Querying Recombination Junctions of Replication-Competent Adeno-Associated Viruses in Gene Therapy Vector Preparations with Single Molecule, Real-Time Sequencing
Abstract
1. Introduction
2. Materials and Methods
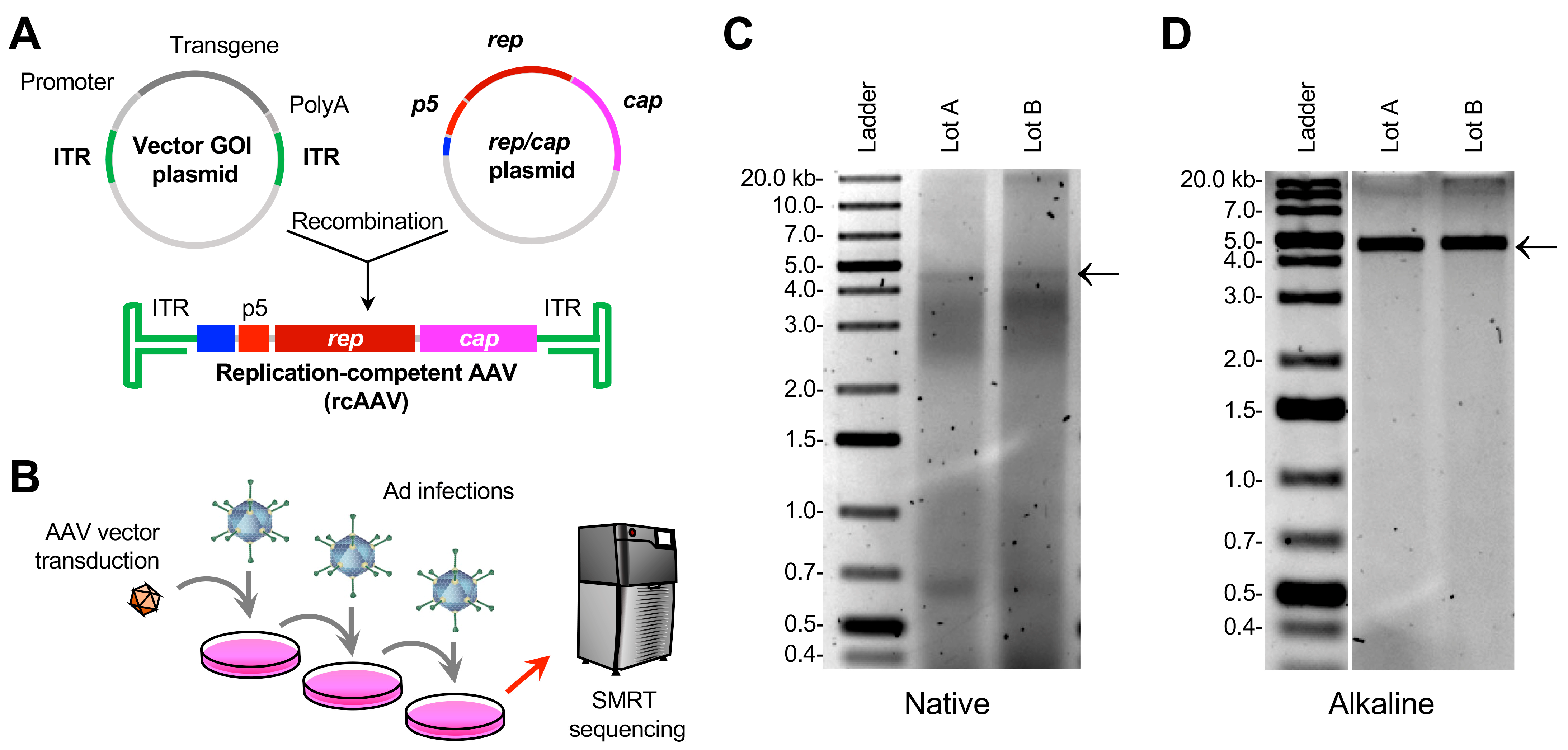
2.1. Plasmid and Vector Production
2.2. Propagation of rcAAV
2.3. Vector DNA Extraction and Agarose Gel Electrophoresis
2.4. SMRT Sequencing and AAV-GPseq
2.5. Bioinformatics
3. Results
3.1. Amplification of rcAAVs
3.2. Isolation of DNA from Crude Lysates of Cells Transduced with Expanded rcAAVs Yields Heterogeneous ~5-kb Genomes
3.3. Transgene-Related Sequences Were Rare in rcAAV-Enriched Lysates
3.4. Mapping Reads to a Reconstructed rcAAV Reference Revealed ITRs That Were Contiguous with rep and cap Sequences
3.5. Formation of rcAAVs Occur via Non-Specific Recombination Events at the 5′ and 3′ Ends of the rep/cap Genes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conroy, G. How gene therapy is emerging from its ‘dark age’. Nature 2022, 612, S24–S26. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Liu, S.; Ou, L. rAAV immunogenicity, toxicity, and durability in 255 clinical trials: A meta-analysis. Front. Immunol. 2022, 13, 1001263. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves first haemophilia B gene therapy. Nat. Rev. Drug Discov. 2023, 22, 7. [Google Scholar] [CrossRef]
- Penaud-Budloo, M.; Francois, A.; Clement, N.; Ayuso, E. Pharmacology of Recombinant Adeno-associated Virus Production. Mol. Ther. Methods Clin. Dev. 2018, 8, 166–180. [Google Scholar] [CrossRef]
- Wright, J.F. Product-Related Impurities in Clinical-Grade Recombinant AAV Vectors: Characterization and Risk Assessment. Biomedicines 2014, 2, 80–97. [Google Scholar] [CrossRef]
- Gombold, J.; Karakasidis, S.; Niksa, P.; Podczasy, J.; Neumann, K.; Richardson, J.; Sane, N.; Johnson-Leva, R.; Randolph, V.; Sadoff, J.; et al. Systematic evaluation of in vitro and in vivo adventitious virus assays for the detection of viral contamination of cell banks and biological products. Vaccine 2014, 32, 2916–2926. [Google Scholar] [CrossRef] [PubMed]
- Samulski, R.J.; Chang, L.S.; Shenk, T. Helper-free stocks of recombinant adeno-associated viruses: Normal integration does not require viral gene expression. J. Virol. 1989, 63, 3822–3828. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Khuntirat, B.; Qing, K.; Ponnazhagan, S.; Kube, D.M.; Zhou, S.; Dwarki, V.J.; Srivastava, A. Characterization of wild-type adeno-associated virus type 2-like particles generated during recombinant viral vector production and strategies for their elimination. J. Virol. 1998, 72, 5472–5480. [Google Scholar] [CrossRef]
- Allen, J.M.; Debelak, D.J.; Reynolds, T.C.; Miller, A.D. Identification and elimination of replication-competent adeno-associated virus (AAV) that can arise by nonhomologous recombination during AAV vector production. J. Virol. 1997, 71, 6816–6822. [Google Scholar] [CrossRef]
- Meier, A.F.; Fraefel, C.; Seyffert, M. The Interplay between Adeno-Associated Virus and its Helper Viruses. Viruses 2020, 12, 662. [Google Scholar] [CrossRef]
- Spengler, U.; Fischer, H.-P.; Caselmann, W.H. Liver Disease Associated with Viral Infections. In Zakim and Boyer’s Hepatology; Elsevier: Amsterdam, The Netherlands, 2012; pp. 629–643. [Google Scholar] [CrossRef]
- Lalazar, G.; Ilan, Y. Viral Diseases of the Liver. In Liver Immunology: Principles and Practice; Springer: Berlin/Heidelberg, Germany, 2013; pp. 159–171. [Google Scholar] [CrossRef]
- Gao, G.; Qu, G.; Burnham, M.S.; Huang, J.; Chirmule, N.; Joshi, B.; Yu, Q.C.; Marsh, J.A.; Conceicao, C.M.; Wilson, J.M. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 2000, 11, 2079–2091. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.A.; Lerch, T.F.; Hare, J.T.; Chapman, M.S. A pseudo-plaque method for infectious particle assay and clonal isolation of adeno-associated virus. J. Virol. Methods 2010, 170, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Tai, P.W.L.; Xie, J.; Fong, K.; Seetin, M.; Heiner, C.; Su, Q.; Weiand, M.; Wilmot, D.; Zapp, M.L.; Gao, G. Adeno-associated Virus Genome Population Sequencing Achieves Full Vector Genome Resolution and Reveals Human-Vector Chimeras. Mol. Ther. Methods Clin. Dev. 2018, 9, 130–141. [Google Scholar] [CrossRef]
- Tran, N.T.; Heiner, C.; Weber, K.; Weiand, M.; Wilmot, D.; Xie, J.; Wang, D.; Brown, A.; Manokaran, S.; Su, Q.; et al. AAV-Genome Population Sequencing of Vectors Packaging CRISPR Components Reveals Design-Influenced Heterogeneity. Mol. Ther. Methods Clin. Dev. 2020, 18, 639–651. [Google Scholar] [CrossRef]
- Tran, N.T.; Lecomte, E.; Saleun, S.; Namkung, S.; Robin, C.; Weber, K.; Devine, E.; Blouin, V.; Adjali, O.; Ayuso, E.; et al. Human and insect cell-produced rAAVs show differences in genome heterogeneity. Hum. Gene Ther. 2022, 33, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, M.; Terova, O. Downstream purification of adeno-associated virus for large-scale manufacturing of gene therapies. Cell Gene Ther. Insights 2020, 6, 955–963. [Google Scholar] [CrossRef]
- Joseph, S.; Russell, D. Molecular Cloning: A Laboratory Manual. In Alkaline Agarose Gel Electrohoresis; Green, M.R., Sambrook, J., Eds.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012; Volume 1, pp. 636–666. [Google Scholar]
- Afgan, E.; Sloggett, C.; Goonasekera, N.; Makunin, I.; Benson, D.; Crowe, M.; Gladman, S.; Kowsar, Y.; Pheasant, M.; Horst, R.; et al. Genomics Virtual Laboratory: A Practical Bioinformatics Workbench for the Cloud. PLoS ONE 2015, 10, e0140829. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, X.; Feng, Z.; Li, G.; Fisher, J.T.; Stewart, Z.A.; Engelhardt, J.F. Optimization of Recombinant Adeno-Associated Virus-Mediated Expression for Large Transgenes, Using a Synthetic Promoter and Tandem Array Enhancers. Hum. Gene Ther. 2015, 26, 334–346. [Google Scholar] [CrossRef]
- Excoffon, K.J.; Koerber, J.T.; Dickey, D.D.; Murtha, M.; Keshavjee, S.; Kaspar, B.K.; Zabner, J.; Schaffer, D.V. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc. Natl. Acad. Sci. USA 2009, 106, 3865–3870. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Tattersall, P. The autonomously replicating parvoviruses of vertebrates. Adv. Virus Res. 1987, 33, 91–174. [Google Scholar] [PubMed]
- Nguyen, G.N.; Everett, J.K.; Kafle, S.; Roche, A.M.; Raymond, H.E.; Leiby, J.; Wood, C.; Assenmacher, C.A.; Merricks, E.P.; Long, C.T.; et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 2021, 39, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Dalwadi, D.A.; Calabria, A.; Tiyaboonchai, A.; Posey, J.; Naugler, W.E.; Montini, E.; Grompe, M. AAV integration in human hepatocytes. Mol. Ther. 2021, 29, 2898–2909. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, D.E.; Bushman, F.D.; Chandler, R.J.; Crystal, R.G.; Davidson, B.L.; Dolmetsch, R.; Eggan, K.C.; Gao, G.; Gil-Farina, I.; Kay, M.A.; et al. Evaluating the state of the science for adeno-associated virus integration: An integrated perspective. Mol. Ther. 2022, 30, 2646–2663. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, P.; Yu, X.; Frabutt, D.A.; Lam, A.K.; Mulcrone, P.L.; Chrzanowski, M.; Firrman, J.; Pouchnik, D.; Sang, N.; et al. Subgenomic particles in rAAV vectors result from DNA lesion/break and non-homologous end joining of vector genomes. Mol. Ther. Nucleic Acids 2022, 29, 852–861. [Google Scholar] [CrossRef]



| Reference | Lot A | Lot B |
|---|---|---|
| Total reads | 1,839,036 | 6,499,874 |
| hg38 (human genome) | 1,415,770 (76.98%) | 5,393,393 (82.98%) |
| rep/cap plasmid | 230,787 (12.55%) | 217,501 (3.35%) |
| AAV-GOI | 103,613 (5.63%) | 31,127 (0.49%) |
| Lot A Forms | Sequence | Read Count (%) Total 3147 |
|---|---|---|
| 5′-end rep/cap |  | |
| A-5.1 |  | 3073 (97.65%) |
| A-5.2 |  | 23 (0.73%) |
| A-5.3 |  | 20 (0.64%) |
| A-5.4 |  | 11 (0.35%) |
| A-5.5 |  | 6 (0.19%) |
| A-5.6 |  | 6 (0.19%) |
| A-5.7 |  | 5 (0.16%) |
| Lot B forms | Read count (%) Total 1019 | |
| B-5.1 |  | 487 (47.79%) |
| B-5.2 |  | 395 (38.76%) |
| B-5.3 |  | 44 (4.32%) |
| B-5.4 |  | 21 (2.06%) |
| B-5.5 |  | 11 (1.08%) |
| B-5.6 |  | 9 (0.88%) |
| B-5.7 |  | 8 (0.79%) |
| B-5.8 |  | 7 (0.69%) |
| B-5.9 |  | 7 (0.69%) |
| B-5.10 |  | 3 (0.29%) |
| B-5.11 |  | 2 (0.20%) |
| 5′-ITR (green) Partial Ad ITR (blue) AAV2 p5 promoter (red) | ||
| Lot A Form | Sequence | Read Count (%) Total 3159 |
|---|---|---|
| 3′-end rep/cap |  | |
| A-3.1 |  | 3,136 (99.27%) |
| Lot B forms | Read count (%) Total 1058 | |
| B-3.1 |  | 947 (89.51%) |
| B-3.2 |  | 86 (8.13%) |
| B-3.3 |  | 8 (0.76%) |
| AAV2 3′ end (magenta) AAV2 p5 partial insert (red) 3’-ITR (green) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yip, M.; Chen, J.; Zhi, Y.; Tran, N.T.; Namkung, S.; Pastor, E.; Gao, G.; Tai, P.W.L. Querying Recombination Junctions of Replication-Competent Adeno-Associated Viruses in Gene Therapy Vector Preparations with Single Molecule, Real-Time Sequencing. Viruses 2023, 15, 1228. https://doi.org/10.3390/v15061228
Yip M, Chen J, Zhi Y, Tran NT, Namkung S, Pastor E, Gao G, Tai PWL. Querying Recombination Junctions of Replication-Competent Adeno-Associated Viruses in Gene Therapy Vector Preparations with Single Molecule, Real-Time Sequencing. Viruses. 2023; 15(6):1228. https://doi.org/10.3390/v15061228
Chicago/Turabian StyleYip, Mitchell, Jing Chen, Yan Zhi, Ngoc Tam Tran, Suk Namkung, Eric Pastor, Guangping Gao, and Phillip W. L. Tai. 2023. "Querying Recombination Junctions of Replication-Competent Adeno-Associated Viruses in Gene Therapy Vector Preparations with Single Molecule, Real-Time Sequencing" Viruses 15, no. 6: 1228. https://doi.org/10.3390/v15061228
APA StyleYip, M., Chen, J., Zhi, Y., Tran, N. T., Namkung, S., Pastor, E., Gao, G., & Tai, P. W. L. (2023). Querying Recombination Junctions of Replication-Competent Adeno-Associated Viruses in Gene Therapy Vector Preparations with Single Molecule, Real-Time Sequencing. Viruses, 15(6), 1228. https://doi.org/10.3390/v15061228







