False-Positive Screening and Confirmatory HIV Diagnostic Test in a Patient with Cured SARS-CoV-2 Infection Is Not Mediated by Env/Spike Cross-Reactive Antibodies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples and Data
2.2. Certified Diagnostic Tests
2.3. Protein Production
2.4. Antigen-Specific Ab Depletion
2.5. Enzyme-Linked Immunosorbent Assays (ELISAs)
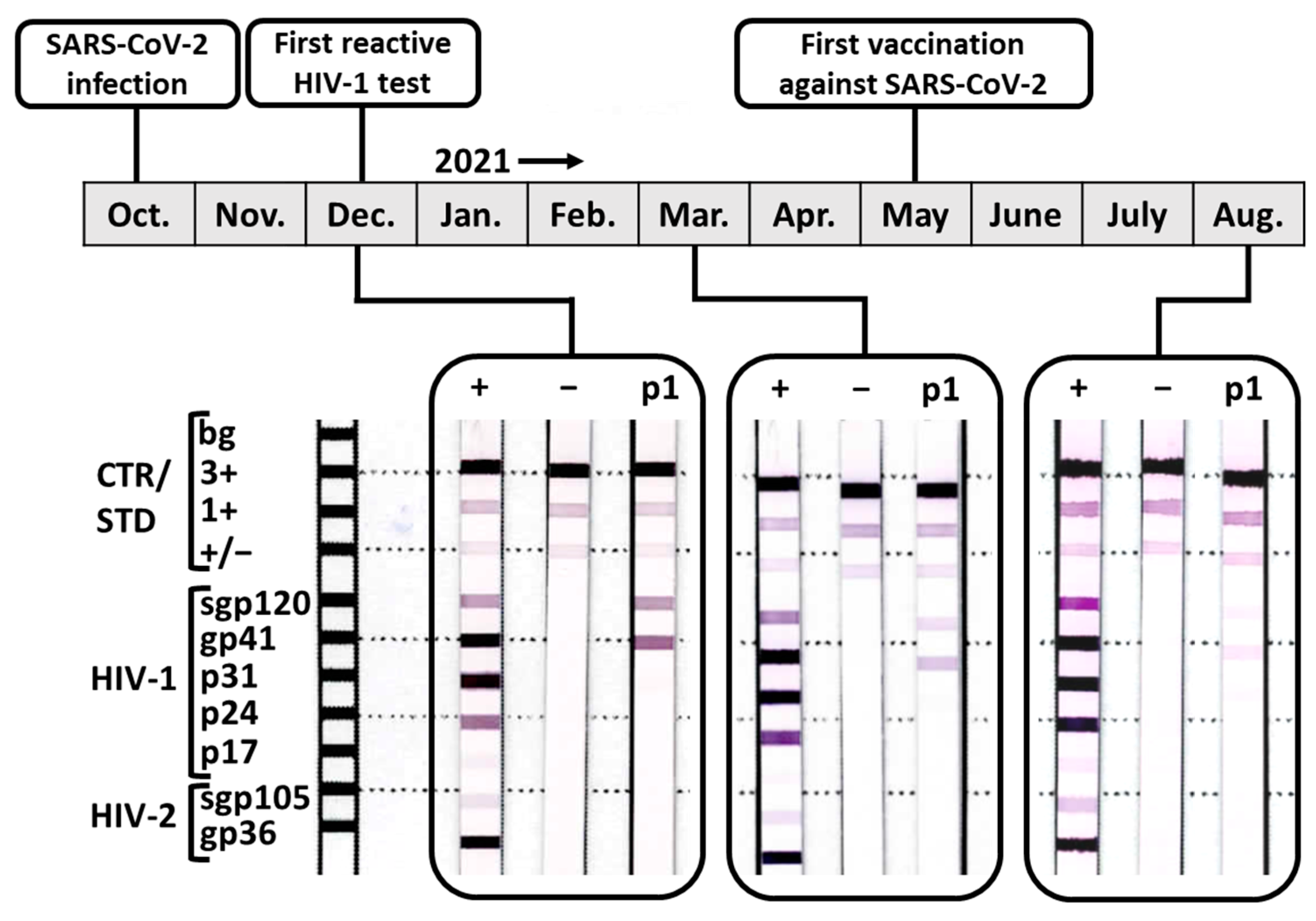
3. Case Description
4. Results
4.1. The HIV Diagnostic Test Interference after Acute SARS-CoV-2 Infection Resolved over Time
4.2. The Assessment of Common Types of Interferences Could Not Disprove a Potential Relationship between SARS-CoV-2 Infection and Subsequent False-Positive HIV Diagnostic Tests
4.3. Cross-Reactive Abs to SARS-CoV-2 Spike and HIV-1 gp120 Did Not Cause HIV Diagnostic Test Interference
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- German-Austrian Guideline for Antiretroviral Treatments of HIV-1 Infections, 9th ed.; German AIDS-society (DAIG) and Austrian AIDS-Society (OEAG); DAIG: Hamburg, Germany, 2020.
- Haleyur Giri Setty, M.K.; Lee, S.; Lathrop, J.; Hewlett, I.K. Biotin Interference in Point of Care HIV Immunoassay. Biores. Open Access 2020, 9, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Güler, E.; Arıkan, A.; Abobakr, M.; Sayan, M.; Süer, K.; Şanlıdağ, T. Positive Anti-HIV ELISA Results in Pregnancy: Is It Reliable? Infect. Dis. Obstet. Gynecol. 2022, 2022, 1157793. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Sayedy, N.; Iqbal, J. A False-Positive HIV Test: Severe Lupus Flare in Disguise. Cureus 2022, 14, e24349. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Dirweesh, A.; Amodu, A.; Nadeem, M.; Wallach, S.L. A Case of False-Positive HIV Test in a Patient with Newly Diagnosed Hodgkin Lymphoma and Literature Review. Cureus 2020, 12, e10884. [Google Scholar] [CrossRef]
- Erickson, C.P.; McNiff, T.; Klausner, J.D. Influenza vaccination and false positive HIV results. N. Engl. J. Med. 2006, 354, 1422–1423. [Google Scholar] [CrossRef]
- Araujo, P.R.; Albertoni, G.; Arnoni, C.; Almeida, K.; Ribeiro, J.; Rizzo, S.R.; Carvalho, F.O.; Baretto, J.A.; Mangueira, C. Rubella vaccination and transitory false-positive test results for human immunodeficiency virus Type 1 in blood donors. Transfusion 2009, 49, 2516–2517. [Google Scholar] [CrossRef]
- Monos, D.S.; Frank, T.S.; Senior, M.B.; Gadson, M.; Zmijewski, C.M.; Prystowsky, M.B.; Goodman, D.B. Delineation of false-positive HIV antibody response in patients with renal failure and history of multiple transfusions. Transfusion 1989, 29, 119–123. [Google Scholar] [CrossRef]
- Ladizinski, B.; Sankey, C. Acute Epstein-Barr virus infection and human immunodeficiency virus antibody cross-reactivity. Am. J. Med. 2014, 127, e9–e10. [Google Scholar] [CrossRef]
- Huffman, C.A.; Barts, R.N.; Berry-Cabán, C.S.; Choi, Y.S. Report of a False Positive Rapid HIV Test Due to Hepatitis A in a U.S. Army Soldier. J. Clin. Res. HIV AIDS Prev. 2014, 2, 13–17. [Google Scholar] [CrossRef]
- Wai, C.T.; Tambyah, P.A. False-positive HIV-1 ELISA in patients with hepatitis B. Am. J. Med. 2002, 112, 737. [Google Scholar] [CrossRef]
- Baskar, P.V.; Collins, G.D.; Dorsey-Cooper, B.A.; Pyle, R.S.; Nagel, J.E.; Dwyer, D.; Dunston, G.; Johnson, C.E.; Kendig, N.; Israel, E.; et al. Serum antibodies to HIV-1 are produced post-measles virus infection: Evidence for cross-reactivity with HLA. Clin. Exp. Immunol. 1998, 111, 251–256. [Google Scholar] [CrossRef]
- Watt, G.; Chanbancherd, P.; Brown, A.E. Human immunodeficiency virus type 1 test results in patients with malaria and dengue infections. Clin. Infect. Dis. 2000, 30, 819. [Google Scholar] [CrossRef]
- Shallal, A.; Gudipati, S.; Peterson, E.; Cook, B.; Tibbetts, R.; Markowitz, N. Increase in false positive 4th generation HIV tests in patients with COVID-19 disease. In Proceedings of the CROI 2022, Virtual, 13–16 February 2022. [Google Scholar]
- Hayat, L.; Beker, C.; Karaca, A.; Hafizoglu, N.; Kinik, K.; Yilmaz, F. Antibody False Positivity Among COVID-19 Convalescent Plasma Donors: A Comparative Study from the Turkish Red Crescent Blood Center. Med. Bull. Haseki 2021, 59, 353–357. [Google Scholar] [CrossRef]
- Alfie, L.G.; Longueira, Y.S.; Pippo, M.; Cruces, L.; Quiroga, M.F.; Turk, G.; Laufer, N. Increased risk of false positive HIV ELISA results after COVID-19. AIDS 2023, 37, 947–950. [Google Scholar] [CrossRef]
- Salih, R.Q.; Salih, G.A.; Abdulla, B.A.; Ahmed, A.D.; Mohammed, H.R.; Kakamad, F.H.; Salih, A.M. False-positive HIV in a patient with SARS-CoV-2 infection; a case report. Ann. Med. Surg. 2021, 71, 103027. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, P.; Malhotra, R.; Mathur, P. False-Positive Human Immunodeficiency Virus Reactivity in COVID Patients: A Word of Caution. J. Glob. Infect. Dis. 2022, 14, 43–44. [Google Scholar] [CrossRef]
- Cipitelli, M.D.C.; Dornelas-Ribeiro, M.; Santos, C.G.M. Letter to the editor: Additional evidence to support a cross-reactivity of SARS-CoV-2 with HIV chemiluminescent assay. J. Clin. Pathol. 2021, 75, 503–504. [Google Scholar] [CrossRef]
- Tan, S.S.; Chew, K.L.; Saw, S.; Jureen, R.; Sethi, S. Cross-reactivity of SARS-CoV-2 with HIV chemiluminescent assay leading to false-positive results. J. Clin. Pathol. 2021, 74, 614. [Google Scholar] [CrossRef]
- Papamanoli, A.; Psevdos, G. False-positive HIV screening test in a patient with pulmonary embolism because of severe acute respiratory syndrome coronavirus 2 infection. AIDS 2021, 35, 1521–1522. [Google Scholar] [CrossRef]
- Hakobyan, N.; Yadav, R.; Abaza, K.; Friedman, A. False-Positive Human Immunodeficiency Virus Results in COVID-19 Patients. Cureus 2023, 15, e34096. [Google Scholar] [CrossRef]
- Miller, M.E.; Cevigney, R.; Ayyash, M.; Shaman, M.; Kole, M. Higher rates of false-positive HIV antigen/antibody screens during the COVID-19 pandemic: Implications for pregnant patients. Am. J. Obstet. Gynecol. 2023, 228, S690. [Google Scholar] [CrossRef]
- Yamaniha, K.; Kinjo, T.; Akamine, M.; Setoguchi, M.; Tateyama, M.; Fujita, J. False-positive for SARS-CoV-2 antigen test in a man with acute HIV infection. J. Infect. Chemother. 2021, 27, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Duerr, R.; Crosse, K.M.; Valero-Jimenez, A.M.; Dittmann, M. SARS-CoV-2 Portrayed against HIV: Contrary Viral Strategies in Similar Disguise. Microorganisms 2021, 9, 1389. [Google Scholar] [CrossRef] [PubMed]
- Perween, R.; PraveenKumar, M.; Shrivastava, T.; Parray, H.A.; Singh, V.; Singh, S.; Chiranjivi, A.; Jakhar, K.; Sonar, S.; Tiwari, M.; et al. The SARS CoV-2 spike directed non-neutralizing polyclonal antibodies cross-react with Human immunodeficiency virus (HIV-1) gp41. Int. Immunopharmacol. 2021, 101, 108187. [Google Scholar] [CrossRef]
- Mishra, N.; Kumar, S.; Singh, S.; Bansal, T.; Jain, N.; Saluja, S.; Kumar, R.; Bhattacharyya, S.; Palanichamy, J.K.; Mir, R.A.; et al. Cross-neutralization of SARS-CoV-2 by HIV-1 specific broadly neutralizing antibodies and polyclonal plasma. PLoS Pathog. 2021, 17, e1009958. [Google Scholar] [CrossRef]
- Mannar, D.; Leopold, K.; Subramaniam, S. Glycan reactive anti-HIV-1 antibodies bind the SARS-CoV-2 spike protein but do not block viral entry. Sci. Rep. 2021, 11, 12448. [Google Scholar] [CrossRef]
- Acharya, P.; Williams, W.; Henderson, R.; Janowska, K.; Manne, K.; Parks, R.; Deyton, M.; Sprenz, J.; Stalls, V.; Kopp, M.; et al. A glycan cluster on the SARS-CoV-2 spike ectodomain is recognized by Fab-dimerized glycan-reactive antibodies. bioRxiv 2020. [Google Scholar] [CrossRef]
- Keating, S.M.; Mizrahi, R.A.; Adams, M.S.; Asensio, M.A.; Benzie, E.; Carter, K.P.; Chiang, Y.; Edgar, R.C.; Gautam, B.K.; Gras, A.; et al. Generation of recombinant hyperimmune globulins from diverse B-cell repertoires. Nat. Biotechnol. 2021, 39, 989–999. [Google Scholar] [CrossRef]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.I.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2021, 15, e0244126. [Google Scholar] [CrossRef]
- Marcotte, H.; Piralla, A.; Zuo, F.; Du, L.; Cassaniti, I.; Wan, H.; Kumagai-Braesh, M.; Andrell, J.; Percivalle, E.; Sammartino, J.C.; et al. Immunity to SARS-CoV-2 up to 15 months after infection. iScience 2022, 25, 103743. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. Cell Rep. Med. 2021, 2, 100354. [Google Scholar] [CrossRef]
- Greipp, P.R. Hypergammaglobulinemia. In Encyclopedia of Immunology, 2nd ed.; Delves, P.J., Ed.; Elsevier: Oxford, UK, 1998; pp. 1161–1166. [Google Scholar]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rojas, M.; Rodriguez, Y.; Zapata, E.; Ramirez-Santana, C.; Anaya, J.M. Persistent Autoimmune Activation and Proinflammatory State in Post-Coronavirus Disease 2019 Syndrome. J. Infect. Dis. 2022, 225, 2155–2162. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse functional autoantibodies in patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Anaya, J.M.; Monsalve, D.M.; Rojas, M.; Rodriguez, Y.; Montoya-Garcia, N.; Mancera-Navarro, L.M.; Villadiego-Santana, A.M.; Rodriguez-Leguizamon, G.; Acosta-Ampudia, Y.; Ramirez-Santana, C. Latent rheumatic, thyroid and phospholipid autoimmunity in hospitalized patients with COVID-19. J. Transl. Autoimmun. 2021, 4, 100091. [Google Scholar] [CrossRef]
- Li, Y.C.; Yang, F.; Ji, X.Y.; Fang, Z.J.; Liu, J.; Wang, Y. False human immunodeficiency virus test results associated with rheumatoid factors in rheumatoid arthritis. Chin. Med. Sci. J. 2014, 29, 103–106. [Google Scholar] [CrossRef]
- Gehin, J.E.; Klaasen, R.A.; Norli, E.S.; Warren, D.J.; Syversen, S.W.; Goll, G.L.; Bjoro, T.; Kvien, T.K.; Mjaavatten, M.D.; Bolstad, N. Rheumatoid factor and falsely elevated results in commercial immunoassays: Data from an early arthritis cohort. Rheumatol. Int. 2021, 41, 1657–1665. [Google Scholar] [CrossRef]


| Characteristics | Total | Female | Male |
| Number of patients, n (%) | 65 (100) | 42 (64.6) | 23 (35.4) |
| Age in years, median (IQR) | 51 (19) | 50.5 (18) | 52 (20.5) |
| Time post SARS-CoV-2 diagnosis, median months (IQR) | 9 (5) * | 9 (4.3) | 9 (4.8) |
| Vaccination status | |||
| Nonvaccinated, n (%) | 4 (6.2) | 2 (4.8) | 2 (8.7) |
| Vaccinated, n (%) | 53 (81.5) | 32 (76.1) | 21 (91.3) |
| Vaccination status unknown, n (%) | 8 (12.3) | 8 (19) | 0 (0) |
| Laboratory investigation | Total | Female | Male |
| SARS-CoV-2 spike-specific IgG Liaison SARS-CoV-2 TrimericS IgG assay (DiaSorin) | |||
| Positive, n (%) | 63 (96.9) | 40 (95.2) | 23 (100) |
| Negative, n (%) | 2 (3.1) | 2 (4.8) | 0 (0) |
| SARS-CoV-2 N protein-specific IgG SARS-CoV-2 IgG assay (Abbott) | |||
| Positive, n (%) | 27 (41.5) | 16 (38.1) | 11 (47.8) |
| Borderline, n (%) | 9 (13.8) | 7 (16.7) | 2 (8.7) |
| Negative, n (%) | 29 (44.6) | 19 (45.2) | 10 (43.5) |
| HIV-1/2 screening assay Elecsys HIV combi PT (Roche) | |||
| Positive, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Negative, n (%) | 65 (100) | 42 (100) | 23 (100) |
| Architect HIV Ag/Ab Combo test (Abbott) | |||
| Positive, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Negative, n (%) | 65 (100) | 42 (100) | 23 (100) |
| HIV-1/2 confirmatory immunoblot INNO-LIA HIV I/II Score (Fujirebio) | |||
| Positive, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Negative, n (%) | 65 (100) | 42 (100) | 23 (100) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsner, C.; Appeltrath, G.A.; Konik, M.; Parreuter, J.; Broecker-Preuss, M.; Krawczyk, A.; Esser, S.; Sammet, S.; Karsten, C.B. False-Positive Screening and Confirmatory HIV Diagnostic Test in a Patient with Cured SARS-CoV-2 Infection Is Not Mediated by Env/Spike Cross-Reactive Antibodies. Viruses 2023, 15, 1161. https://doi.org/10.3390/v15051161
Elsner C, Appeltrath GA, Konik M, Parreuter J, Broecker-Preuss M, Krawczyk A, Esser S, Sammet S, Karsten CB. False-Positive Screening and Confirmatory HIV Diagnostic Test in a Patient with Cured SARS-CoV-2 Infection Is Not Mediated by Env/Spike Cross-Reactive Antibodies. Viruses. 2023; 15(5):1161. https://doi.org/10.3390/v15051161
Chicago/Turabian StyleElsner, Carina, Gwenllian A. Appeltrath, Margarethe Konik, Janine Parreuter, Martina Broecker-Preuss, Adalbert Krawczyk, Stefan Esser, Stefanie Sammet, and Christina B. Karsten. 2023. "False-Positive Screening and Confirmatory HIV Diagnostic Test in a Patient with Cured SARS-CoV-2 Infection Is Not Mediated by Env/Spike Cross-Reactive Antibodies" Viruses 15, no. 5: 1161. https://doi.org/10.3390/v15051161
APA StyleElsner, C., Appeltrath, G. A., Konik, M., Parreuter, J., Broecker-Preuss, M., Krawczyk, A., Esser, S., Sammet, S., & Karsten, C. B. (2023). False-Positive Screening and Confirmatory HIV Diagnostic Test in a Patient with Cured SARS-CoV-2 Infection Is Not Mediated by Env/Spike Cross-Reactive Antibodies. Viruses, 15(5), 1161. https://doi.org/10.3390/v15051161








