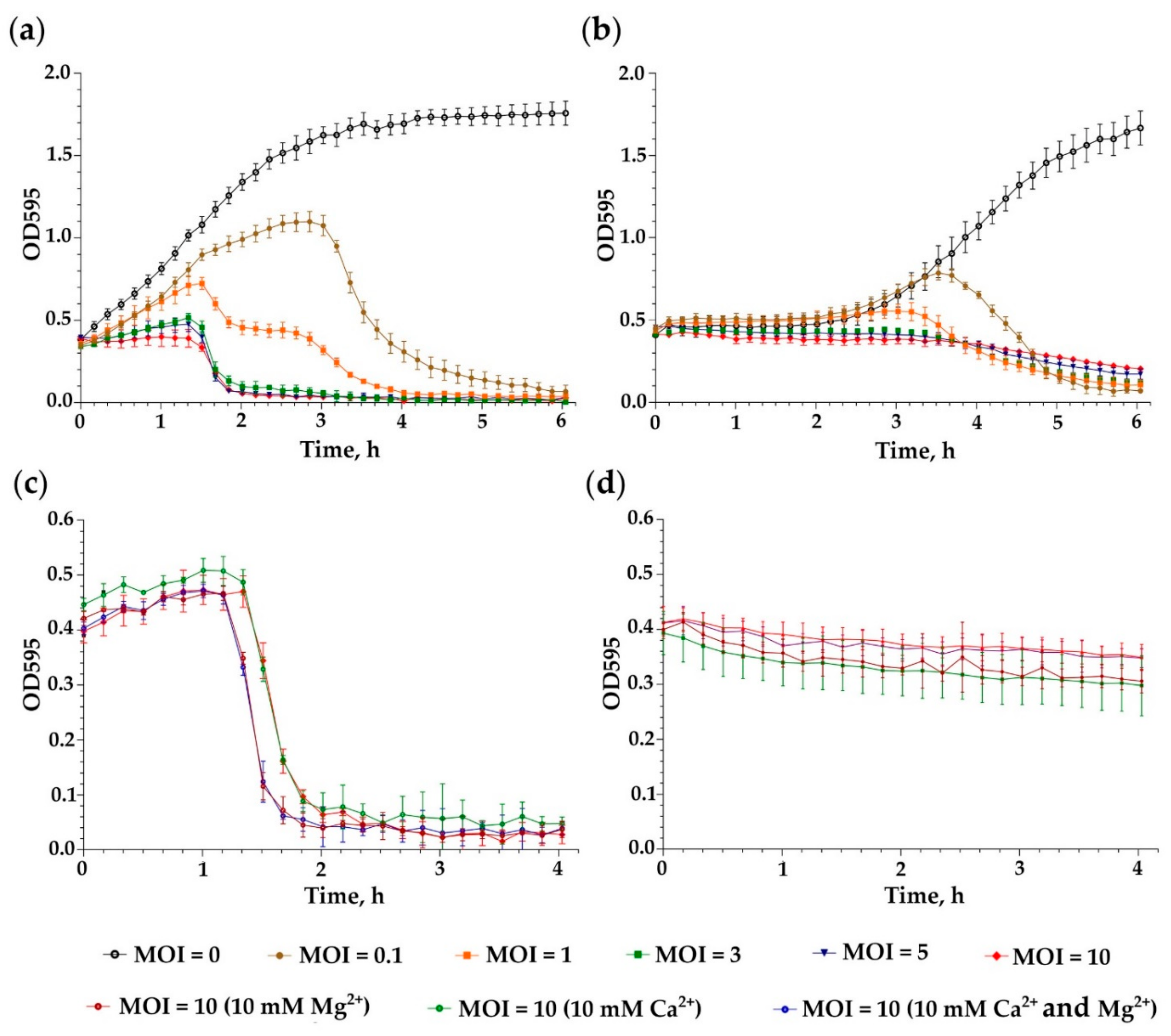
3.3. Killing Assay
3.4. Adsorption Assay and One-Step Growth Curve
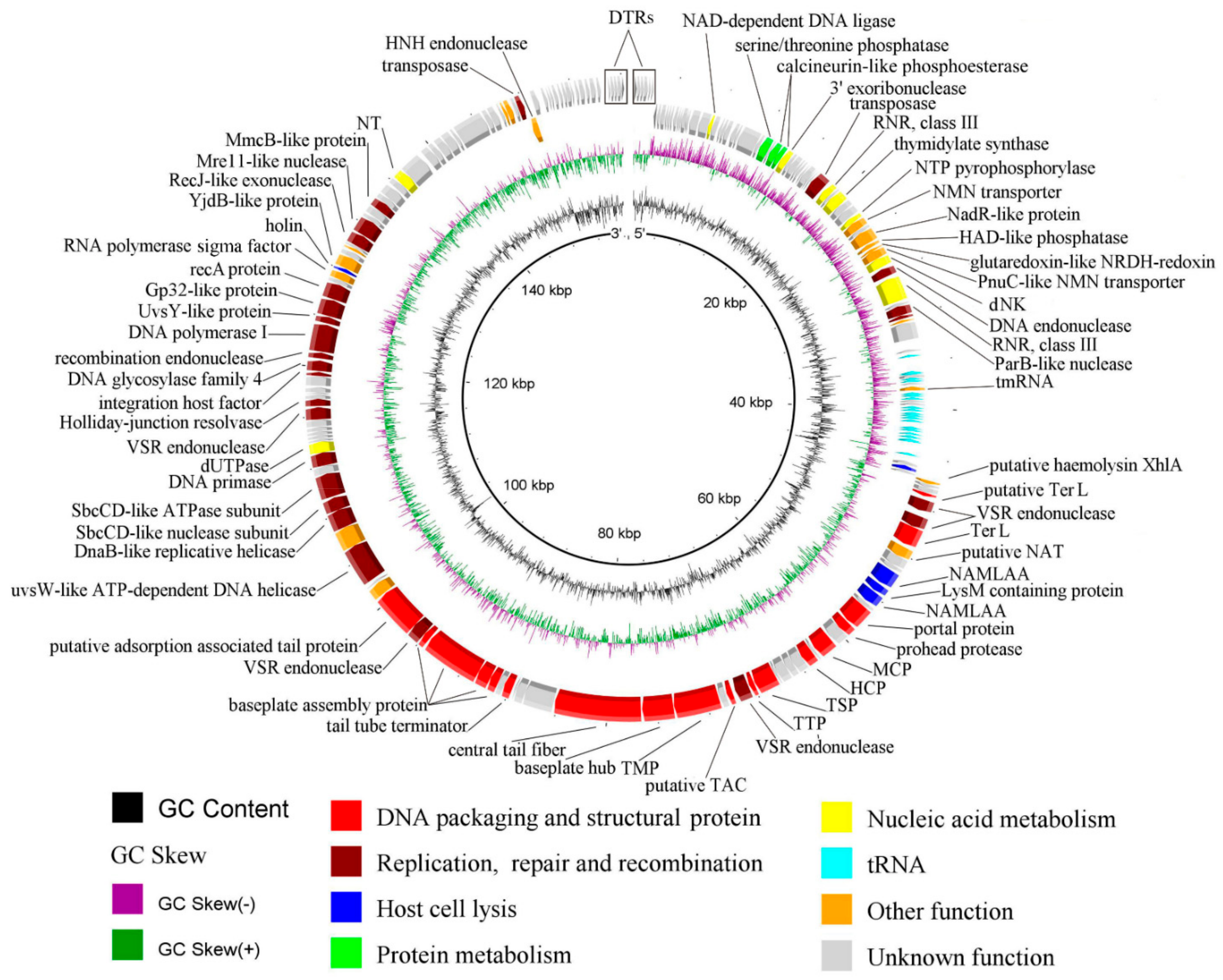
3.5. General Genome Organization of iF6
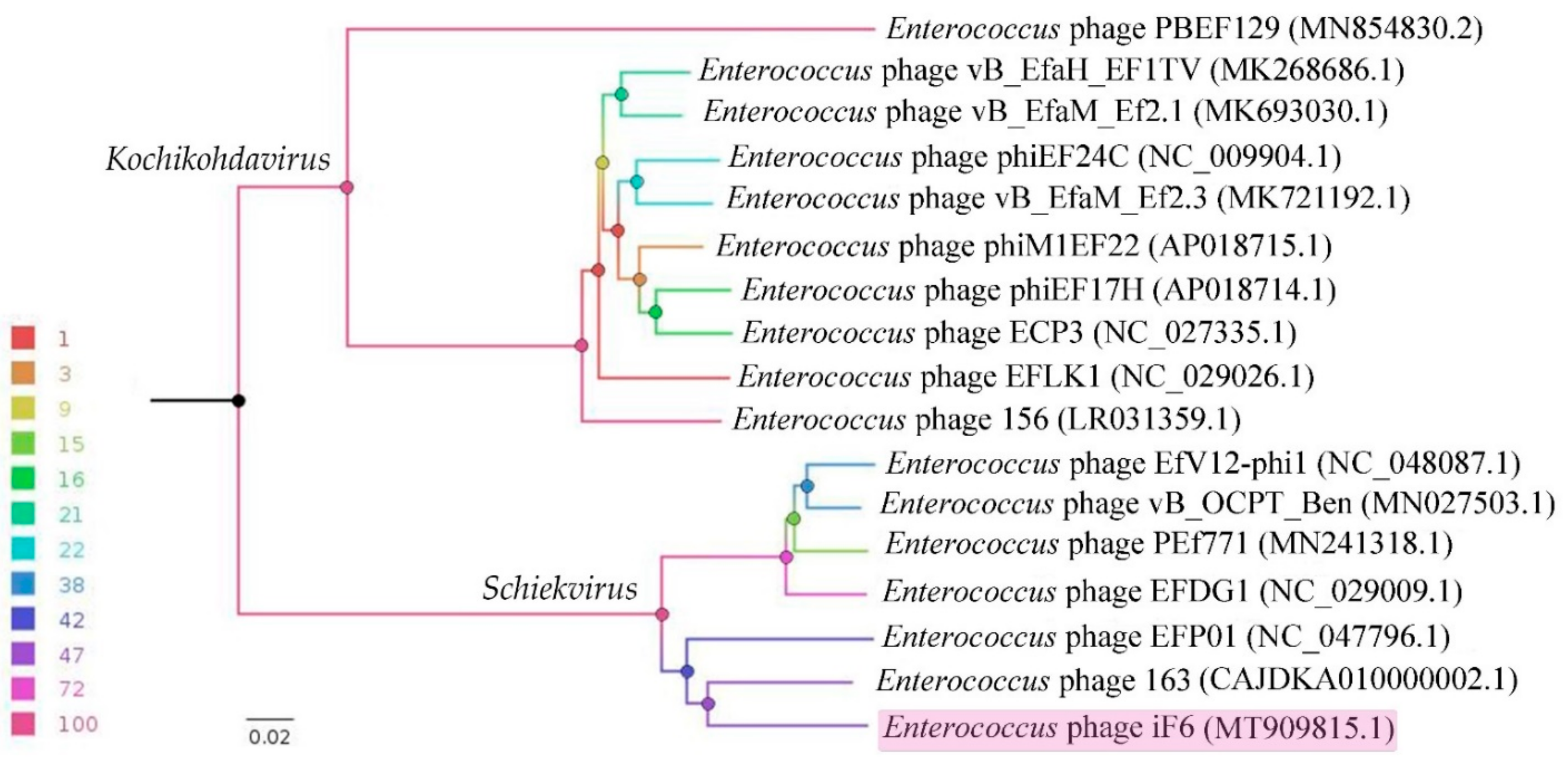
3.6. Comparative Genomics
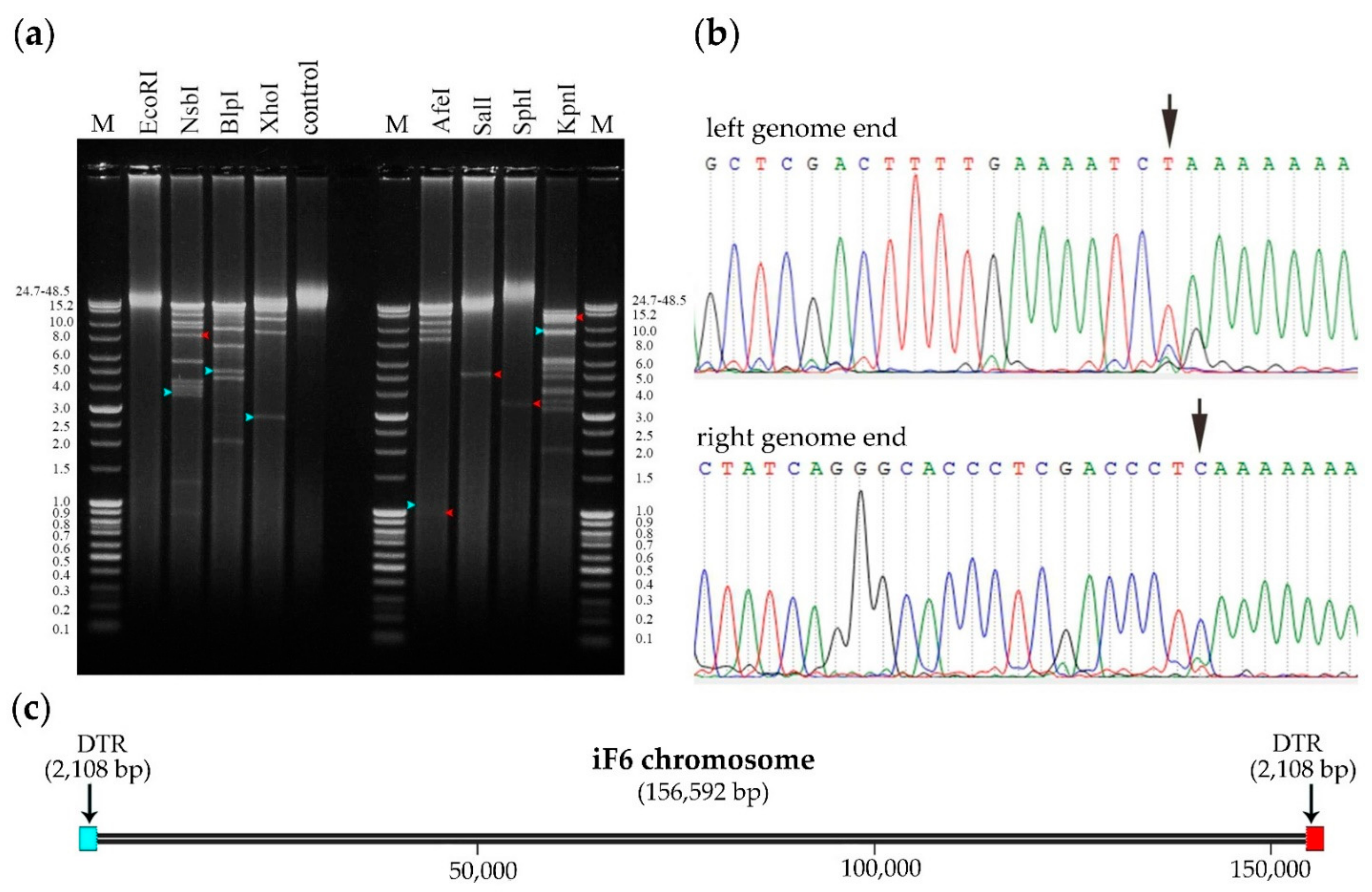
3.7. Determination of Packaging Strategy
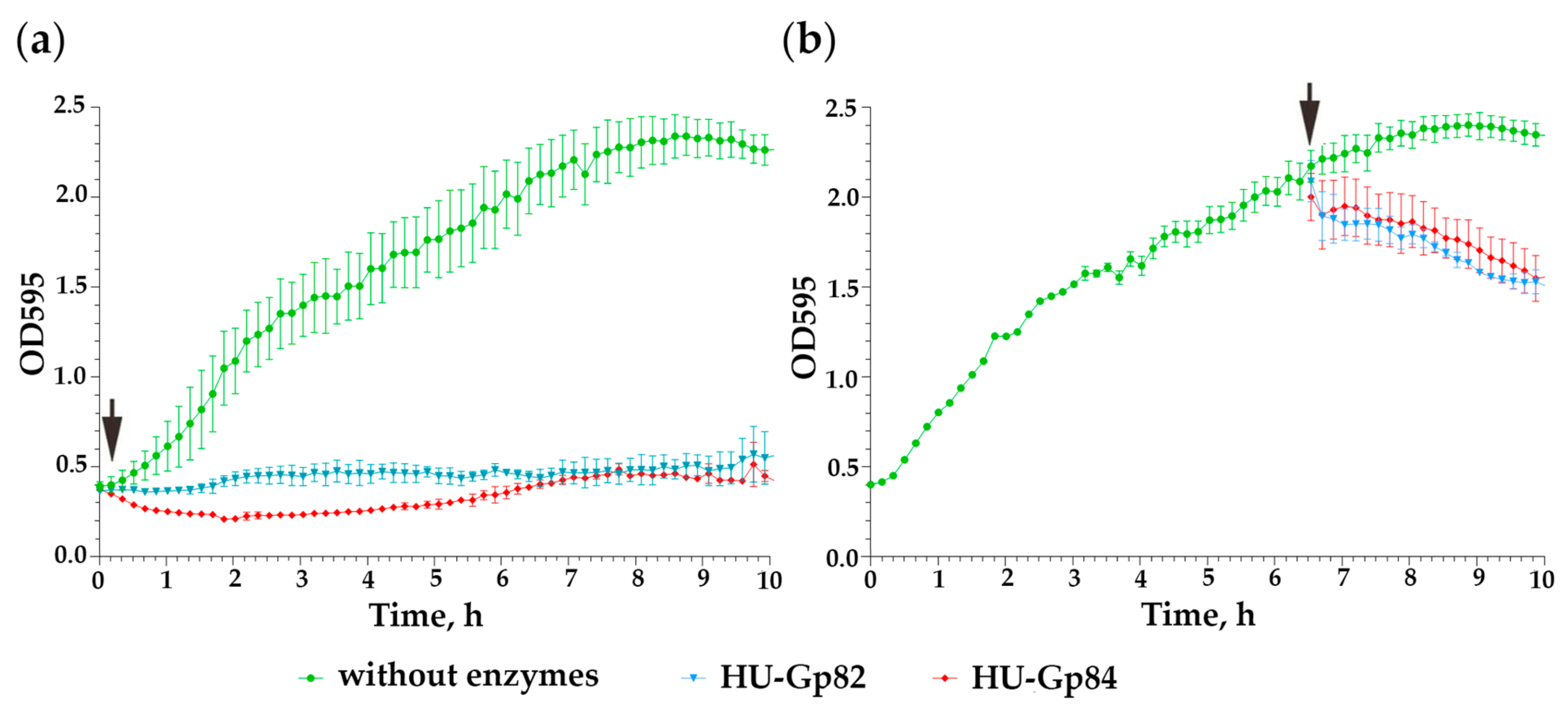
3.8. Cloning and Characterization of Recombinant Endolysins
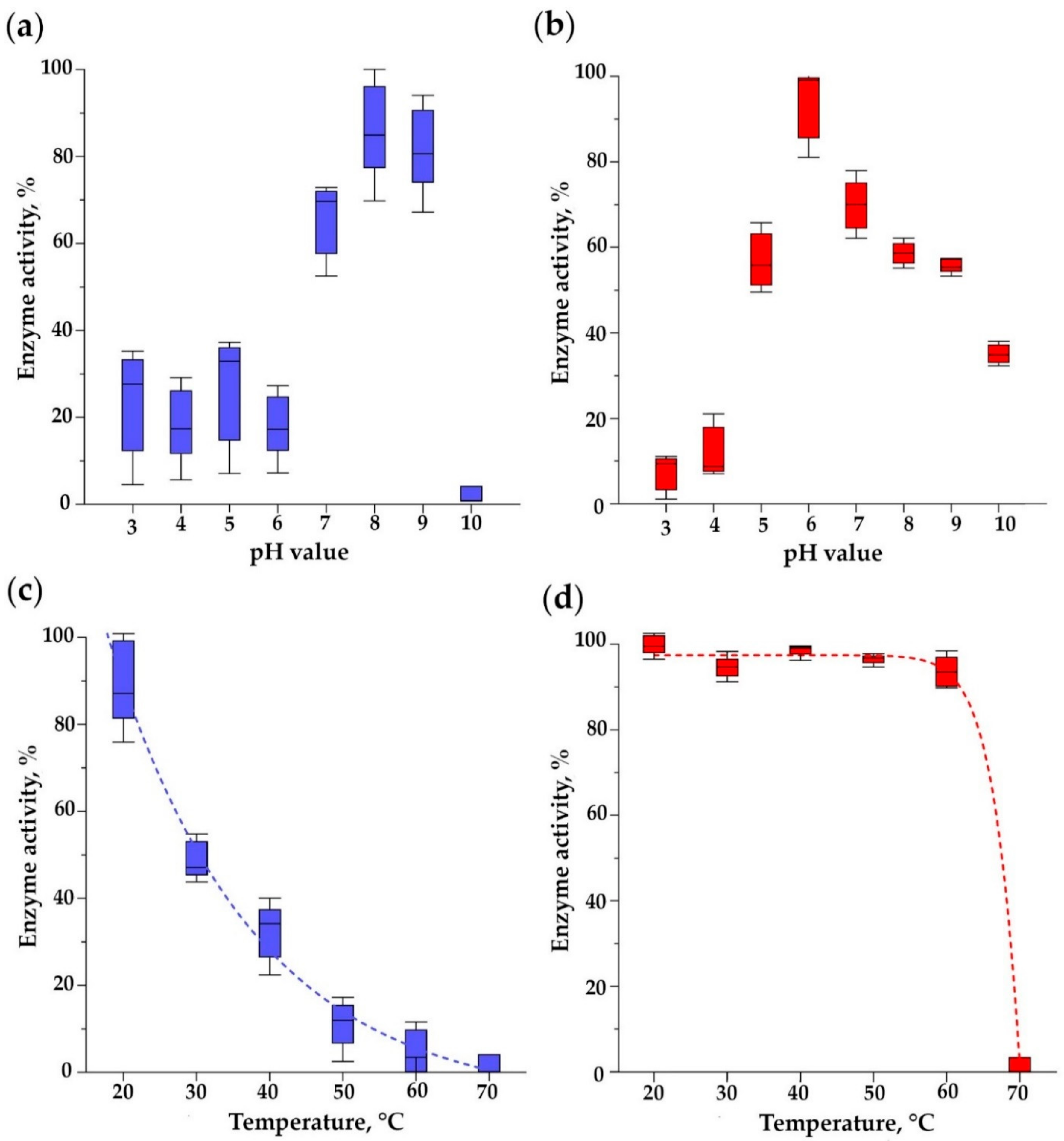
3.9. pH Optimum of Bacteriolytic Activity and Thermal Stability of Endolysins
3.10. Spectrum of Lytic Activity of Endolysins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Geneva: World Health Organization 2020 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021.
- Bittencourt De Marques, E.B.; Suzart, S. Occurrence of Virulence-Associated Genes in Clinical Enterococcus Faecalis Strains Isolated in Londrina, Brazil. J. Med. Microbiol. 2004, 53, 1069–1073. [Google Scholar] [CrossRef] [Green Version]
- Maiti, T.K.; Nagarathna, S.; Kumari, H.B.V.; Shukla, D.P. A Series of Enterococcal Brain Abscesses. J. Neurosci. Rural. Pract. 2015, 6, 434–437. [Google Scholar] [CrossRef] [Green Version]
- Sood, S.; Malhotra, M.; Das, B.K.; Kapil, A. Enterococcal Infections & Antimicrobial Resistance. Indian J. Med. Res. 2008, 128, 111–121. [Google Scholar]
- Pasero, D.; Cossu, A.P.; Terragni, P. Multi-Drug Resistance Bacterial Infections in Critically Ill Patients Admitted with COVID-19. Microorganisms 2021, 9, 1773. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, D.R.; Labate, L.; Tutino, S.; Baldi, F.; Russo, C.; Robba, C.; Ball, L.; Dettori, S.; Marchese, A.; Dentone, C.; et al. Enterococcal Bloodstream Infections in Critically Ill Patients with COVID-19: A Case Series. Ann. Med. 2021, 53, 1779–1786. [Google Scholar] [CrossRef] [PubMed]
- Amarsy, R.; Trystram, D.; Cambau, E.; Monteil, C.; Fournier, S.; Oliary, J.; Junot, H.; Sabatier, P.; Porcher, R.; Robert, J.; et al. Surging Bloodstream Infections and Antimicrobial Resistance during the First Wave of COVID–19: A Study in a Large Multihospital Institution in the Paris Region. Int. J. Infect. Dis. 2022, 114, 90–96. [Google Scholar] [CrossRef]
- Tannhäuser, R.; Nickel, O.; Lindner, M.; Bethge, A.; Wolf, J.; Borte, S.; Lübbert, C. Bacterial Contamination of the Smartphones of Healthcare Workers in a German Tertiary-Care Hospital before and during the COVID-19 Pandemic. Am. J. Infect. Control 2021, 50, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Suttle, C.A. Marine Viruses—Major Players in the Global Ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Fischetti, V.A. Development of Phage Lysins as Novel Therapeutics: A Historical Perspective. Viruses 2018, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of Bacteriophages by Double Agar Overlay Plaque Assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar] [CrossRef]
- Buzikov, R.M.; Piligrimova, E.G.; Shadrin, A.M. Complete Genome Sequence of Enterococcus Faecium FS86, Used for Propagation of Bacteriophages with Therapeutic Potential. Microbiol. Resour. Announc. 2020, 9, e00776-20. [Google Scholar] [CrossRef]
- Ferreira, T.A.; Rasband, W. The ImageJ User Guide Version 1.45. Image Process. Anal. Java. 2011. Available online: https://imagej.nih.gov/ij/docs/user-guide-USbooklet.pdf (accessed on 1 June 2021).
- Piligrimova, E.G.; Kazantseva, O.A.; Nikulin, N.A.; Shadrin, A.M. Bacillus Phage VB_BtS_b83 Previously Designated as a Plasmid May Represent a New Siphoviridae Genus. Viruses 2019, 11, 624. [Google Scholar] [CrossRef] [Green Version]
- Swift, M.L. GraphPad Prism, Data Analysis, and Scientific Graphing. J. Chem. Inf. Comput. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Miller, J.M. GraphPad PRISM. Analysis 2003, 52. Available online: https://cdn.graphpad.com/faq/2/file/Prism_v4_Examples.pdf (accessed on 1 June 2021).
- Kazantseva, O.A.; Piligrimova, E.G.; Shadrin, A.M. VB_BcM_Sam46 and VB_BcM_Sam112, Members of a New Bacteriophage Genus with Unusual Small Terminase Structure. Sci. Rep. 2021, 11, 12173. [Google Scholar] [CrossRef]
- Hyman, P.; Abedon, S.T. Practical Methods for Determining Phage Growth Parameters. Methods Mol. Biol. 2009, 501, 175–202. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Bushnell, B. BBMap Short-Read Aligner, and Other Bioinformatics Tools. Available online: https://Sourceforge.Net/Projects/Bbmap/ (accessed on 1 November 2020).
- Andrews, S. FastQC—A Quality Control Tool for High Throughput Sequence Data. Babraham Bioinformatics. 2010. Available online: http://www.Bioinformatics.Babraham.Ac.Uk/Projects/Fastqc/ (accessed on 1 November 2020).
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A Modular and Extensible Implementation of the RAST Algorithm for Building Custom Annotation Pipelines and Annotating Batches of Genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred Interactive Server for Protein Homology Detection and Structure Prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laslett, D.; Canback, B. ARAGORN, a Program to Detect TRNA Genes and TmRNA Genes in Nucleotide Sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.F.; Petty, N.K.; ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A Genome Comparison Visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras-Moreira, B.; Vinuesa, P. GET_HOMOLOGUES, a Versatile Software Package for Scalable and Robust Microbial Pangenome Analysis. Appl. Environ. Microbiol. 2013, 79, 7696–7701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, D.M.; Kannan, L.; Coleman, M.K.; Wolf, Y.I.; Sorokin, A.; Koonin, E.V.; Mushegian, A. A Low-Polynomial Algorithm for Assembling Clusters of Orthologous Groups from Intergenomic Symmetric Best Matches. Bioinformatics 2010, 26, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M. VICTOR: Genome-Based Phylogeny and Classification of Prokaryotic Viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. FigTree, a Graphical Viewer of Phylogenetic Trees; Institute of Evolutionary Biology University of Edinburgh: Edinburgh, UK, 2009. [Google Scholar]
- Casjens, S.R.; Gilcrease, E.B. Determining DNA Packaging Strategy by Analysis of the Termini of the Chromosomes in Tailed-Bacteriophage Virions. Methods Mol. Biol. 2009, 502, 91–111. [Google Scholar] [CrossRef] [Green Version]
- Cormack, R.S.; Somssich, I.E. Rapid Amplification of Genomic Ends (RAGE) as a Simple Method to Clone Flanking Genomic DNA. Gene 1997, 194, 273–276. [Google Scholar] [CrossRef]
- Skorynina, A.V.; Piligrimova, E.G.; Kazantseva, O.A.; Kulyabin, V.A.; Baicher, S.D.; Ryabova, N.A.; Shadrin, A.M. Bacillus-Infecting Bacteriophage Izhevsk Harbors Thermostable Endolysin with Broad Range Specificity. PLoS ONE 2020, 15, e0242657. [Google Scholar] [CrossRef]
- Dokland, T. Scaffolding Proteins and Their Role in Viral Assembly. Cell. Mol. Life Sci. 1999, 56, 580–603. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, J.; Rashel, M.; Maeda, Y.; Takemura, I.; Sugihara, S.; Akechi, K.; Muraoka, A.; Wakiguchi, H.; Matsuzaki, S. Isolation and Characterization of a Novel Enterococcus Faecalis Bacteriophage ΦEF24C as a Therapeutic Candidate. FEMS Microbiol. Lett. 2008, 278, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, L.; Gelman, D.; Shlezinger, M.; Dessal, A.L.; Coppenhagen-Glazer, S.; Beyth, N.; Hazan, R. Defeating Antibiotic- and Phage-Resistant Enterococcus Faecalis Using a Phage Cocktail in Vitro and in a Clot Model. Front. Microbiol. 2018, 9, 326. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, T.; Ishida, W.; Nasukawa, T.; Ujihara, T.; Nakajima, I.; Suzuki, T.; Uchiyama, J.; Todokoro, D.; Daibata, M.; Fukushima, A.; et al. In Vitro and in Vivo Evaluation of Three Newly Isolated Bacteriophage Candidates, Phief7h, Phief14h1, Phief19g, for Treatment of Enterococcus Faecalis Endophthalmitis. Microorganisms 2021, 9, 212. [Google Scholar] [CrossRef] [PubMed]
- Proença, D.; Fernandes, S.; Leandro, C.; Silva, F.A.; Santos, S.; Lopes, F.; Mato, R.; Cavaco-Silva, P.; Pimentel, M.; São-José, C. Phage Endolysins with Broad Antimicrobial Activity against Enterococcus Faecalis Clinical Strains. Microb. Drug Resist. 2012, 18, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pradal, I.; Casado, A.; del Rio, B.; Rodriguez-Lucas, C.; Fernandez, M.; Alvarez, M.A.; Ladero, V. Enterococcus Faecium Bacteriophage VB_EfaH_163, a New Member of the Herelleviridae Family, Reduces the Mortality Associated with an E. Faecium VanR Clinical Isolate in a Galleria Mellonella Animal Model. Viruses 2023, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Tkachev, P.V.; Pchelin, I.M.; Azarov, D.V.; Gorshkov, A.N.; Shamova, O.V.; Dmitriev, A.V.; Goncharov, A.E. Two Novel Lytic Bacteriophages Infecting Enterococcus Spp. Are Promising Candidates for Targeted Antibacterial Therapy. Viruses 2022, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, A.W.; Collins, L.J.; Ackermann, H.W. A Study of Five Bacteriophages of the Myoviridae Family Which Replicate on Different Gram-Positive Bacteria. Arch. Virol. 1993, 133, 75–84. [Google Scholar] [CrossRef]
- Uchiyama, J.; Takemura, I.; Satoh, M.; Kato, S.I.; Ujihara, T.; Akechi, K.; Matsuzaki, S.; Daibata, M. Improved Adsorption of an Enterococcus Faecalis Bacteriophage ΦEF24C with a Spontaneous Point Mutation. PLoS ONE 2011, 6, e26648. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, M.S.; Clewell, D.B.; Ike, Y.; Shankar, N. Enterococci: From Commensals to Leading Causes of Drug. Mass. Eye Ear Infirm. 2014, 674, 5–63. [Google Scholar]
- D’Andrea, M.M.; Frezza, D.; Romano, E.; Marmo, P.; Henrici De Angelis, L.; Perini, N.; Thaller, M.C.; di Lallo, G. The Lytic Bacteriophage VB_EfaH_EF1TV, a New Member of the Herelleviridae Family, Disrupts Biofilm Produced by Enterococcus Faecalis Clinical Strains. J. Glob. Antimicrob. Resist. 2019, 21, 68–75. [Google Scholar] [CrossRef]
- Khalifa, L.; Brosh, Y.; Gelman, D.; Coppenhagen-Glazer, S.; Beyth, S.; Poradosu-Cohen, R.; Que, Y.A.; Beyth, N.; Hazan, R. Targeting Enterococcus Faecalis Biofilms with Phage Therapy. Appl. Environ. Microbiol. 2015, 81, 2696–2705. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, J.; Rashel, M.; Takemura, I.; Wakiguchi, H.; Matsuzaki, S. In Silico and in Vivo Evaluation of Bacteriophage ΦEF24C, a Candidate for Treatment of Enterococcus Faecalis Infections. Appl. Environ. Microbiol. 2008, 74, 4149–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlezinger, M.; Friedman, M.; Houri-Haddad, Y.; Hazan, R.; Beyth, N. Phages in a Thermoreversible Sustainedrelease Formulation Targeting E. Faecalis in Vitro and in Vivo. PLoS ONE 2019, 14, e0219599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, J.; Takemura, I.; Hayashi, I.; Matsuzaki, S.; Satoh, M.; Ujihara, T.; Murakami, M.; Imajoh, M.; Sugai, M.; Daibata, M. Characterization of lytic enzyme open reading frame 9 (ORF9) derived from Enterococcus faecalis bacteriophage phiEF24C. Appl. Environ. Microbiol. 2011, 77, 580–585. [Google Scholar] [CrossRef] [Green Version]
- Proença, D.; Velours, C.; Leandro, C.; Garcia, M.; Pimentel, M.; São-José, C. A Two-Component, Multimeric Endolysin Encoded by a Single Gene. Mol. Microbiol. 2015, 95, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Yoong, P.; Schuch, R.; Nelson, D.; Fischetti, V.A. Identification of a Broadly Active Phage Lytic Enzyme with Lethal Activity against Antibiotic-Resistant Enterococcus Faecalis and Enterococcus Faecium. J. Bacteriol. 2004, 186, 4730–4739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Binte Muhammad Jai, H.S.; Dam, L.C.; Tay, L.S.; Koh, J.J.W.; Loo, H.L.; Kline, K.A.; Goh, B.C. Engineered Lysins With Customized Lytic Activities Against Enterococci and Staphylococci. Front. Microbiol. 2020, 11, 574739. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buzikov, R.M.; Kazantseva, O.A.; Piligrimova, E.G.; Ryabova, N.A.; Shadrin, A.M. Bacteriolytic Potential of Enterococcus Phage iF6 Isolated from “Sextaphag®” Therapeutic Phage Cocktail and Properties of Its Endolysins, Gp82 and Gp84. Viruses 2023, 15, 767. https://doi.org/10.3390/v15030767
Buzikov RM, Kazantseva OA, Piligrimova EG, Ryabova NA, Shadrin AM. Bacteriolytic Potential of Enterococcus Phage iF6 Isolated from “Sextaphag®” Therapeutic Phage Cocktail and Properties of Its Endolysins, Gp82 and Gp84. Viruses. 2023; 15(3):767. https://doi.org/10.3390/v15030767
Chicago/Turabian StyleBuzikov, Rustam M., Olesya A. Kazantseva, Emma G. Piligrimova, Natalya A. Ryabova, and Andrey M. Shadrin. 2023. "Bacteriolytic Potential of Enterococcus Phage iF6 Isolated from “Sextaphag®” Therapeutic Phage Cocktail and Properties of Its Endolysins, Gp82 and Gp84" Viruses 15, no. 3: 767. https://doi.org/10.3390/v15030767




