Expanding the Repertoire of the Plant-Infecting Ophioviruses through Metatranscriptomics Data
Abstract
:1. Introduction
2. Material and Methods
2.1. Identification of Ophiovirus Sequences from Public Plant RNA-Seq Datasets
2.2. Sequence Assembly and Virus Identification
2.3. Bioinformatics Tools and Analyses
2.3.1. Sequence Analyses
2.3.2. Pairwise Sequence Identity
2.3.3. Phylogenetic Analysis
3. Results
3.1. Summary of Discovered Ophiovirus Genomic Sequences
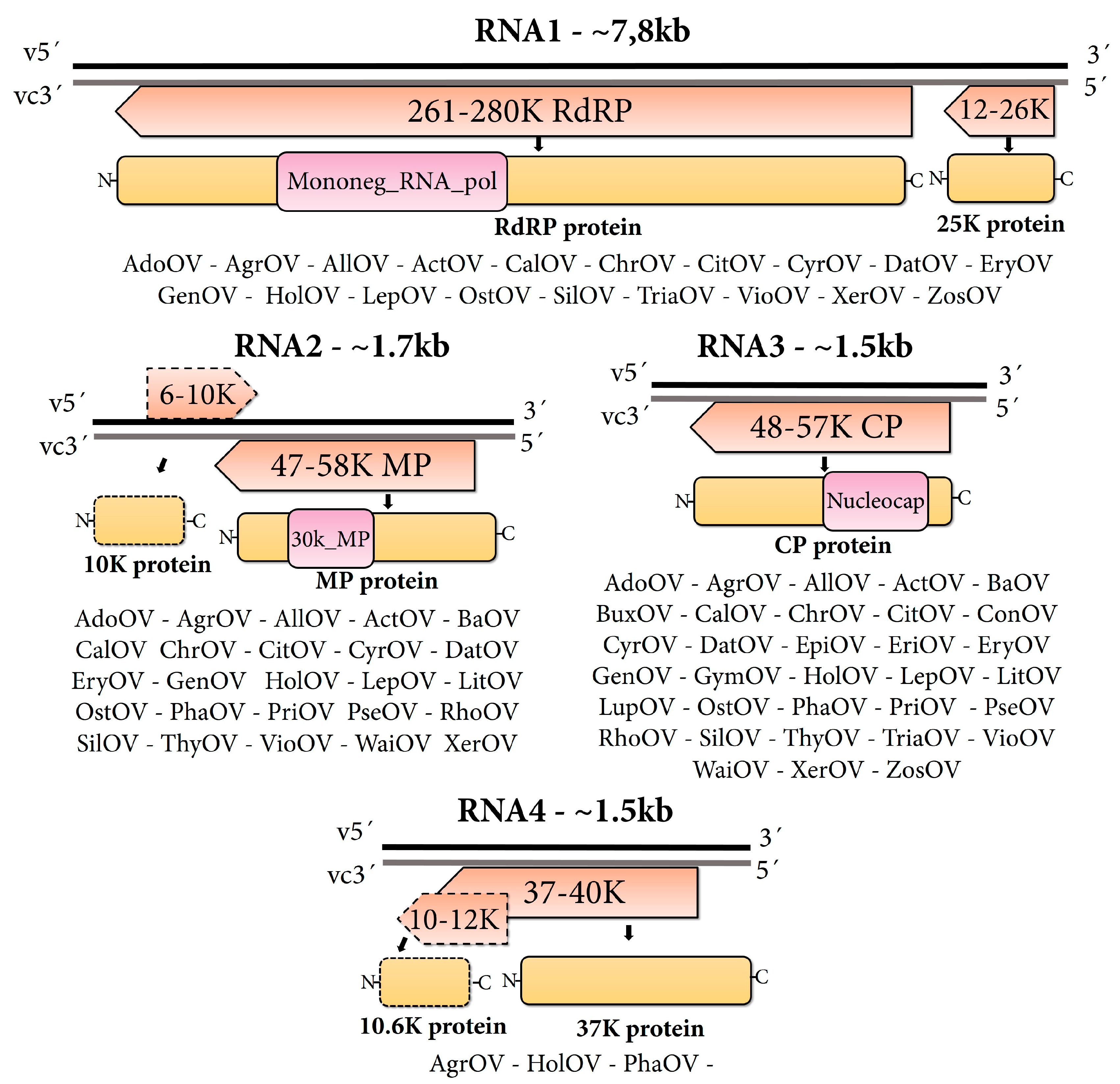
3.2. Structural and Functional Annotation of Ophiovirus Sequences
3.3. Pairwise Identities of Ophiovirus Sequences and Species Demarcation Criteria
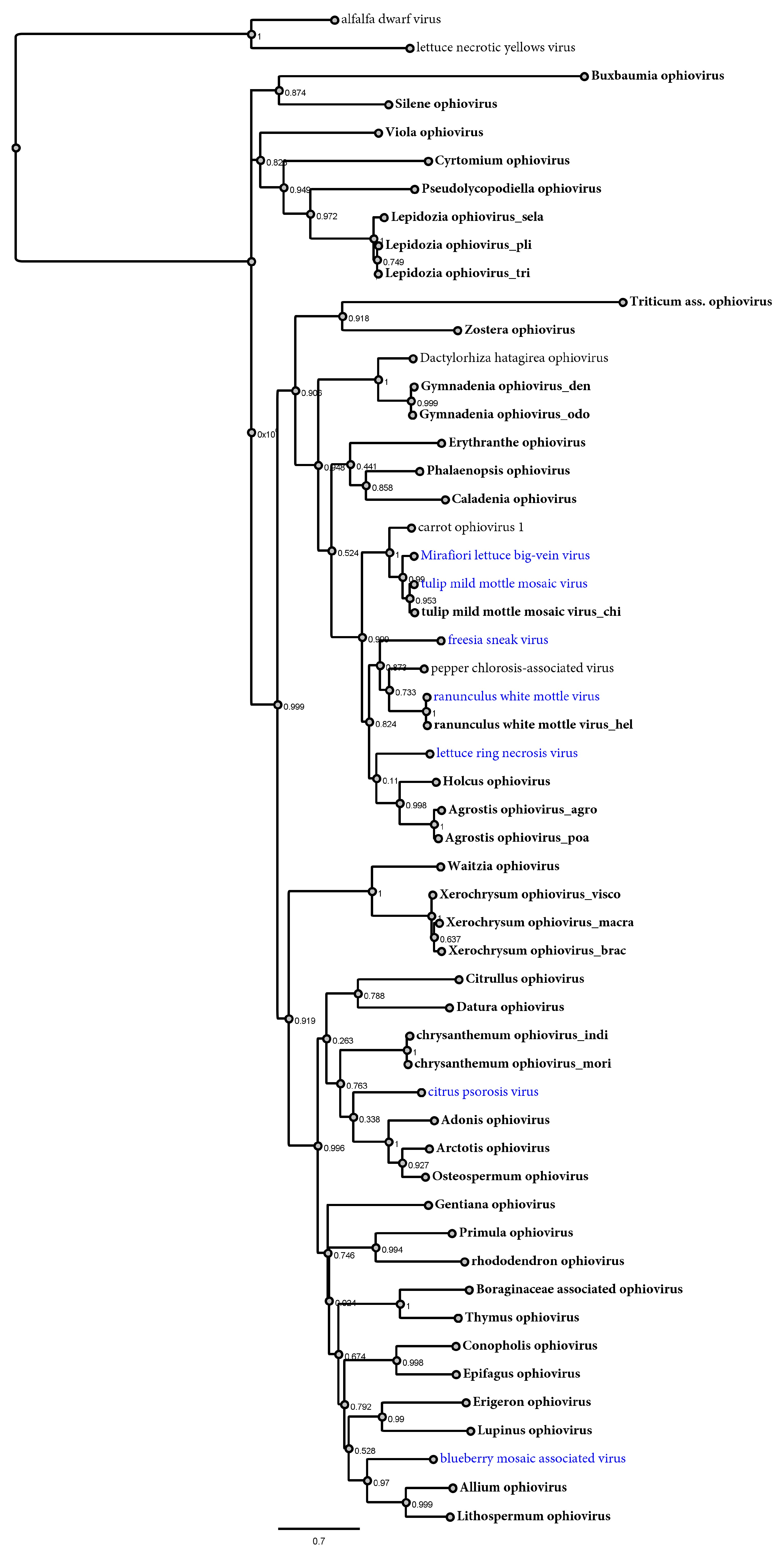
3.4. Phylogenetic Relationships between Ophioviruses and Hosts
4. Discussion
4.1. Discovery of Novel Ophioviruses Expands Their Diversity and Evolutionary History
4.2. Host Range and Genomic Organization of the Novel Ophioviruses
4.3. Genomic Features of the Discovered Ophioviruses
4.4. Sequence Diversity and Evolutionary Clues of Identified Ophioviruses
4.5. Ophiovirus Tentative Taxonomical Classification
4.6. Potential Vectors and Transmission Modes
4.7. Limitations of Sequence Discovery through Data Mining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Kuhn, J.H. Viruses Defined by the Position of the Virosphere within the Replicator Space. Microbiol. Mol. Biol. Rev. 2021, 85, e0019320. [Google Scholar] [CrossRef] [PubMed]
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Varsani, A.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global Organization and Proposed Megataxonomy of the Virus World. Microbiol. Mol. Biol. Rev. 2020, 84, e00061-19. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, J.L.; Holmes, E.C. Predicting virus emergence amid evolutionary noise. Open Biol. 2017, 7, 170–189. [Google Scholar] [CrossRef] [Green Version]
- Dolja, V.V.; Krupovic, M.; Koonin, E.V. Deep Roots and Splendid Boughs of the Global Plant Virome. Annu. Rev. Phytopathol. 2020, 58, 23–53. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Taylor, J.; Lin, V.; Altman, T.; Barbera, P.; Meleshko, D.; Lohr, D.; Novakovsky, G.; Buchfink, B.; Al-Shayeb, B.; et al. Petabase-scale sequence alignment catalyses viral discovery. Nature 2022, 602, 142–147. [Google Scholar] [CrossRef]
- Mifsud, J.C.O.; Gallagher, R.V.; Holmes, E.C.; Geoghegan, J.L. Transcriptome Mining Expands Knowledge of RNA Viruses across the Plant Kingdom. J. Virol. 2022, 96, e00260-22. [Google Scholar] [CrossRef]
- Bejerman, N.; Debat, H.; Dietzgen, R.G. The Plant Negative-Sense RNA Virosphere: Virus Discovery Through New Eyes. Front. Microbiol. 2020, 11, 588427. [Google Scholar] [CrossRef]
- Lauber, C.; Seitz, S. Opportunities and Challenges of Data-Driven Virus Discovery. Biomolecules 2022, 12, 1073. [Google Scholar] [CrossRef]
- Simmonds, P.; Adams, M.J.; Benkő, M.; Breitbart, M.; Brister, J.R.; Carstens, E.B.; Davison, A.J.; Delwart, E.; Gorbalenya, A.E.; Harrach, B.; et al. Virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017, 15, 161–168. [Google Scholar] [CrossRef]
- García, M.L.; Bó, E.D.; da Graça, J.V.; Gago-Zachert, S.; Hammond, J.; Moreno, P.; Natsuaki, T.; Pallás, V.; Navarro, J.A.; Reyes, C.A.; et al. ICTV Virus Taxonomy Profile: Ophioviridae. J. Gen. Virol. 2017, 98, 1161. [Google Scholar] [CrossRef]
- Fox, A.; Gibbs, A.J.; Fowkes, A.R.; Pufal, H.; McGreig, S.; Jones, R.A.C.; Boonham, N.; Adams, I.P. Enhanced Apiaceous Potyvirus Phylogeny, Novel Viruses, and New Country and Host Records from Sequencing Apiaceae Samples. Plants 2022, 11, 1951. [Google Scholar] [CrossRef] [PubMed]
- Shimomoto, Y.; Takemura, C.; Yanagisawa, H.; Neriya, Y.; Sasaya, T. Complete genome sequence of a novel ophiovirus associated with chlorotic disease of pepper (Capsicum annuum L.) in Japan. Arch. Virol. 2023, 168, 48. [Google Scholar] [CrossRef]
- Sidharthan, V.K.; Kalaivanan, N.; Baranwal, V. Discovery of putative novel viruses in the transcriptomes of endangered plant species native to India and China. Gene 2021, 786, 145626. [Google Scholar] [CrossRef]
- Leebens-Mack, J.H.; Barker, M.S.; Carpenter, E.J.; Deyholos, M.K.; Gitzendanner, M.A.; Graham, S.W.; Szövényi, P. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 2019, 574, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Bejerman, N.; Dietzgen, R.G.; Debat, H. Unlocking the Hidden Genetic Diversity of Varicosaviruses, the Neglected Plant Rhabdoviruses. Pathogens 2022, 11, 1127. [Google Scholar] [CrossRef]
- Bejerman, N.; Debat, H. Exploring the tymovirales landscape through metatranscriptomics data. Arch. Virol. 2022, 167, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Sun, H.; Dai, S.; Feng, S.; Zhang, J.; Gong, S.; Wang, J. Identification of Transcription Factors Involved in the Regulation of Flowering in Adonis Amurensis Through Combined RNA-seq Transcriptomics and iTRAQ Proteomics. Genes 2019, 10, 305. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.Q.; Zhang, J.; Burgess, P.; Rosso, S.; Huang, B. Interactive effects of melatonin and cytokinin on alleviating drought-induced leaf senescence in creeping bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Chen, S.; McElroy, J.S.; Dane, F.; Goertzen, L.R. Transcriptome assembly and comparison of an Allotetraploid weed species, annual bluegrass, with its two diploid progenitor species, Schrad and Kunth. Plant Genome. 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Fajkus, P.; Peska, V.; Zavodnik, M.; Fojtova, M.; Fulneckova, J.; Dobias, S.; Kilar, A.; Dvorackova, M.; Zachova, D.; Necasova, I.; et al. Telomerase rnas in land plants. Nucleic Acids Res. 2019, 47, 9842–9856. [Google Scholar] [CrossRef] [Green Version]
- Jayasena, A.S.; Fisher, M.F.; Panero, J.L.; Secco, D.; Bernath-Levin, K.; Berkowitz, O.; Taylor, N.L.; Schilling, E.E.; Whelan, J.; Mylne, J.S. Stepwise evolution of a buried inhibitor peptide over 45 My. Mol. Biol. Evol. 2017, 34, 1505–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Huang, J.; Wang, Y.; Zhao, X.; Su, L.; Thomas, G.W.; Zhao, M.; Zhang, X.; Jungreis, I.; Kellis, M. Genus-wide characterization of bumblebee genomes provides insights into their evolution and variation in ecological and behavioral traits. Mol. Biol. Evol. 2021, 38, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Bohman, B.; Wong, D.C.J.; Rodriguez-Delgado, C.; Scaffidi, A.; Flematti, G.R.; Phillips, R.D.; Pichersky, E.; Peakall, R. Complex Sexual Deception in an Orchid Is Achieved by Co-opting Two Independent Biosynthetic Pathways for Pollinator Attraction. Curr. Biol. 2017, 27, 1867–1877.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Ma, X.; Wei, M.; Zhao, T.; Zhan, R.; Chen, W. SSR marker development and intraspecific genetic divergence exploration of Chrysanthemum indicum based on transcriptome analysis. BMC Genom. 2018, 19, 291. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Dong, Q.; Chen, H.; Cong, Q.; Ding, K. Structural characterization of a polysaccharide from Chrysanthemum morifolium flowers and its antioxidant activity. Carbohydr. Polym. 2015, 130, 113–121. [Google Scholar] [CrossRef]
- Garcia-Lozano, M.; Dutta, S.K.; Natarajan, P.; Tomason, Y.R.; Lopez, C.; Katam, R.; Levi, A.; Nimmakayala, P.; Reddy, U.K. Transcriptome changes in reciprocal grafts involving watermelon and bottle gourd reveal molecular mechanisms involved in increase of the fruit size, rind toughness and soluble solids. Plant Mol. Biol. 2020, 102, 213–223. [Google Scholar] [CrossRef]
- You, C.; Cui, J.; Wang, H.; Qi, X.; Kuo, L.Y.; Ma, H.; Gao, L.; Mo, B.; Chen, X. Conservation and divergence of small RNA pathways and microRNAs in land plants. Genome Biol. 2017, 18, 158. [Google Scholar] [CrossRef]
- Chen, R.B.; Liu, J.H.; Xiao, Y.; Zhang, F.; Chen, J.F.; Ji, Q.; Tan, H.X.; Huang, X.; Feng, H.; Huang, B.K.; et al. Deep sequencing reveals the effect of MeJA on scutellarin biosynthesis in Erigeron breviscapus. PLoS ONE 2015, 10, e0143881. [Google Scholar] [CrossRef] [Green Version]
- Flores-Vergara, M.A.; Oneal, E.; Costa, M.; Villarino, G.; Roberts, C.; De Luis Balaguer, M.A.; Coimbra, S.; Willis, J.; Franks, R.G. Developmental analysis of Mimulus seed transcriptomes reveals functional gene expression clusters and four imprinted, endosperm-expressed genes. Front Plant Sci 2020, 11, 132. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zhang, Q.; Yang, Y.; Wang, Q.; He, Y.; Dong, N. Synergetic effect on methylene blue adsorption to biochar with gentian violet in dyeing and printing wastewater under competitive adsorption mechanism. Case Stud. Therm. Eng. 2021, 26, 101099. [Google Scholar] [CrossRef]
- Piñeiro Fernández, L.; Byers, K.J.R.P.; Cai, J.; Sedeek, K.E.M.; Kellenberger, R.T.; Russo, A.; Qi, W.; Aquino Fournier, C.; Schlüter, P.M. A phylogenomic analysis of the floral transcriptomes of sexually deceptive and rewarding European orchids, Ophrys and Gymnadenia. Front. Plant Sci. 2019, 29, 1553. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Carey, M.; Meharg, A.A.; Meharg, C. Microbiome and ecotypic adaption of Holcus lanatus (L.) to extremes of its soil pH range, investigated through transcriptome sequencing. Microbiome 2018, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, A.M.; Jayasena, A.S.; Zhang, J.; Berkowitz, O.; Secco, D.; Knott, G.J.; Whelan, J.; Bond, C.S.; Mylne, J.S. Evidence for ancient origins of bowman-birk inhibitors from Selaginella moellendorffii. Plant Cell 2017, 29, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.I. De novo Sequencing and comparative transcriptomics of floral development of the distylous species Lithospermum multiflorum. Front. Plant Sci. 2016, 7, 1934. [Google Scholar] [CrossRef] [Green Version]
- Cannon, S.B.; McKain, M.R.; Harkess, A.; Nelson, M.N.; Dash, S.; Deyholos, M.K.; Peng, Y.; Joyce, B.; Stewart, C.N., Jr.; Rolf, M. Multiple polyploidy events in the early radiation of nodulating and nonnodulating legumes. Mol. Biol. Evol. 2015, 32, 193–210. [Google Scholar] [CrossRef]
- Chao, Y.T.; Yen, S.H.; Yeh, J.H.; Chen, W.C.; Shih, M.C. Orchidstra 2.0-a transcriptomics resource for the orchid family. Plant Cell Physiol. 2017, 58, e9. [Google Scholar] [CrossRef] [Green Version]
- Muyle, A.; Bachtrog, D.; Marais, G.; Turner, J. Epigenetics drive the evolution of sex chromosomes in animals and plants. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200124. [Google Scholar] [CrossRef]
- Mollion, M.; Ehlers, B.K.; Figuet, E.; Santoni, S.; Lenormand, T.; Maurice, S.; Galtier, N.; Bataillon, T. Patterns of genome-wide nucleotide diversity in the gynodioecious plant Thymus vulgaris are compatible with recent sweeps of cytoplasmic genes. Genome Biol. Evol. 2018, 10, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Iquebal, M.A.; Sharma, P.; Jasrotia, R.S.; Jaiswal, S.; Kaur, A.; Saroha, M.; Angadi, U.B.; Sheoran, S.; Singh, R.; Singh, G.P.; et al. RNAseq analysis reveals drought-responsive molecular pathways with candidate genes and putative molecular markers in root tissue of wheat. Sci. Rep. 2019, 9, 13917. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Zhu, X.; Yang, Y.; Wang, Y.; Arens, P.; Liu, H. De novo transcriptome analysis of Viola ×wittrockiana exposed to high temperature stress. PLoS ONE 2019, 14, e0222344. [Google Scholar] [CrossRef]
- Crump, B.C.; Wojahn, J.M.; Tomas, F.; Mueller, R.S. Metatranscriptomics and amplicon sequencing reveal mutualisms in seagrass microbiomes. Front. Microbiol. 2018, 9, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhire, B.M.; Varsani, A.; Martin, D.P. SDT: A Virus Classification Tool Based on Pairwise Sequence Alignment and Identity Calculation. PLoS ONE 2014, 9, e108277. [Google Scholar] [CrossRef] [PubMed]
- Peña, E.J.; Luna, G.R.; Zanek, M.C.; Borniego, M.B.; Reyes, C.A.; Heinlein, M.; García, M.L. Citrus psorosis and Mirafiori lettuce big-vein ophiovirus coat proteins localize to the cytoplasm and self interact in vivo. Virus Res. 2012, 170, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.A.; Ocolotobiche, E.E.; Marmisollé, F.E.; Luna, G.R.; Borniego, M.B.; Bazzini, A.A.; Asurmendi, S.; García, M.L. Citrus psorosis virus 24K protein interacts with citrus miRNA precursors, affects their processing and subsequent miRNA accumulation and target expression. Mol. Plant Pathol. 2015, 17, 317–329. [Google Scholar] [CrossRef]
- Luna, G.R.; Reyes, C.A.; Peña, E.J.; Ocolotobiche, E.; Baeza, C.; Borniego, M.B.; Kormelink, R.; García, M.L. Identification and characterization of two RNA silencing suppressors encoded by ophioviruses. Virus Res. 2017, 235, 96–105. [Google Scholar] [CrossRef]
- Luna, G.R.; Peña, E.J.; Borniego, M.B.; Heinlein, M.; Garcia, M.L. Ophioviruses CPsV and MiLBVV movement protein is encoded in RNA 2 and interacts with the coat protein. Virology 2013, 441, 152–161. [Google Scholar] [CrossRef]
- Hiraguri, A.; Ueki, S.; Kondo, H.; Nomiyama, K.; Shimizu, T.; Ichiki-Uehara, T.; Omura, T.; Sasaki, N.; Nyunoya, H.; Sasaya, T. Identification of a movement protein of Mirafiori lettuce big-vein ophiovirus. J. Gen. Virol. 2013, 94, 1145–1150. [Google Scholar] [CrossRef] [Green Version]
- Borniego, M.B.; Karlin, D.; Peña, E.J.; Luna, G.R.; García, M.L. Bioinformatic and mutational analysis of ophiovirus movement proteins, belonging to the 30K superfamily. Virology 2016, 498, 172–180. [Google Scholar] [CrossRef]
- van der Wilk, F.; Dullemans, A.M.; Verbeek, M.; Van Den Heuvel, J.F.J.M. Nucleotide sequence and genomic organization of an ophiovirus associated with lettuce big-vein disease. J. Gen. Virol. 2002, 83, 2869–2877. [Google Scholar] [CrossRef]
- Marsile-Medun, S.; Debat, H.J.; Gifford, R.J. Identification of the first endogenous Ophiovirus sequence. bioRxiv 2018, 235044. [Google Scholar] [CrossRef] [Green Version]
- Postler, T.S.; Rubino, L.; Adriaenssens, E.M.; Dutilh, B.E.; Harrach, B.; Junglen, S.; Kropinski, A.M.; Krupovic, M.; Wada, J.; Crane, A.; et al. Guidance for creating individual and batch latinized binomial virus species names. J. Gen. Virol. 2022, 103, 001800. [Google Scholar] [CrossRef] [PubMed]
- Fetters, A.M.; Cantalupo, P.G.; Na Wei, N.; Robles, M.T.S.; Stanley, A.; Stephens, J.D.; Pipas, J.M.; Ashman, T.-L. The pollen virome of wild plants and its association with variation in floral traits and land use. Nat. Commun. 2022, 13, 523. [Google Scholar] [CrossRef] [PubMed]
- Lot, H.; Campbell, R.N.; Souche, S.; Milne, R.G.; Roggero, P. Transmission by Olpidium brassicae of Mirafiori lettuce virus and Lettuce big-vein virus, and Their Roles in Lettuce Big-Vein Etiology. Phytopathology 2002, 92, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meekes, E.; Verbeek, M. New Insights in Freesia Leaf Necrosis Disease. Acta Hortic. 2011, 901, 231–236. [Google Scholar] [CrossRef]

| Plant Host | Taxa/ Family | Virus Name/ Abbreviation | Bioproject ID/ Data Citation | Segment/Virus Reads (Total/RPKM) | Length (nt) | Accession Number | Protein ID | Length (aa) | Highest-Scoring Virus Protein | Blastp E-Value | Blastp Query Coverage%/ | Blastp Identity% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amur adonis (Adonis amurensis) | dicot/ Ranunculaceae | Adonis ophiovirus/ AdoOV | PRJNA521968/ [17] | RNA1 (693/2.1) RNA2 (1032/14.6) RNA3 (2406/37.5) | 7425 * 1595 1448 | BK062646 BK062647 BK062648 | RdRp MP CP | 2411 * 467 450 | CPsV-RdRp CPsV-MP CPsV-CP | 0.0 3e−108 6e−110 | 93 94 100 | 46.79 40.44 |
| Creeping bentgrass (Agrostis stolonifera) | monocot/ Poaceae | Agrostis ophiovirus_agro/ AgrOV_agro | PRJNA324407/ [18] | RNA1 (403/0.2) RNA2 (214/0.4) RNA3 (404/0.9) RNA4 (269/0.5) | 7710 * 1863 1540 1907 | BK062649 BK062650 BK062651 BK062652 | RdRp 22kDa MP CP 37kDa | 2294 * 174 499 453 322 | MLBVV-RdRp RWMV-22kDa MLBVV-MP MLBVV-CP LNRV-37kDa | 0.0 1e−21 1e−155 3e−138 4e−69 | 84 75 98 99 90 | 59.61 40.91 49 49 37.54 |
| Annual bluegrass (Poa annua) | monocot/ Poaceae | Agrostis ophiovirus_poa/ AgrOV_poa | PRJNA265116/ [19] | RNA 1 (36/0.2) RNA2 (903/1.9) RNA3 (932/2.4) RNA4 (206/0.6) | 824 * 1828 1525 1428 | BK062653 BK062654 BK062655 BK062656 | 22kDa MP CP 37kDa | 174 499 453 323 | RWMV-22kDa MLBVV-MP MLBVV-CP LRNV-37kDa | 7e−21 5e−155 1e−138 7e−69 | 77 98 99 90 | 40.96 49 49 37.59 |
| Wild garlic (Allium ursinum) | monocot/ Amaryllidaceae | Allium ophiovirus/ AllOV | PRJNA542932/ [20] | RNA1 (15956/14.8) RNA2 (11305/42.3) RNA3 (16494/75.6) | 7380 * 1832 1495 | BK062657 BK062658 BK062659 | RdRp MP CP | 2338 478 454 | BlMaV-RdRp BlMaV-MP BlMaV-CP | 0.0 4e−111 5e−125 | 99 93 81 | 52.96 38 49.6 |
| Silver actotis (Arctotis venusta) | dicot/ Asteraceae | Arctotis ophiovirus/ ActOP | PRJNA371565/ [21] | RNA1 (30281/99.0) RNA2 (18388/287.8) RNA3 (4558/84.8) | 8319 1738 1462 | BK062660 BK062661 BK062662 | RdRp 22kDa MP CP | 2406 222 486 446 | CPsV-RdRp no hits CPsV-MP CPsV-CP | 0.0 - 2e−142 1e−121 | 99 - 97 100 | 45.18 48 43.56 |
| Borage (Boranginaceae) | dicot/ Boranginaceae | Boranginaceae associated ophiovirus/BaOV | PRJNA659133/ [22] | RNA2 (625/4.8) RNA3 (1684/14.2) | 1737 1589 | BK062663 BK062664 | MP CP | 486 471 | BlMaV-MP BlMaV-CP | 2e−110 2e−86 | 91 76 | 39.74 41.92 |
| Bug moss (Buxbaumia aphylla) | Bryophyta/ Buxbaumiaceae | Buxbaumia ophiovirus/ BuxOV | PRJEB21674/ 1000 Plant (1KP) Transcriptomes Initiative | RNA3 (1296/27.8) | 1590 | BK062665 | CP | 485 | MLBVV-CP | 4e−18 | 53 | 25.82 |
| Crab-lipped spider orchid (Caladenia plicata) | monocot/ Orchidaceae | Caladenia ophiovirus/ CalOV | PRJNA384875/ [23] | RNA1 (5541/15.3) RNA2 (1126/13.2) RNA3 (447/6.5) | 7488 1760 1423 | BK062666 BK062667 BK062668 | RdRp 22kDa MP CP | 2247 163 445 438 | RWMV-RdRp RWMV-22kDa LRNV-MP RWMV-CP | 0.0 7e−10 9e−100 4e−101 | 100 61 99 94 | 49.48 32.67 39.47 39.66 |
| Indian chrysanthemum (Chrysanthemum indicum) | dicot/ Asteraceae | Chrysanthemum ophiovirus_indi/ChrOV_indi | PRJNA361213/ [24] | RNA1 (1318/2.8) RNA2 (1242/10.1) RNA3 (597/6.6) | 8240 2143 1572 | BK062669 BK062670 BK062671 | RdRp 22kDa MP CP | 2379 222 483 457 | BlMaV-RdRp BlMaV-22kDa CPsV-MP BlMaV-CP | 0.0 0.002 4e−117 3e−109 | 97 62 94 99 | /47.45 30.54 42.61 42.19 |
| Garden mum (Chrysanthemum morifolium) | dicot/ Asteraceae | Chrysanthemum ophiovirus_mori/ChrOV_mori | PRJNA315793/ [25] | RNA1 (12382/4.8) RNA2 (4371/6.5) RNA3 (1635/3.3) | 8255 2164 1573 | BK062672 BK062663 BK062674 | RdRp 22kDa MP CP | 2379 222 483 457 | BlMaV-RdRp BlMaV-22kDa CPsV-MP BlMaV-CP | 0.0 0.002 4e−117 3e−109 | 97 62 94 99 | 47.49 30.50 42.64 42.15 |
| Watermelon (Citrullus lanatus) | dicot/ Cucurbitaceae | Citrullus ophiovirus/CitOV | PRJNA576654/ [26] | RNA1 (10212/21.4) RNA2 (32771/332.7) RNA3 (18763/219.4) | 8510 1760 1528 | BK062675 BK062676 BK062677 | RdRp 22kDa MP CP | 2418 245 483 464 | CPsV-RdRp no hits CPsV-MP CPsV-CP | 0.0 - 9e−127 8e−82 | 99 - 97 93 | 42.77 43.19 38.66 |
| Bear corn (Conopholis americana) | dicot/ Orobanchaceae | Conopholis ophiovirus/ConOV | PRJEB21674/ 1000 Plant (1KP) Transcriptomes Initiative | RNA3 (1148/22.5) | 1684 | BK062678 | CP | 481 | BlMaV-CP | 4e−99 | 69 | 45.35 |
| Holly fern (Cyrtomium fortunei) | Polypodiophyta/ Dryopteridaceae | Cyrtomium ophiovirus/CyrOV | PRJNA384992/ [27] | RNA1 (16605/59.5) RNA2 (18411/261.7) RNA3 (42710/660.2) | 7548 1902 1749 | BK062679 BK062680 BK062681 | RdRp 22kDa MP CP | 2357 105 409 500 | BlMaV-RdRp no hits BlMaV-MP MLBVV-CP | 0.0 - 4e−06 4e−50 | 97 - 67 67 | 36.83 22.92 33.90 |
| Sacred datura (Datura wrightii) | Dicot/ Solanaceae | Datura ophiovirus/DatOV | PRJNA473174/ Sun, University of California, USA | RNA1 (3286/13.0) RNA2 (6830/122.1) RNA3 (18608/349.7) | 8055 1788 1701 | BK062682 BK062683 BK062684 | RdRp 22kDa MP CP | 2366 186 481 511 | BlMaV-RdRp no hits BlMaV-MP CPsV-CP | 0.0 - 1e−86 1e−88 | 97 - 95 69 | 50.09 35.43 39.50 |
| Beech drops (Epifagus virginiana) | dicot/ Orobanchaceae | Epifagus ophiovirus/EpiOV | PRJEB21674/ 1000 Plant (1KP) Transcriptomes Initiative | RNA3 (250/7.8) | 1371 * | BK062685 | CP | 328 * | BlMaV-CP | 3e−59 | 88 | 42.81 |
| Lifeflower (Erigeron breviscapus) | dicot/ Asteraceae | Erigeron ophiovirus/EriOV | PRJNA293262/ [28] | RNA3 (817/2.1) | 1837 | BK062686 | CP | 463 | BlMaV-CP | 2e−92 | 74 | 44.09 |
| Pardus monkey-flower (Erythranthe pardalis) | dicot/ Phrymaceae | Erythranthe ophiovirus/EryOV | PRJNA508749/ [29] | RNA1 (611/2.6) RNA2 (539/11.0) RNA3 (3995/78.3) | 7643 1587 1651 | BK062687 BK062688 BK062689 | RdRp 22kDa MP CP | 2271 195 436 490 | LRNV-RdRp BlMaV-22kDa LRNV-MP RWMV-CP | 0.0 2e−11 1e−97 1e−100 | 99 58 90 99 | 51.73 30.43 39.33 36.68 |
| Tube gentian (Gentiana siphonantha) | dicot/ Gentianaceae | Gentiana ophiovirus/ (GenOV) | PRJNA555883/ [30] | RNA1 (9973/23.4) RNA2 (1620/14.7) RNA3 (1561/20.0) | 8043 2077 1473 | BK062690 BK062691 BK062692 | RdRp 22kDa MP CP | 2254 190 516 450 | BlMaV-RdRp BlMaV-22kDa BlMaV-MP BlMaV-CP | 0.0 0.007 3e−47 2e−92 | 99 75 52 80 | 45.32 24.32 37.86 42.66 |
| Marsh fragrant orchid (Gymnadenia densiflora) | monocot/ Orchidaceae | Gymnadenia ophiovirus_den/GymOV_den | PRJNA504609/ [31] | RNA3 (336/5.6) | 1431 | BK062693 | CP | 446 | DhOV-CP | 0.0 | 94 | 60.05 |
| Short-spurred fragrant orchid (Gymnadenia odorattissima) | monocot/ Orchidaceae | Gymnadenia ophiovirus_odo/GymOV_odo | PRJNA504609/ [31] | RNA3 (172/3.0) | 1339 * | BK062694 | CP | 425 | DhOV-CP | 0.0 | 87 | 58.25 |
| Common velvetgrass (Holcus lanatus) | monocot/ Poaceae | Holcus ophiovirus/HolOV | PRJEB3994/ [32] | RNA 1 (2879/3.1) RNA2 (3959/18.5) RNA3 (3501/19.4) RNA4 (2272/13.1) | 7627 * 1770 1495 1436 | BK062695 BK062696 BK062697 BK062698 | RdRp 22kDa MP CP 37kDa | 2194 * 162 459 444 322 | RWMV-RdRp MLBVV-22kDa MLBVV-MP RWMV-CP LRNV-37kDa | 0.0 3e−24 2e−176 4e−148 1e−73 | 89 82 99 100 98 | 65.13 40 53.43 49.77 39.06 |
| Hairy liverwort (Lepidozia trichodes) | Marchantiophyta/ Lepidoziaceae | Lepidozia ophiovirus_tri/LepOV_tri | PRJNA505755/ Fairylake Botanical Garden, China | RNA1 (38067/93.9) RNA2 (10558/106.3) RNA3 (28205/336.2) | 7644 1872 1581 | BK062699 BK062700 BK062701 | RdRp 22kDa MP CP | 2357 109 460 471 | BlMaV-RdRp no hits BlMaV-MP MLBVV-CP | 0.0 - 3e−19 6e−58 | 96 - 49 71 | 37.15 25.96 30.99 |
| Basket liverwort (Plicanthus hirtellus) | Marchantiophyta/ Anastrophyllaceae | Lepidozia ophiovirus_pli/LepOV_pli | PRJNA505755/ Fairylake Botanical Garden, China | RNA1 (1358/3.8) RNA2 (128/1.8) RNA3 (1057/14.3) | 7546 1497 1555 | BK062702 BK062703 BK062704 | RdRp 22kDa MP CP | 2357 109 460 471 | BlMaV-RdRp no hits BlMaV-MP MLBVV-CP | 0.0 - 2e−19 8e−58 | 96 - 49 71 | 37.19 25.94 30.92 |
| Krauss’ spike moss (Selaginella kraussiana) | Lycophyta/ Selaginellaceae | Lepidozia ophiovirus_sela/LepOV_sela | PRJNA351923/ [33] | RNA1 (556211 /499.8) RNA2 (75738/277.9) RNA3(288058/1251.5) | 7644 1872 1581 | BK062705 BK062706 BK062707 | RdRp 22kDa MP CP | 2357 109 460 471 | BlMaV-RdRp no hits BlMaV-MP MLBVV-CP | 0.0 - 4e−19 5e−58 | 96 - 49 71 | 37.11 25.99 30.95 |
| Manyflowered gromwell (Lithospermum multiflorum) | dicot/ Boraginaceae | Lithospermum ophiovirus/LitOV | PRJNA353131/ [34] | RNA2 (449/6.1) RNA3 (2370/28.6) | 1498 * 1693 | BK062708 BK062709 | MP CP | 470* 460 | BlMaV-MP MLBVV-CP | 7e−118 1e−51 | 92 64 | 42.61 32.70 |
| Garden lupin (Lupinus polyphyllus) | dicot/ Fabaceae | Lupinus ophiovirus/LupOV | PRJEB8056/ [35] | RNA3 (1631/45.8) | 1838 | BK062710 | CP | 448 | BlMaV-CP | 1e−95 | 73 | 44.31 |
| Trailing pink daisy (Osteospermum jucundum) | dicot/ Asteraceae | Osteospermum ophiovirus/OstOV | PRJNA371565/ [21] | RNA1 (11077/35.9) RNA2 (11158/181.4) RNA3 (12821/234.5) | 8521 1701 1512 | BK062711 BK062712 BK062713 | RdRp 22kDa MP CP | 2407 204 482 449 | CPsV-RdRp no hits CPsV-MP CPsV-CP | 0.0 - 2e−143 2e−120 | 100 - 100 100 | 46.10 47.89 43.65 |
| Moth orchid (Phalaenopsis lueddemanniana) | monocot/ Orchidaceae | Phalaenopsis ophiovirus/PhaOV | PRJNA345261/ [36] | RNA2 (709/8.6) RNA3 (1173/16.3) RNA4 (388/6.8) | 1867 1630 1296 | BK062714 BK062715 BK062716 | MP CP 37kDa | 489 431 360 | LRNV-MP MLBVV-CP LRNV-37kDa | 1e−92 7e−120 1e−14 | 95 100 47 | 37.58 44.87 31.76 |
| Clammy primrose (Primula pumilio) | dicot/ Primulaceae | Primula ophiovirus/PriOV | PRJNA544345/ Hao, D., Chengdu, China | RNA2 (6291/82.6) RNA3 (8866/115.9) | 1565 1572 | BK062717 BK062718 | MP CP | 450 455 | LRNV-MP CPsV-CP | 2e−56 1e−86 | 91 93 | 30.64 37.15 |
| Slender bog club-moss (Pseudolycopodiella caroliniana) | Lycophyta/ Lycopodiaceae | Pseudolycopodiella ophiovirus/PseOV | PRJEB4921/ 1000 Plant (1KP) Transcriptomes Initiative | RNA2 (695/20.5) RNA3 (1402/47.4) | 1829 1594 | BK062719 BK062720 | MP CP | 464 466 | MLBVV-MP MLBVV-CP | 1e−22 2e−55 | 57 71 | 28.37 31.86 |
| Firecracker rhododendron (Rhododendron spinuliferum) | dicot/ Ericaceae | Rhododendron ophiovirus/RhoOV | PRJNA530078/ Xue Zhang, Yunnan University, China | RNA2 (1008/11.5) RNA3 (590/9.0) | 1867 1406 | BK062725 BK062726 | MP CP | 452 441 | BlMaV-MP BlMaV-CP | 3e−49 6e−78 | 95 95 | 31.25 37.07 |
| Diclinis campion (Silene diclinis) | dicot/ Caryophyllaceae | Silene ophiovirus/ SilOV | PRJEB39526/ [37] | RNA1 (382/2.8) RNA2 (438/12.7) RNA3 (250/7.2) | 6036 * 1511 1532 | BK062727 BK062728 BK062729 | RdRp MP CP | 1993 * 426 446 | BlMaV-RdRp LRNV-MP TMMMV-CP | 0.0 2e−29 2e−53 | 98 69 80 | 40.51 29.30 35.12 |
| Thyme (Thymus vulgaris) | dicot/ Lamiaceae | Thymus ophiovirus/ ThyOV | PRJNA417241/ [38] | RNA2 (1885/24.2) RNA3 (38453/495.5) | 1598 1589 | BK062730 BK062731 | MP CP | 480 477 | BlMaV-MP BlMaV-CP | 3e−114 1e−91 | 95 71 | 40.47 42.98 |
| Wheat (Triticum aestivum) | monocot/ Poaceae | Triticum associated ophiovirus/TriaOV | PRJNA432496/ [39] | RNA1 (17636/41.4) RNA3 (476/5.0) | 5377 * 1192 * | BK062733 BK062734 | RdRp CP | 1792 * 377 * | RWMV-RdRp DhOV-CP | 0.0 8e−15 | 95 57 | 34.62 30.77 |
| Pansies (Viola x wittrockiana) | dicot/ Violaceae | Viola ophiovirus/VioOV | PRJNA552204/ [40] | RNA1 (2761/5.5) RNA2 (188/1.8) RNA3 (126/1.2) | 7671 1570 1576 | BK062735 BK062736 BK062737 | RdRp 22kDa MP CP | 2308 173 435 492 | MLBVV-RdRp no hits BlMaV-MP MLBVV-CP | 0.0 - 4e−16 4e−52 | 94 - 54 69 | 37.76 27.17 33.62 |
| Golden waitzia (Waitzia nitida) | dicot/ Asteraceae | Waitzia ophiovirus /(WaiOV) | PRJNA371565/ [21] | RNA2 (219/1.8) RNA3 (208/1.8) | 1570 1486 | BK062738 BK062739 | MP CP | 453 460 | BlMaV-MP CPsV-CP | 5e−35 7e−63 | 83 97 | 29.84 31.32 |
| Strawflower (Xerochrysum bracteatum) | dicot/ Asteraceae | Xerochrysum ophiovirus_brac/ XerOV_brac_ | PRJNA371565/ [21] | RNA1 (15362/46.6) RNA2 (7601/112.2) RNA3 (11398/169.0) | 7681 1577 1570 | BK062740 BK062741 BK062742 | RdRp 22kDa MP CP | 2266 199 444 461 | BlMaV-RdRp no hits LRNV-MP CPsV-CP | 0.0 - 4e−39 7e−56 | 99 - 90 94 | 40.75 28.76 30.16 |
| White strawflower (Xerochrysum macranthum) | dicot/ Asteraceae | Xerochrysum ophiovirus_macra/ XerOV_macra | PRJNA371565/ [21] | RNA1 (3999/13.0) RNA2 (5003/76.2) RNA3 (2662/44.1) | 7692 1646 1513 | BK062743 BK062744 BK062745 | RdRP 22kDa MP CP | 2264 199 444 459 | BlMaV-RdRp no hits LRNV-MP CPsV-CP | 0.0 - 4e−40 7e−57 | 99 - 90 95 | 40.75 29.82 30.11 |
| Sticky everlasting (Xerochrysum viscosum) | dicot/ Asteraceae | Xerochrysum ophiovirus_visco/ XerOV_visco | PRJNA371565/ [21] | RNA1 (4099/13.7) RNA2 (346/5.5) RNA3 (304/5.1) | 7591 1577 1522 | BK062746 BK062747 BK062748 | RdRp 22kDa MP CP | 2266 199 441 459 | BlMaV-RdRp no hits LRNV-MP CPsV-CP | 0.0 - 2e−41 3e−58 | 0.0 - 91 96 | 41.45 30.05 30.44 |
| Dwarf eelgrass (Zostera japonica) | monocot/ Zosteraceae | Zostera ophiovirus/ ZosOV | PRJNA419030/ [41] | RNA1 (42459/165.7) RNA3 (15392/284.8) | 7748 1634 | BK062749 BK062750 | RdRp 22kDa CP | 2281 216 452 | LRNV-RdRp no hits RWMV-CP | 0.0 - 4e−52 | 93 - 99 | 41.84 30.31 |
| Virus Name/Abbreviation | Species Name |
|---|---|
| Adonis ophiovirus/AdoOV | Ophiovirus adonidis |
| Agrostis ophiovirus_agro/AgrOV_agro | Ophiovirus agrostis |
| Agrostis ophiovirus_poa/AgrOV_poa | Ophiovirus agrostis |
| Allium ophiovirus/AllOV | Ophiovirus alli |
| Arctotis ophiovirus/ActOP | Ophiovirus arctotis |
| Boranginaceae associated ophiovirus/BaOV | Ophiovirusboranginaceae |
| Buxbaumia ophiovirus/BuxOV | Ophiovirus buxbaumiae |
| Caladenia ophiovirus/CalOV | Ophiovirus caladeniae |
| chrysanthemum ophiovirus_indi/ChrOV_indi | Ophiovirus chrysanthemi |
| chrysanthemum ophiovirus_mori/ChrOV_mori | Ophiovirus chrysanthemi |
| Citrullus ophiovirus/CitOV | Ophiovirus citrullus |
| Conopholis ophiovirus/ConOV | Ophiovirus conopholis |
| Cyrtomium ophiovirus/CyrOV | Ophiovirus cyrtomii |
| Datura ophiovirus/DatOV | Ophiovirus daturi |
| Epifagus ophiovirus/EpiOV | Ophiovirus epifagus |
| Erigeron ophiovirus/EriOV | Ophiovirus erigeron |
| Erythranthe ophiovirus/EryOV | Ophiovirus erythranthis |
| Gentiana ophiovirus/ (GenOV) | Ophiovirus gentianae |
| Gymnadenia ophiovirus_den/GymOV_den | Ophiovirus gymnadeniae |
| Gymnadenia ophiovirus_odo/GymOV_odo | Ophiovirus gymnadeniae |
| Holcus ophiovirus/HolOV | Ophiovirus holci |
| Lepidozia ophiovirus_tri/LepOV_tri | Ophiovirus lepidoziae |
| Lepidozia ophiovirus_pli/LepOV_pli | Ophiovirus lepidoziae |
| Lepidozia ophiovirus_sela/LepOV_sela | Ophiovirus lepidoziae |
| Lithospermum ophiovirus/LitOV | Ophiovirus lithospermi |
| Lupinus ophiovirus/LupOV | Ophiovirus lupini |
| Osteospermum ophiovirus/OstOV | Ophiovirus osteospermi |
| Phalaenopsis ophiovirus/PhaOV | Ophiovirus phalaenopsis |
| Primula ophiovirus/PriOV | Ophiovirus primuli |
| Pseudolycopodiella ophiovirus/PseOV | Ophiovirus pseudolycopodiellae |
| rhododendron ophiovirus/RhoOV | Ophiovirus rhododendri |
| Silene ophiovirus/SilOV | Ophiovirus sileni |
| Thymus ophiovirus/ ThyOV | Ophiovirus thymi |
| Triticum associated ophiovirus/TriaOV | Ophiovirus tritici |
| Viola ophiovirus/VioOV | Ophiovirus violae |
| Waitzia ophiovirus /(WaiOV) | Ophiovirus waitziae |
| Xerochrysum ophiovirus_brac/ XerOV_brac_ | Ophiovirus xerochrysi |
| Xerochrysum ophiovirus_macra/ XerOV_macra | Ophiovirus xerochrysi |
| Xerochrysum ophiovirus_visco/ XerOV_visco | Ophiovirus xerochrysi |
| Zostera ophiovirus/ZosOV | Ophiovirus zosterae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debat, H.; Garcia, M.L.; Bejerman, N. Expanding the Repertoire of the Plant-Infecting Ophioviruses through Metatranscriptomics Data. Viruses 2023, 15, 840. https://doi.org/10.3390/v15040840
Debat H, Garcia ML, Bejerman N. Expanding the Repertoire of the Plant-Infecting Ophioviruses through Metatranscriptomics Data. Viruses. 2023; 15(4):840. https://doi.org/10.3390/v15040840
Chicago/Turabian StyleDebat, Humberto, Maria Laura Garcia, and Nicolas Bejerman. 2023. "Expanding the Repertoire of the Plant-Infecting Ophioviruses through Metatranscriptomics Data" Viruses 15, no. 4: 840. https://doi.org/10.3390/v15040840
APA StyleDebat, H., Garcia, M. L., & Bejerman, N. (2023). Expanding the Repertoire of the Plant-Infecting Ophioviruses through Metatranscriptomics Data. Viruses, 15(4), 840. https://doi.org/10.3390/v15040840







