HIV-1 Drug Resistance among Treatment-Naïve Patients in Russia: Analysis of the National Database, 2006–2022
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Data Collection
2.2. RNA Extraction and HIV-1 Sequencing
2.3. Sequence Quality Control
2.4. HIV-1 Subtyping
2.5. HIV-1 Drug Resistance Interpretation
- nucleoside reverse transcriptase inhibitors (NRTIs): abacavir (ABC), zidovudine (AZT), emtricitabine (FTC), lamivudine (3TC), tenofovir (TDF), stavudine (d4T), didanosine (ddI);
- non-nucleoside reverse transcriptase inhibitors (NNRTIs): doravirine (DOR), efavirenz (EFV), etravirine (ETR), nevirapine (NVP), rilpivirine (RPV);
- protease inhibitors (PIs): atazanavir (ATV), darunavir (DRV), lopinavir (LPV), fosamprenavir (FPV), indinavir (IDV), nelfinavir (NFV), saquinavir (SQV), tipranavir (TPV);
- integrase strand transfer inhibitors (INSTIs): bictegravir (BIC), cabotegravir (CAB), dolutegravir (DTG), elvitegravir (EVG), raltegravir (RAL).
2.6. Statistical Analysis
2.7. Ethics
3. Results
3.1. Characteristics of the Study Population
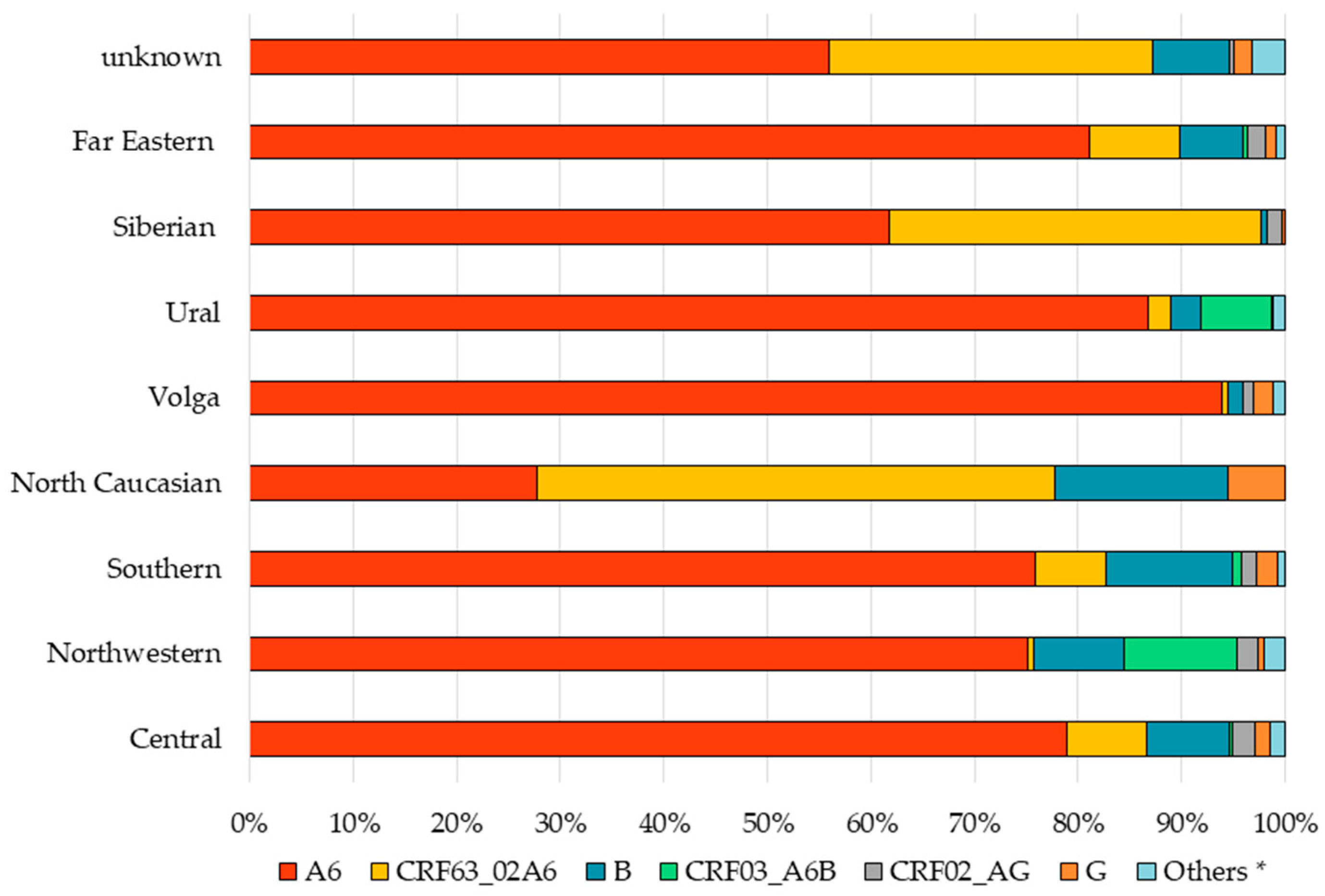
3.2. Prevalence of HIV-1 Genetic Variants
3.3. Prevalence of Major DRMs and SDRMs
3.4. Prevalence of DR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2021–2020 Data; ECDC: Solna, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/hiv-aids-surveillance-europe-2021-2020-data (accessed on 1 December 2022).
- Ladnaia, N.N.; Pokrovsky, V.V.; Sokolova, E.V.; Chekryzhova, D.G.; Kirzhanova, V.V. Prevalence of human immune deficiency virus infection in the territories of the Russian Federation in 2021. Epidemiol. Infect. Dis. Curr. Items 2022, 12, 12–18. (In Russian) [Google Scholar] [CrossRef]
- Palella, F.J.; Delaney, K.M.; Moorman, A.C.; Loveless, M.O.; Fuhrer, J.; Satten, G.A.; Aschman, D.J.; Holmberg, S.D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 1998, 338, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Chen, Y.Q.; McCauley, M.; Gamble, T.; Hosseinipour, M.C.; Kumarasamy, N.; Hakim, J.G.; Kumwenda, J.; Grinsztejn, B.; Pilotto, J.H.; et al. Prevention of HIV-1 infection with early antiretroviral therapy. N. Engl. J. Med. 2011, 365, 493–505. [Google Scholar] [CrossRef]
- Keller, S.C.; Yehia, B.R.; Eberhart, M.G.; Brady, K.A. Accuracy of definitions for linkage to care in persons living with HIV. J. Acquir. Immune Defic. Syndr. 2013, 63, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Samji, H.; Cescon, A.; Hogg, R.S.; Modur, S.P.; Althoff, K.N.; Buchacz, K.; Burchell, A.N.; Cohen, M.; Gebo, K.A.; Gill, M.J. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE 2013, 8, e81355. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health of the Russian Federation. Clinical Guidelines “HIV Infection in Adults”. 2017. Available online: http://rushiv.ru/wp-content/uploads/2019/03/kr79.pdf (accessed on 1 December 2022). (In Russian).
- Ministry of Health of the Russian Federation. Clinical Guidelines “HIV Infection in Adults”. 2020. Available online: http://rushiv.ru/wp-content/uploads/2022/11/KR79.pdf (accessed on 1 December 2022). (In Russian).
- Al-Salama, Z.T. Elsulfavirine: First global approval. Drugs 2017, 77, 1811–1816. [Google Scholar] [CrossRef]
- International Treatment Preparedness Coalition Eastern Europe and Central Asia. Analysis of Procurement of ARV Drugs in the Russian Federation in 2021; ITPC-EECA: Kyrgyzstan, Ukraine, 2022; Available online: https://itpc-eeca.org/wp-content/uploads/2022/07/otchet_po_monitoringu_goszakupok_arvp_vich_2021_god_pdf.pdf (accessed on 1 December 2022). (In Russian)
- Federal Russian AIDS Center. Report 2006. Available online: http://www.hivrussia.info/wp-content/uploads/2019/01/Byulleten-30-VICH-infektsiya-2006-g.pdf (accessed on 1 December 2022). (In Russian).
- Heath, K.; Levi, J.; Hill, A. The Joint United Nations Programme on HIV/AIDS 95-95-95 targets: Worldwide clinical and cost benefits of generic manufacture. AIDS 2021, 35 (Suppl. S2), 197–203. [Google Scholar] [CrossRef] [PubMed]
- Clutter, D.S.; Jordan, M.R.; Bertagnolio, S.; Shafer, R.W. HIV-1 drug resistance and resistance testing. Infect. Genet. Evol. 2016, 46, 292–307. [Google Scholar] [CrossRef]
- Standard of Primary Health Care for Adults with HIV Infection. Available online: http://rushiv.ru/wp-content/uploads/2022/07/0001202207260029.pdf (accessed on 1 December 2022). (In Russian).
- International Treatment Preparedness Coalition Eastern Europe and Central Asia. Analysis of Procurement of Diagnostics for HIV Treatment in Russia in 2020–2021; ITPC-EECA: Kyrgyzstan, Ukraine, 2022; Available online: https://itpc-eeca.org/wp-content/uploads/2022/07/monitoring-testov-vich-2020-21-gg-1.pdf (accessed on 1 December 2022). (In Russian)
- Pokrovskaya, A.; Kireev, D.; Emerole, K.; Kirichenko, A.; Pokrovsky, V. Pilot model of the HIV drug resistance testing coverage cascade. In Proceedings of the HIV Glasgow Conference, Glasgow, UK, 23–26 October 2022; pp. 191–192. [Google Scholar]
- Bennett, D.E.; Camacho, R.J.; Otelea, D.; Kuritzkes, D.R.; Fleury, H.; Kiuchi, M.; Heneine, W.; Kantor, R.; Jordan, M.R.; Schapiro, J.M.; et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE 2009, 4, e4724. [Google Scholar] [CrossRef] [PubMed]
- Nosik, M.N.; Ryzhov, K.A.; Kravchenko, A.V.; Sevostyanihin, S.E.; Kuimova, U.A.; Potapova, A.B.; Sobkin, A.L. Analysis of prevalence of HIV-1 primary resistance to antiretroviral drugs in the territory of Moscow and Moscow region. J. Microbiol. Epid. Immun. 2019, 96, 27–31. (In Russian) [Google Scholar] [CrossRef]
- Antonova, A.A.; Tumanov, A.S.; Lebedev, A.V.; Kozyennova, E.V.; Glinkina, L.N.; Kulagin, V.V.; Shemshura, A.B.; Lebedev, P.V.; Khoteleva, L.V.; Bobkova, M.R. Genetic profile and characteristics of HIV-1 drug resistance mutation in the Krasnodar region over the 2014 to 2019. HIV Inf. Immunosup. Disord. 2022, 14, 20–30. (In Russian) [Google Scholar] [CrossRef]
- Kirichenko, A.A.; Kireev, D.E.; Lopatukhin, A.E.; Murzakova, A.V.; Lapovok, I.A.; Ladnaya, N.N.; Pokrovsky, V.V. The level and structure of HIV-1 drug resistance among patients with no experience of taking antiretroviral drugs since the start of antiretroviral therapy in the Russian Federation. HIV Inf. Immunosup. Disord. 2019, 11, 75–83. (In Russian) [Google Scholar] [CrossRef]
- Kirichenko, A.; Kireev, D.; Lopatukhin, A.; Murzakova, A.; Lapovok, I.; Saleeva, D.; Ladnaya, N.; Gadirova, A.; Ibrahimova, S.; Safarova, A.; et al. Prevalence of HIV-1 drug resistance in Eastern European and Central Asian countries. PLoS ONE 2022, 17, e0257731. [Google Scholar] [CrossRef]
- Kirichenko, A.; Lapovok, I.; Baryshev, P.; van de Vijver, D.A.M.C.; van Kampen, J.J.A.; Boucher, C.A.B.; Paraskevis, D.; Kireev, D. Genetic Features of HIV-1 Integrase Sub-Subtype A6 Predominant in Russia and Predicted Susceptibility to INSTIs. Viruses 2020, 12, 838. [Google Scholar] [CrossRef]
- Decree of the Chief State Sanitary Doctor of the Russian Federation of January 28, 2021 N 4 “On Approval of Sanitary Rules and Norms SanPiN 3.3686-21 “Sanitary and Epidemiological Requirements for the Prevention of Infectious Diseases””. Available online: https://www.rospotrebnadzor.ru/files/news/SP_infections_compressed.pdf (accessed on 1 December 2022). (In Russian).
- Stanford HIV Data Base. Available online: http://hivdb.stanford.edu (accessed on 1 December 2022).
- The Joint United Nations Programme on HIV/AIDS. Understanding Fast-Track: Accelerating Action to End the AIDS Epidemic by 2030; UNAIDS: Geneva, Switzerland, 2014; Available online: https://www.unaids.org/en/resources/documents/2014/JC2686_WAD2014report (accessed on 1 December 2022).
- World Health Organization. HIV Drug Resistance Report; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241512831 (accessed on 1 December 2022).
- World Health Organization. HIV Drug Resistance Surveillance Guidance—2015 Update; WHO: Geneva, Switzerland, 2016; Available online: https://apps.who.int/iris/handle/10665/204471 (accessed on 1 December 2022).
- Wittkop, L.; Günthard, H.F.; de Wolf, F.; Dunn, D.; Cozzi-Lepri, A.; de Luca, A.; Kücherer, C.; Obel, N.; von Wyl, V.; Masquelier, B.; et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect. Dis. 2011, 11, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Hamers, R.L.; Schuurman, R.; Sigaloff, K.C.; Wallis, C.L.; Kityo, C.; Siwale, M.; Mandaliya, K.; Ive, P.; Botes, M.E.; Wellington, M.; et al. Effect of pretreatment HIV-1 drug resistance on immunological, virological, and drug-resistance outcomes of first-line antiretroviral treatment in sub-Saharan Africa: A multicentre cohort study. Lancet Infect Dis. 2012, 12, 307–317. [Google Scholar] [CrossRef]
- Phillips, A.N.; Stover, J.; Cambiano, V.; Nakagawa, F.; Jordan, M.R.; Pillay, D.; Doherty, M.; Revill, P.; Bertagnolio, S. Impact of HIV drug resistance on HIV/AIDS-associated mortality, new infections, and antiretroviral therapy program costs in sub-Saharan Africa. J. Infect. Dis. 2017, 215, 1362–1365. [Google Scholar] [CrossRef]
- Hofstra, L.M.; Sauvageot, N.; Albert, J.; Alexiev, I.; Garcia, F.; Struck, D.; Van de Vijver, D.A.M.C.; Åsjö, B.; Beshkov, D.; Coughlan, S.; et al. Transmission of HIV Drug Resistance and the Predicted Effect on Current First-line Regimens in Europe. Clin. Infect. Dis. 2016, 62, 655–663. [Google Scholar] [CrossRef]
- Cambiano, V.; Bertagnolio, S.; Jordan, M.R.; Lundgren, J.D.; Phillips, A. Transmission of drug resistant HIV and its potential impact on mortality and treatment outcomes in resource-limited settings. J. Infect. Dis. 2013, 207 (Suppl. S2), S57–S62. [Google Scholar] [CrossRef] [PubMed]
- Bobkov, A.; Kazennova, E.; Selimova, L.; Bobkova, M.; Khanina, T.; Ladnaya, N.; Kravchenko, A.; Pokrovsky, V.; Cheingsong-Popov, R.; Weber, J. A sudden epidemic of HIV type 1 among injecting drug users in the former Soviet Union: Identification of subtype A, subtype B, and novel gagA/envB recombinants. AIDS Res. Hum. Retrovir. 1998, 14, 669–676. [Google Scholar] [CrossRef]
- Foley, B.T.; Leitner, T.; Paraskevis, D.; Peeters, M. Primate immunodeficiency virus classification and nomenclature: Review. Infect. Genet. Evol. 2016, 46, 150–158. [Google Scholar] [CrossRef]
- Novitsky, V.A.; Montano, M.A.; Essex, M. Molecular epidemiology of an HIV-1 subtype A subcluster among injection drug users in the Southern Ukraine. AIDS Res. Hum. Retrovir. 1998, 14, 1079–1085. [Google Scholar] [CrossRef]
- Van de Klundert, M.A.A.; Antonova, A.; Di Teodoro, G.; Ceña Diez, R.; Chkhartishvili, N.; Heger, E.; Kuznetsova, A.; Lebedev, A.; Narayanan, A.; Ozhmegova, E.; et al. Molecular Epidemiology of HIV-1 in Eastern Europe and Russia. Viruses 2022, 14, 2099. [Google Scholar] [CrossRef]
- Kostaki, E.G.; Karamitros, T.; Bobkova, M.; Oikonomopoulou, M.; Magiorkinis, G.; Garcia, F.; Hatzakis, A.; Paraskevis, D. Spatiotemporal Characteristics of the HIV-1 CRF02_AG/CRF63_02A1 Epidemic in Russia and Central Asia. AIDS Res. Hum. Retrovir. 2018, 34, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Baryshev, P.B.; Bogachev, V.V.; Gashnikova, N.M. HIV-1 genetic diversity in Russia: CRF63_02A1, a new HIV type 1 genetic variant spreading in Siberia. AIDS Res. Hum. Retrovir. 2014, 30, 592–597. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Developing a Reporting System for the Surveillance of HIV Drug Resistance in Europe; ECDC: Stockholm, Sweden, 2019; Available online: https://www.ecdc.europa.eu/en/publications-data/developing-reporting-system-surveillance-hiv-drug-resistance-europe (accessed on 11 April 2023).
- Pasechnik, O.A.; Blokh, A.I. The prevalence of HIV recombinant forms in Russia and countries of the CIS: Systematic review and meta-analysis. Rus. J. Infect. Immun. 2018, 8, 127–138. (In Russian) [Google Scholar] [CrossRef]
- Moskaleychik, F.F.; Laga, V.Y.; Delgado, E.; Vega, Y.; Fernandez-Garcia, A.; Perez-Alvarez, L.; Kornilaeva, G.V.; Pronin, A.Y.; Zhernov, Y.V.; Thomson, M.M.; et al. Rapid spread of the HIV-1 circular recombinant CRF02-AG in Russia and neighboring countries. Vopr. Virusol. 2015, 60, 14–19. (In Russian) [Google Scholar] [PubMed]
- Little, S.J.; Frost, S.D.; Wong, J.K.; Smith, D.M.; Pond, S.L.; Ignacio, C.C.; Parkin, N.T.; Petropoulos, C.J.; Richman, D.D. Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J. Virol. 2008, 82, 5510–5518. [Google Scholar] [CrossRef]
- Xu, H.T.; Colby-Germinario, S.P.; Asahchop, E.L.; Oliveira, M.; McCallum, M.; Schader, S.M.; Han, Y.; Quan, Y.; Sarafianos, S.G.; Wainberg, M.A. Effect of mutations at position E138 in HIV-1 reverse transcriptase and their interactions with the M184I mutation on defining patterns of resistance to nonnucleoside reverse transcriptase inhibitors rilpivirine and etravirine. Antimicrob. Agents Chemother. 2013, 57, 3100–3109. [Google Scholar] [CrossRef]
- Picchio, G.R.; Rimsky, L.T.; Van Eygen, V.; Haddad, M.; Napolitano, L.A.; Vingerhoets, J. Prevalence in the USA of rilpivirine resistance-associated mutations in clinical samples and effects on phenotypic susceptibility to rilpivirine and etravirine. Antivir. Ther. 2014, 19, 819–823. [Google Scholar] [CrossRef]
- Porter, D.P.; Toma, J.; Tan, Y.; Solberg, O.; Cai, S.; Kulkarni, R.; Andreatta, K.; Lie, Y.; Chuck, S.K.; Palella, F.; et al. Clinical Outcomes of Virologically-Suppressed Patients with Pre-existing HIV-1 Drug Resistance Mutations Switching to Rilpivirine/Emtricitabine/Tenofovir Disoproxil Fumarate in the SPIRIT Study. HIV Clin. Trials 2016, 17, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Lebedev, A.; Gromov, K.; Kazennova, E.; Zazzi, M.; Incardona, F.; Sönnerborg, A.; Bobkova, M. Pre-existing singleton E138A mutations in the reverse transcriptase gene do not affect the efficacy of first-line antiretroviral therapy regimens using rilpivirine in human immunodeficiency virus-infected patients. Clin. Case Rep. 2022, 10, e05373. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on HIV Drug Resistance 2017–2021; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/978-92-4-151284-8 (accessed on 1 December 2022).
- World Health Organization. Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241550055 (accessed on 1 December 2022).
- Miranda, M.N.S.; Pingarilho, M.; Pimentel, V.; Martins, M.D.R.O.; Kaiser, R.; Seguin-Devaux, C.; Paredes, R.; Zazzi, M.; Incardona, F.; Abecasis, A.B. Trends of Transmitted and Acquired Drug Resistance in Europe From 1981 to 2019: A Comparison Between the Populations of Late Presenters and Non-late Presenters. Front. Microbiol. 2022, 13, 846943. [Google Scholar] [CrossRef] [PubMed]




| Characteristic | Total (n = 4481) |
|---|---|
| Age (years), median (IQR) | 33 (27–39) |
| Sex, n (%) | |
| Male | 2510 (56.0) |
| Female | 1847 (41.2) |
| Unknown | 124 (2.8) |
| Transmission risk group, n (%) | |
| Sexual (without clarification) | 163 (3.6) |
| Heterosexual | 1951 (43.5) |
| MSM | 306 (6.8) |
| IDU | 967 (21.6) |
| Mother-to-child | 18 (0.4) |
| Outbreak | 23 (0.5) |
| Unknown | 1053 (23.5) |
| Viral load (log10 copies/mL), median (IQR) | 4.6 (4.0–5.2) |
| CD4+ T-cell count (cells/mm3), median (IQR) | 401 (264–559.5) |
| FD, n (%) | |
| Central | 1661 (37.1) |
| Northwestern | 297 (6.6) |
| Southern | 666 (14.9) |
| North Caucasian | 18 (0.4) |
| Volga | 617 (13.8) |
| Ural | 432 (9.6) |
| Siberian | 353 (7.9) |
| Far Eastern | 217 (4.8) |
| Unknown | 220 (4.9) |
| Year of diagnosis, n (%) | |
| 1997–1999 | 61 (1.4) |
| 2000 | 48 (1.1) |
| 2001 | 79 (1.8) |
| 2002 | 43 (1.0) |
| 2003 | 32 (0.7) |
| 2004 | 46 (1.0) |
| 2005 | 50 (1.1) |
| 2006 | 70 (1.6) |
| 2007 | 361 (8.1) |
| 2008 | 201 (4.5) |
| 2009 | 155 (3.5) |
| 2010 | 125 (2.8) |
| 2011 | 144 (3.2) |
| 2012 | 176 (3.9) |
| 2013 | 183 (4.1) |
| 2014 | 340 (7.6) |
| 2015 | 416 (9.3) |
| 2016 | 495 (11.0) |
| 2017 | 384 (8.6) |
| 2018 | 345 (7.7) |
| 2019 | 337 (7.5) |
| 2020 | 107 (2.4) |
| 2021 | 145 (3.2) |
| 2022 | 78 (1.7) |
| Unknown | 60 (1.3) |
| Sampling year, n (%) | |
| 2006 | 9 (0.2) |
| 2007 | 254 (5.7) |
| 2008 | 295 (6.6) |
| 2009 | 50 (1.1) |
| 2010 | 29 (0.6) |
| 2011 | 67 (1.5) |
| 2012 | 253 (5.6) |
| 2013 | 162 (3.6) |
| 2014 | 251 (5.6) |
| 2015 | 409 (9.1) |
| 2016 | 469 (10.5) |
| 2017 | 433 (9.7) |
| 2018 | 681 (15.2) |
| 2019 | 508 (11.3) |
| 2020 | 246 (5.5) |
| 2021 | 245 (5.5) |
| 2022 | 120 (2.7) |
| Genetic Variant, n (%) | |||||||
|---|---|---|---|---|---|---|---|
| Transmission Risk Group | A6 | CRF63_02A6 | B | CRF03_A6B | CRF02_AG | G | Others * |
| Sexual (without clarification) | 117 (71.8) | 13 (8.0) | 25 (15.3) | 2 (1.2) | 2 (1.2) | 4 (2.5) | 0 |
| Heterosexual | 1648 (84.5) | 130 (6.7) | 90 (4.6) | 31 (1.6) | 26 (1.3) | 21 (1.1) | 5 (0.3) |
| MSM | 156 (51.0) | 5 (1.6) | 103 (33.7) | 1 (0.3) | 11 (3.6) | 17 (5.6) | 13 (4.2) |
| IDU | 789 (81.6) | 121 (12.5) | 9 (0.9) | 21 (2.2) | 16 (1.7) | 2 (0.2) | 9 (0.9) |
| Mother-to-child | 17 (94.4) | 1 (5.6) | 0 | 0 | 0 | 0 | 0 |
| Outbreak | 16 (69.6) | 5 (21.7) | 0 | 0 | 0 | 1 (4.3) | 1 (4.3) |
| Unknown | 771 (73.1) | 139 (13.2) | 68 (6.5) | 18 (1.7) | 15 (1.4) | 13 (1.2) | 29 (2.8) |
| All | 3514 (78.4) | 414 (9.2) | 295 (6.6) | 73 (1.6) | 70 (1.6) | 58 (1.3) | 57 (1.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirichenko, A.; Kireev, D.; Lapovok, I.; Shlykova, A.; Lopatukhin, A.; Pokrovskaya, A.; Bobkova, M.; Antonova, A.; Kuznetsova, A.; Ozhmegova, E.; et al. HIV-1 Drug Resistance among Treatment-Naïve Patients in Russia: Analysis of the National Database, 2006–2022. Viruses 2023, 15, 991. https://doi.org/10.3390/v15040991
Kirichenko A, Kireev D, Lapovok I, Shlykova A, Lopatukhin A, Pokrovskaya A, Bobkova M, Antonova A, Kuznetsova A, Ozhmegova E, et al. HIV-1 Drug Resistance among Treatment-Naïve Patients in Russia: Analysis of the National Database, 2006–2022. Viruses. 2023; 15(4):991. https://doi.org/10.3390/v15040991
Chicago/Turabian StyleKirichenko, Alina, Dmitry Kireev, Ilya Lapovok, Anastasia Shlykova, Alexey Lopatukhin, Anastasia Pokrovskaya, Marina Bobkova, Anastasiia Antonova, Anna Kuznetsova, Ekaterina Ozhmegova, and et al. 2023. "HIV-1 Drug Resistance among Treatment-Naïve Patients in Russia: Analysis of the National Database, 2006–2022" Viruses 15, no. 4: 991. https://doi.org/10.3390/v15040991
APA StyleKirichenko, A., Kireev, D., Lapovok, I., Shlykova, A., Lopatukhin, A., Pokrovskaya, A., Bobkova, M., Antonova, A., Kuznetsova, A., Ozhmegova, E., Shtrek, S., Sannikov, A., Zaytseva, N., Peksheva, O., Piterskiy, M., Semenov, A., Turbina, G., Filoniuk, N., Shemshura, A., ... Akimkin, V. (2023). HIV-1 Drug Resistance among Treatment-Naïve Patients in Russia: Analysis of the National Database, 2006–2022. Viruses, 15(4), 991. https://doi.org/10.3390/v15040991






