Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America
Abstract
:1. Introduction
2. State of the Art on the Seven Main Arboviruses in French Guiana
2.1. Dengue Virus
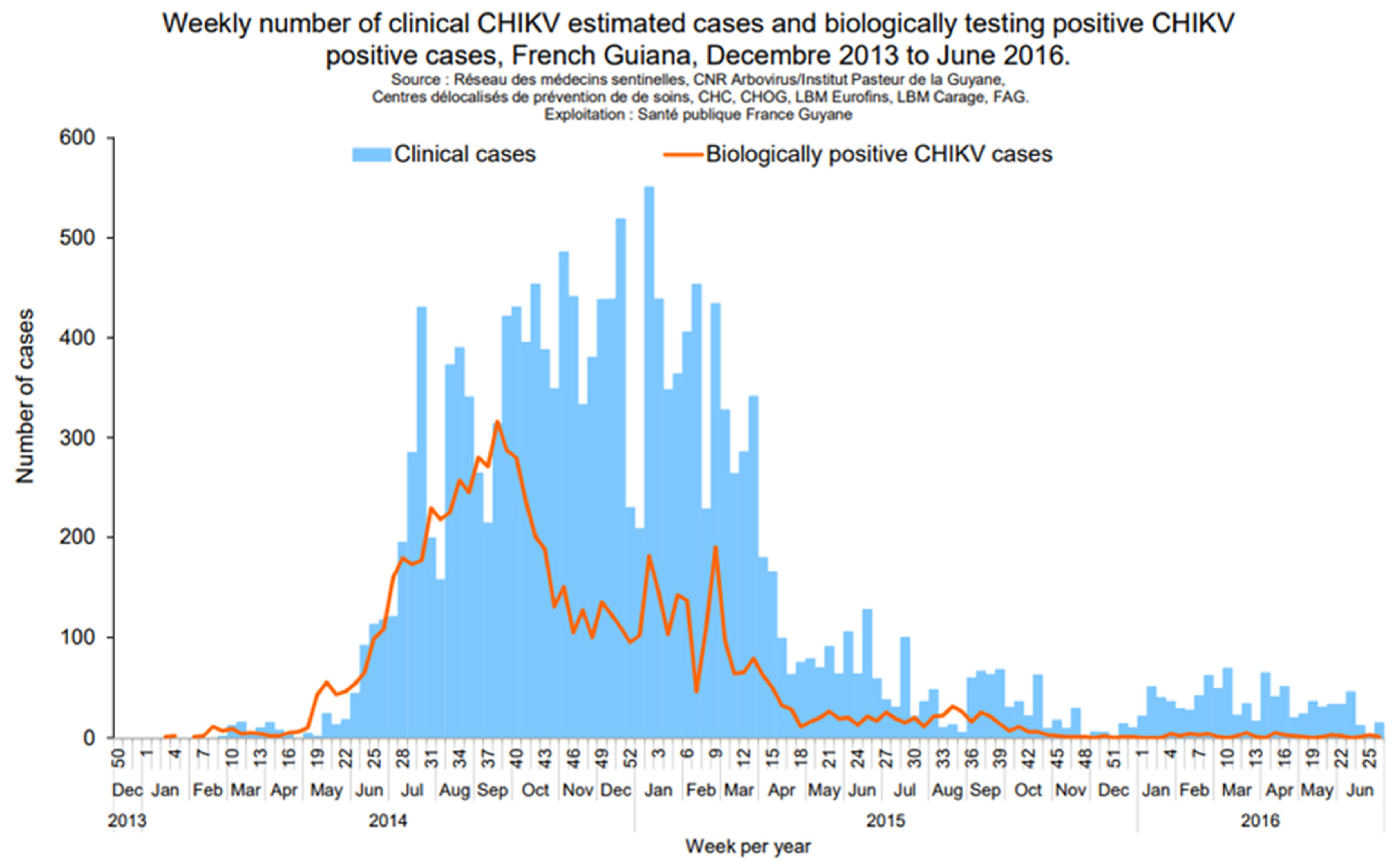
2.2. Chikungunya Virus
2.3. Zika Virus
2.4. Yellow Fever Virus
2.5. Mayaro Virus
2.6. Tonate Virus
2.7. Oropouche Virus
3. Challenges for the Future
3.1. Increase in Factors Favoring Arbovirus Epidemics
3.2. Despite Common Characteristics, a Great Heterogeneity
3.3. Vector Control
3.4. Risks of Introduction of Aedes Albopictus in French Guiana
3.5. Zoonotic Arboviruses
3.6. Potential Emergence of New Arbovirus
3.7. Potential Introduction of Arboviruses from French Guiana to Europe and the West Indies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Musso, D.; Rousset, D.; Peyrefitte, C. Special Issue “Endemic Arboviruses”. Viruses 2022, 14, 645. [Google Scholar] [CrossRef] [PubMed]
- de Thoisy, B.; Duron, O.; Epelboin, L.; Musset, L.; Quénel, P.; Roche, B.; Binetruy, F.; Briolant, S.; Carvalho, L.; Chavy, A.; et al. Ecology, Evolution, and Epidemiology of Zoonotic and Vector-Borne Infectious Diseases in French Guiana: Transdisciplinarity Does Matter to Tackle New Emerging Threats. Infect. Genet. Evol. 2021, 93, 104916. [Google Scholar] [CrossRef] [PubMed]
- Esser, H.J.; Mögling, R.; Cleton, N.B.; van der Jeugd, H.; Sprong, H.; Stroo, A.; Koopmans, M.P.G.; de Boer, W.F.; Reusken, C.B.E.M. Risk Factors Associated with Sustained Circulation of Six Zoonotic Arboviruses: A Systematic Review for Selection of Surveillance Sites in Non-Endemic Areas. Parasites Vectors 2019, 12, 265. [Google Scholar] [CrossRef] [Green Version]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging Arboviruses: Why Today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bonifay, T.; Godaert, L.; Epelboin, Y.; Rousset, D.; Douine, M.; Hilderal, H.; Clavel, C.; Abel, S.; Najioullah, F.; Fagour, L.; et al. Contribution of Research in the West Indies and Northeast Amazonia to Knowledge of the 2014–2015 Chikungunya Epidemic in the Americas. Curr. Trop. Med. Rep. 2021, 8, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Bonifay, T.; Walter, G.; Abboud, P.; Epelboin, L.; Bellaud, G.; Bleibtreu, A.; Dournon, N.; Henn, A.; Lemaignen, A.; Nguyen, L.B.L.; et al. Analyse rétrospective des épidémies d’arbovirus du XIXe siècle: Alors dengue ou chikungunya? Méd. Mal. Infect. 2019, 49, S120–S121. [Google Scholar] [CrossRef]
- Fouque, F.; Reynes, J.M.; Moreau, J.P. Dengue in French Guiana, 1965–1993. Bull. Pan Am. Health Organ. 1995, 29, 147–155. [Google Scholar]
- Robin, Y.; Lhuillier, M.; Girault, G.; Pajot, F.; Dégallier, N. Dengue Viruses and Other Arboviruses in French Guiana. In Proceedings of the Internacional Simposium on Tropical Arboviruses and Haemorrhagic Fevers, Academia Brasiliera de ciencias, Rio de Janeiro, Brazil, 14 April 1980. [Google Scholar]
- L’Azou, M.; Taurel, A.-F.; Flamand, C.; Quénel, P. Recent Epidemiological Trends of Dengue in the French Territories of the Americas (2000–2012): A Systematic Literature Review. PLoS Negl. Trop. Dis. 2014, 8, e3235. [Google Scholar] [CrossRef]
- Santé Publique France Situation Epidémiologique de la Dengue en Guyane. Point au 9 Février 2023. Available online: https://www.santepubliquefrance.fr/regions/guyane/documents/bulletin-regional/2023/situation-epidemiologique-de-la-dengue-en-guyane.-point-au-9-fevrier-2023 (accessed on 16 May 2023).
- de Thoisy, B.; Lacoste, V.; Germain, A.; Muñoz-Jordán, J.; Colón, C.; Mauffrey, J.-F.; Delaval, M.; Catzeflis, F.; Kazanji, M.; Matheus, S.; et al. Dengue Infection in Neotropical Forest Mammals. Vector Borne Zoonotic Dis. 2009, 9, 157–170. [Google Scholar] [CrossRef] [Green Version]
- Fritzell, C.; Rousset, D.; Adde, A.; Kazanji, M.; Kerkhove, M.D.V.; Flamand, C. Current Challenges and Implications for Dengue, Chikungunya and Zika Seroprevalence Studies Worldwide: A Scoping Review. PLoS Negl. Trop. Dis. 2018, 12, e0006533. [Google Scholar] [CrossRef] [Green Version]
- Bailly, S.; Rousset, D.; Fritzell, C.; Hozé, N.; Ben Achour, S.; Berthelot, L.; Enfissi, A.; Vanhomwegen, J.; Salje, H.; Fernandes-Pellerin, S.; et al. Spatial Distribution and Burden of Emerging Arboviruses in French Guiana. Viruses 2021, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Le Turnier, P.; Bonifay, T.; Mosnier, E.; Schaub, R.; Jolivet, A.; Demar, M.; Bourhy, P.; Nacher, M.; Djossou, F.; Epelboin, L. Usefulness of C-Reactive Protein in Differentiating Acute Leptospirosis and Dengue Fever in French Guiana. Open. Forum Infect. Dis. 2019, 6, ofz323. [Google Scholar] [CrossRef] [PubMed]
- Epelboin, L.; Boullé, C.; Ouar-Epelboin, S.; Hanf, M.; Dussart, P.; Djossou, F.; Nacher, M.; Carme, B. Discriminating Malaria from Dengue Fever in Endemic Areas: Clinical and Biological Criteria, Prognostic Score and Utility of the C-Reactive Protein: A Retrospective Matched-Pair Study in French Guiana. PLoS Negl. Trop. Dis. 2013, 7, e2420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, E.C.; Fonseca, V.; Xavier, J.; Adelino, T.; Claro, I.M.; Fabri, A.; Macario, E.M.; Viniski, A.E.; Souza, C.L.C.; da Costa, E.S.G.; et al. Short Report: Introduction of Chikungunya Virus ECSA Genotype into the Brazilian Midwest and Its Dispersion through the Americas. PLoS Negl. Trop. Dis. 2021, 15, e0009290. [Google Scholar] [CrossRef] [PubMed]
- Van Melle, A.; Cropet, C.; Parriault, M.-C.; Adriouch, L.; Lamaison, H.; Sasson, F.; Duplan, H.; Richard, J.-B.; Nacher, M. Renouncing Care in French Guiana: The National Health Barometer Survey. BMC Health Serv. Res. 2019, 19, 99. [Google Scholar] [CrossRef]
- Bonifay, T.; Prince, C.; Neyra, C.; Demar, M.; Rousset, D.; Kallel, H.; Nacher, M.; Djossou, F.; Epelboin, L.; The Char Chik Working Group. Atypical and Severe Manifestations of Chikungunya Virus Infection in French Guiana: A Hospital-Based Study. PLoS ONE 2018, 13, e0207406. [Google Scholar] [CrossRef] [Green Version]
- da Silva Neto, S.R.; Tabosa de Oliveira, T.; Teixiera, I.V.; Medeiros Neto, L.; Souza Sampaio, V.; Lynn, T.; Endo, P.T. Arboviral Disease Record Data—Dengue and Chikungunya, Brazil, 2013–2020. Sci. Data 2022, 9, 198. [Google Scholar] [CrossRef]
- Flamand, C.; Bailly, S.; Fritzell, C.; Berthelot, L.; Vanhomwegen, J.; Salje, H.; Paireau, J.; Matheus, S.; Enfissi, A.; Fernandes-Pellerin, S.; et al. Impact of Zika Virus Emergence in French Guiana: A Large General Population Seroprevalence Survey. J. Infect. Dis. 2019, 220, 1915–1925. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.; Musso, D.; Ghawche, F. Zika Virus Infection Complicated by Guillain-Barre Syndrome—Case Report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef] [Green Version]
- Cao-Lormeau, V.-M.; Blake, A.; Mons, S.; Lastère, S.; Roche, C.; Vanhomwegen, J.; Dub, T.; Baudouin, L.; Teissier, A.; Larre, P.; et al. Guillain-Barré Syndrome Outbreak Associated with Zika Virus Infection in French Polynesia: A Case-Control Study. Lancet 2016, 387, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Musso, D.; Ko, A.I.; Baud, D. Zika Virus Infection—After the Pandemic. N. Engl. J. Med. 2019, 381, 1444–1457. [Google Scholar] [CrossRef] [PubMed]
- Pomar, L.; Vouga, M.; Lambert, V.; Pomar, C.; Hcini, N.; Jolivet, A.; Benoist, G.; Rousset, D.; Matheus, S.; Malinger, G.; et al. Maternal-Fetal Transmission and Adverse Perinatal Outcomes in Pregnant Women Infected with Zika Virus: Prospective Cohort Study in French Guiana. BMJ 2018, 363, k4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hcini, N.; Kugbe, Y.; Rafalimanana, Z.H.L.; Lambert, V.; Mathieu, M.; Carles, G.; Baud, D.; Panchaud, A.; Pomar, L. Association between Confirmed Congenital Zika Infection at Birth and Outcomes up to 3 Years of Life. Nat. Commun. 2021, 12, 3270. [Google Scholar] [CrossRef] [PubMed]
- Grant, R.; Flechelles, O.; Elenga, N.; Tressières, B.; Gaete, S.; Hebert, J.-C.; Schaub, B.; Djossou, F.; Mallard, A.; Delver, L.; et al. Consequences of In Utero Zika Virus Exposure and Adverse Pregnancy and Early Childhood Outcomes: A Prospective Cohort Study. Viruses 2022, 14, 2755. [Google Scholar] [CrossRef]
- Chippaux, J.-P.; Chippaux, A. Yellow Fever in Africa and the Americas: A Historical and Epidemiological Perspective. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 20. [Google Scholar] [CrossRef] [Green Version]
- Goldani, L.Z. Yellow Fever Outbreak in Brazil, 2017. Braz. J. Infect. Dis. 2017, 21, 123–124. [Google Scholar] [CrossRef]
- Rodríguez-Morales, A.J.; Bonilla-Aldana, D.K.; Suárez, J.A.; Franco-Paredes, C.; Forero-Peña, D.A.; Mattar, S.; Villamil-Gómez, W.E.; Ruíz-Sáenz, J.; Cardona-Ospina, J.A.; Figuera, M.E.; et al. Yellow Fever Reemergence in Venezuela—Implications for International Travelers and Latin American Countries during the COVID-19 Pandemic. Travel. Med. Infect. Dis. 2021, 44, 102192. [Google Scholar] [CrossRef]
- Matheron, S.; Nicand, E.; Rapp, C.; Chidiac, C. Health recommendations for travellers, 2022 (for health professionals). Bull. Epidemiol. Hebdo 2022, BEH Hors-Série, 99. [Google Scholar]
- Flamand, C.; Bailly, S.; Fritzell, C.; Fernandes Pellerin, S.; Toure, A.; Chateau, N.; Saout, M.; Linares, S.; Dubois, F.; Filleul, L.; et al. Vaccination Coverage in the Context of the Emerging Yellow Fever Threat in French Guiana. PLoS Negl. Trop. Dis. 2019, 13, e0007661. [Google Scholar] [CrossRef]
- Garnier, M.A. La Fievre Jaune à La Guyane Avant 1902 et L’épidémie de 1902; Octave Doin: Paris, France, 1903. [Google Scholar]
- Heraud, J.M.; Hommel, D.; Hulin, A.; Deubel, V.; Poveda, J.D.; Sarthou, J.L.; Talarmin, A. First Case of Yellow Fever in French Guiana since 1902. Emerg. Infect. Dis. 1999, 5, 429–432. [Google Scholar] [CrossRef]
- Sanna, A.; Andrieu, A.; Carvalho, L.; Mayence, C.; Tabard, P.; Hachouf, M.; Cazaux, C.-M.; Enfissi, A.; Rousset, D.; Kallel, H. Yellow Fever Cases in French Guiana, Evidence of an Active Circulation in the Guiana Shield, 2017 and 2018. Eurosurveillance 2018, 23, 1800471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.; Michaud, C.; Gaillet, M.; de Thoisy, B.; Lacerda, M.; Duchemin, J. Risques de Réémergence de La Fièvre Jaune Sur Le Plateau Des Guyanes: Une Série de Cas 1990–2020 et Une Revue de La Littérature. 5e Journée Des Travaux Scientifiques Des Soignants de Guyane. Med. Trop. Sante Int. 2022, 2, mtsi.v2i2.2022.248. [Google Scholar] [CrossRef]
- de Thoisy, B.; Dussart, P.; Kazanji, M. Wild Terrestrial Rainforest Mammals as Potential Reservoirs for Flaviviruses (Yellow Fever, Dengue 2 and St Louis Encephalitis Viruses) in French Guiana. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 409–412. [Google Scholar] [CrossRef]
- Long, K.C.; Ziegler, S.A.; Thangamani, S.; Hausser, N.L.; Kochel, T.J.; Higgs, S.; Tesh, R.B. Experimental Transmission of Mayaro Virus by Aedes Aegypti. Am. J. Trop. Med. Hyg. 2011, 85, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Serra, O.P.; Cardoso, B.F.; Ribeiro, A.L.M.; dos Santos, F.A.L.; Slhessarenko, R.D. Mayaro Virus and Dengue Virus 1 and 4 Natural Infection in Culicids from Cuiabá, State of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2016, 111, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Francy, D.B. Laboratory Studies of a Brazilian Strain of Aedes Albopictus as a Potential Vector of Mayaro and Oropouche Viruses. J. Am. Mosq. Control Assoc. 1991, 7, 89–93. [Google Scholar]
- Diagne, C.T.; Bengue, M.; Choumet, V.; Hamel, R.; Pompon, J.; Missé, D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens 2020, 9, 738. [Google Scholar] [CrossRef]
- Talarmin, A.; Chandler, L.J.; Kazanji, M.; de Thoisy, B.; Debon, P.; Lelarge, J.; Labeau, B.; Bourreau, E.; Vié, J.C.; Shope, R.E.; et al. Mayaro Virus Fever in French Guiana: Isolation, Identification, and Seroprevalence. Am. J. Trop. Med. Hyg. 1998, 59, 452–456. [Google Scholar] [CrossRef]
- Mutricy, R.; Matheus, S.; Mosnier, É.; Martinez-Lorenzi, E.; De Laval, F.; Nacher, M.; Niemetzky, F.; Naudion, P.; Djossou, F.; Rousset, D.; et al. Mayaro Virus Infection in French Guiana, a Cross Sectional Study 2003–2019. Infect. Genet. Evol. 2022, 99, 105243. [Google Scholar] [CrossRef]
- Mayaro Virus Disease—French Guiana, France. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/mayaro-virus-disease---french-guiana-france (accessed on 4 April 2023).
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.-M.; Ramírez-Santana, C. Mayaro: An Emerging Viral Threat? Emerg. Microbes Infect. 2018, 7, 163. [Google Scholar] [CrossRef]
- Digoutte, J.P. Ecologie Des Arbovirus et Leur Rôle Pathogène Chez l’homme En Guyane Française; Groupe Institut national de la santé et de la recherche médicale U79; Institut Pasteur de la Guyane Française: Cayenne, French Guiana, 1975. [Google Scholar]
- Monath, T.P.; Lazuick, J.S.; Cropp, C.B.; Rush, W.A.; Calisher, C.H.; Kinney, R.M.; Trent, D.W.; Kemp, G.E.; Bowen, G.S.; Francy, D.B. Recovery of Tonate Virus (“Bijou Bridge” Strain), a Member of the Venezuelan Equine Encephalomyelitis Virus Complex, from Cliff Swallow Nest Bugs (Oeciacus Vicarius) and Nestling Birds in North America. Am. J. Trop. Med. Hyg. 1980, 29, 969–983. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Pontier, D.; Filippi-Codaccioni, O.; Pons, J.-B.; Postigo-Hidalgo, I.; Duhayer, J.; Brünink, S.; Drexler, J.F. Venezuelan Equine Encephalitis Complex Alphavirus in Bats, French Guiana. Emerg. Infect. Dis. 2021, 27, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Digoutte, J.P.; Girault, G. Résultats de l’étude chez la souris du pouvoir protecteur du virus Tonate et de deux souches de virus Cabassou contre la souche neurovirulente everglades du groupe VEE. Ann. Microbiol. 1976, 127B, 429–437. [Google Scholar]
- Talarmin, A.; Trochu, J.; Gardon, J.; Laventure, S.; Hommel, D.; Lelarge, J.; Labeau, B.; Digoutte, J.P.; Hulin, A.; Sarthou, J.L. Tonate Virus Infection in French Guiana: Clinical Aspects and Seroepidemiologic Study. Am. J. Trop. Med. Hyg. 2001, 64, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Mutricy, R.; Djossou, F.; Matheus, S.; Lorenzi-Martinez, E.; De Laval, F.; Demar, M.; Nacher, M.; Rousset, D.; Epelboin, L. Discriminating Tonate Virus from Dengue Virus Infection: A Matched Case-Control Study in French Guiana, 2003–2016. Am. J. Trop. Med. Hyg. 2020, 102, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Lambert, V.; Enfissi, A.; Lefebvre, M.; Pomar, L.; Kedous, S.; Guimiot, F.; Carles, G.; Lavergne, A.; Rousset, D.; Hcini, N. Tonate Virus and Fetal Abnormalities, French Guiana, 2019. Emerg. Infect. Dis. 2022, 28, 445–448. [Google Scholar] [CrossRef]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef] [Green Version]
- Gaillet, M.; Pichard, C.; Restrepo, J.; Lavergne, A.; Perez, L.; Enfissi, A.; Abboud, P.; Lambert, Y.; Ma, L.; Monot, M.; et al. Outbreak of Oropouche Virus in French Guiana. Emerg. Infect. Dis. 2021, 27, 2711–2714. [Google Scholar] [CrossRef]
- Walsh, C.E.S.; Robert, M.A.; Christofferson, R.C. Observational Characterization of the Ecological and Environmental Features Associated with the Presence of Oropouche Virus and the Primary Vector Culicoides Paraenesis: Data Synthesis and Systematic Review. Trop. Med. Infect. Dis. 2021, 6, 143. [Google Scholar] [CrossRef]
- Paixão, M.M.; Ballouz, T.; Lindahl, J.F. Effect of Education on Improving Knowledge and Behavior for Arboviral Diseases: A Systematic Review and Meta-Analysis. Am. J. Trop. Med. Hyg. 2019, 101, 441–447. [Google Scholar] [CrossRef]
- Jolivet, A.; Cadot, E.; Florence, S.; Lesieur, S.; Lebas, J.; Chauvin, P. Migrant Health in French Guiana: Are Undocumented Immigrants More Vulnerable? BMC Public Health 2012, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Meynard, J.-B.; Ardillon, V.; Venturin, C.; Ravachol, F.; Basurko, C.; Matheus, S.; Gaborit, P.; Grenier, C.; Dussart, P.; Quénel, P. First Description of a Dengue Fever Outbreak in the Interior of French Guiana, February 2006. Eur. J. Public Health 2009, 19, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Microbe, T.L. The Lancet Microbe Chikungunya in Brazil… and Beyond? Lancet Microbe 2023, 4, e284. [Google Scholar] [CrossRef] [PubMed]
- Hallet, E.; Flamand, C.; Rousset, D.; Bonifay, T.; Fritzell, C.; Matheus, S.; Dueymes, M.; Ntab, B.; Nacher, M. ZIKA Virus Infection in Pregnant Women in French Guiana: More Precarious-More at Risk. PLoS Negl. Trop. Dis. 2020, 14, e0008193. [Google Scholar] [CrossRef] [PubMed]
- Bonifay, T.; Douine, M.; Bonnefoy, C.; Hurpeau, B.; Nacher, M.; Djossou, F.; Epelboin, L. Poverty and Arbovirus Outbreaks: When Chikungunya Virus Hits More Precarious Populations Than Dengue Virus in French Guiana. Open Forum Infect. Dis. 2017, 4, ofx247. [Google Scholar] [CrossRef]
- Oberlis, M.; Renaud-Grappin, E.; Sevestre, E.; Bauza, J.-L.; Souleymane, G.; Cochet, C. Projet « WASH » En Situation d’épidémie de COVID-19—5e Journée Des Travaux Scientifiques Des Soignants de Guyane. Med. Trop. Sante Int. 2022, 2, mtsi.v2i2.2022.248. [Google Scholar]
- Esu, E.; Lenhart, A.; Smith, L.; Horstick, O. Effectiveness of Peridomestic Space Spraying with Insecticide on Dengue Transmission; Systematic Review. Trop. Med. Int. Health 2010, 15, 619–631. [Google Scholar] [CrossRef]
- Epelboin, Y.; Chaney, S.C.; Guidez, A.; Habchi-Hanriot, N.; Talaga, S.; Wang, L.; Dusfour, I. Successes and Failures of Sixty Years of Vector Control in French Guiana: What Is the next Step? Mem. Inst. Oswaldo Cruz 2018, 113, e170398. [Google Scholar] [CrossRef] [Green Version]
- Dusfour, I.; Thalmensy, V.; Gaborit, P.; Issaly, J.; Carinci, R.; Girod, R. Multiple Insecticide Resistance in Aedes Aegypti (Diptera: Culicidae) Populations Compromises the Effectiveness of Dengue Vector Control in French Guiana. Mem. Inst. Oswaldo Cruz 2011, 106, 346–352. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer IARC Monographs Volume 112: Evaluation of Five Organophosphate Insecticides and Herbicides—IARC. Available online: https://www.iarc.who.int/news-events/iarc-monographs-volume-112-evaluation-of-five-organophosphate-insecticides-and-herbicides/ (accessed on 27 January 2023).
- O’Neill, S.L. The Use of Wolbachia by the World Mosquito Program to Interrupt Transmission of Aedes Aegypti Transmitted Viruses. Adv. Exp. Med. Biol. 2018, 1062, 355–360. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef] [PubMed]
- Chadee, D.D.; Fat, F.H.; Persad, R.C. First Record of Aedes Albopictus from Trinidad, West Indies. J. Am. Mosq. Control Assoc. 2003, 19, 438–439. [Google Scholar]
- Saraiva, J.F.; Maitra, A.; Galardo, A.K.R.; Scarpassa, V.M. First Record of Aedes (Stegomyia) Albopictus in the State of Amapá, Northern Brazil. Acta Amaz. 2019, 49, 71–74. [Google Scholar] [CrossRef]
- Muller, J.N.; Galardo, A.K.R.; dos Santos, W.M.; Ferro, E.P.; Dias, L.d.S.; Corrêa, A.P.S.d.A.; Nunes, M.C.d.L.; Jesus, J.S.; Lima, J.B.P. Expansion of Aedes (Stegomyia) Albopictus (Skuse, 1894) in Northern Brazil: New Records and Distribution in Urban Areas of Macapa City. CheckList 2021, 17, 911–915. [Google Scholar] [CrossRef]
- Talaga, S. Ecologie, Diversité et Évolution Des Moustiques (Diptera Culicidae) de Guyane Française: Implications Dans l’invasion Biologique Du Moustique Aedes aegypti (L.). Ph.D. Thesis, Université de Guyane, Cayenne, French Guiana, France, 2016. [Google Scholar]
- Juliano, S.A. Species Introduction and Replacement Among Mosquitoes: Interspecific Resource Competition or Apparent Competition? Ecology 1998, 79, 255–268. [Google Scholar] [CrossRef]
- Braks, M.A.H.; Honório, N.A.; Lourenço-De-Oliveira, R.; Juliano, S.A.; Lounibos, L.P. Convergent Habitat Segregation of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Southeastern Brazil and Florida. J. Med. Entomol. 2003, 40, 785–794. [Google Scholar] [CrossRef] [Green Version]
- Dégallier, N.; Digoutte, J.P.; Pajot, F.-X.; Kramer, R.; Claustre, J.; Bellony, S.; Chatenay, G.; Alfré, E. Epidémiologie de deux arbovirus du complexe VEE en Guyane française: Données préliminaires sur les relations virus-vecteurs. Cah. ORSTOM Sér. Entomol. Méd. Parasitol. 1978, XVI, 209–221. [Google Scholar]
- Dégallier, N.; Digoutte, J.P.; Pajot, F.-X.; Kramer, R.; Claustre, J.; Bellony, S.; Chatenay, G.; Alfré, E. Epidémiologie de Bunyavirus (arbovirus) des groupes C et Guama en Guyane française: Données préliminaires et comparaison avec les virus du complexe V.E.E. Cah. ORSTOM Sér. Entomol. Méd. Parasitol. 1979, XVII, 3–11. [Google Scholar]
- Panday, R.S.; Digoutte, J.P. Tonate and Guama-Group Viruses Isolated from Mosquitoes in Both a Savannah and Coastal Area in Surinam. Trop. Geogr. Med. 1979, 31, 275–282. [Google Scholar]
- Current ICTV Taxonomy Release|ICTV. Available online: https://ictv.global/taxonomy (accessed on 10 March 2023).
- Dégallier, N. Les Arbovirus Selvatiques en Guyane Française et Leurs Vecteurs. Dr. Thesis, Université Pierre et Marie Curie, Paris, France, 1982. [Google Scholar]
- Panon, G.; Fauran, P.; Digoutte, J.P. Isolation of Ilheus virus in french Guyana. Bull. Soc. Pathol. Exot. Fil. 1979, 72, 315–318. [Google Scholar]
- Lavergne, A.; Lacoste, V.; Germain, A.; Matheus, S.; Dussart, P.; Deparis, X.; de Thoisy, B. Dengue virus infection in neotropical forest mammals: Incidental hosts or potential reservoirs? Med. Trop. (Mars) 2009, 69, 345–350. [Google Scholar]
- Talaga, S.; Dejean, A.; Carinci, R.; Gaborit, P.; Dusfour, I.; Girod, R. Updated Checklist of the Mosquitoes (Diptera: Culicidae) of French Guiana. J. Med. Entomol. 2015, 52, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Talaga, S.; Duchemin, J.-B.; Girod, R.; Dusfour, I. The Culex Mosquitoes (Diptera: Culicidae) of French Guiana: A Comprehensive Review With the Description of Three New Species. J. Med. Entomol. 2021, 58, 182–221. [Google Scholar] [CrossRef] [PubMed]
- Cochet, A.; Calba, C.; Jourdain, F.; Grard, G.; Durand, G.A.; Guinard, A.; Team, I.; Noël, H.; Paty, M.-C.; Franke, F. Autochthonous Dengue in Mainland France, 2022: Geographical Extension and Incidence Increase. Eurosurveillance 2022, 27, 2200818. [Google Scholar] [CrossRef] [PubMed]




| Arbovirus (Acronym) | Family (Genus) | Main Vectors | Sero- Prevalence | Epidemiological Data (Number of Cases) |
|---|---|---|---|---|
| Yellow fever (YFV) | Flaviviridae (Flavivirus) | Haemagogus and Sabethes +/− Aedes aegypti | 95.0% | 1 in 1998 2 deaths in 2017–2018 2 deaths in 2020 |
| Dengue (DENV) | Flaviviridae (Flavivirus) | Ae.aegypti | 73.1% | 2005–2006 (DENV3 > DENV2): 13,700–16,200 2009 (DENV1 > DENV 4): ~7800 2013 (DENV2 > >DENV4): ~16,000 2020–2021 (DENV1 > >DENV2): ~10,000 |
| Zika (ZIKV) | Flaviviridae (Flavivirus) | Ae.aegypti | 23.3% | 2015–2017: ~9700 |
| Chikungunya (CHIKV) | Togaviridae (Alphavirus) | Ae.aegypti | 20.3% | 2014–2015: ~16,000 |
| Mayaro (MAYV) | Togaviridae (Alphavirus) | Haemagogus spp. Ae.aegypti | 3.3% | 17 from 2003 to 2019 ~15 in 2020 |
| Tonate (TONV) | Togaviridae (Alphavirus) | Culex portesi | 11.9% | 45 from 2003 to 2016 |
| Oropouche (OROV) | Peribunyaviridae (Orthobunyavirus) | Culicoides spp. Culicoides paraensis Culex quinquefasciatus? | ND | 41 to 58 in August September 2020 in Saül |
| Genus | Virus | Reference | Date | Number of Isolations | Place | Vector | Vertebrate Hosts | Humans | Comment |
|---|---|---|---|---|---|---|---|---|---|
| Alphavirus | Mucambo virus | [45] | 1972 | 1 | Montsinéry | Phlebotominae Lutzomyia sp. | |||
| [8,45] | 1973 | 5 | Maripasoula | Birds Monasa atra Tachyphonus cristatus Trogon violaceus Turdus nudigenis | |||||
| [45] | NA | NA | NA | Culicidae Aedes sp. Culex portesi Culex sp. Haemagogus sp. Mansonia sp. Sabethini sp. Wyeomyia sp. | Primates Cebus apella (used as sentinel) | ||||
| [45] | NA | NA | Maripasoula | Birds 4 birds sp. | |||||
| [45] | 1972 | NA | Cayenne | Phlebotominae Lutzomyia sp. | |||||
| [45] | 1973 | 3 | Cayenne | Four laboratory workers who had handled the reference strain. Symptoms: sudden onset of high fever, myalgias, headaches, nausea or vomiting, very high asthenia lasting 15 days | Since March 1973, it has never been isolated again and seems to have been replaced by another virus of subtype III, Tonate | ||||
| Tonate virus | [8,45,74] | 1973 | NA | Tonate | Culicidae (n = 71 from 1973 to 1978) Anopheles sp. Coquillettidia sp. Culex portesi Mansonia sp. Uranotaenia sp. Wyeomyia sp. Phlebotominae Lutzomyia sp. | Birds Psarocolius decumanus | See main paragraph | ||
| [45] | 1973 | NA | Gallion Paramana Stoupan Trou Poissons | Culicidae Culex portesi Mansonia titillans | |||||
| [45] | 1975 | NA | Cité Chatenay Gallion La Chaumière Matoury Paramana Tonate Trou Poissons | Culicidae Coquillettidia venezuelensis Culex portesi Culex spissipes Culex zeteki Wyeomyia melanocephala Wyeomyia occulta | |||||
| [45] | 1973–1977 | 16 (birds) 72 (mosquitoes) | Gallion La Chaumière Matoury Paramana Sinnamary Tonate Trou-Poissons | Culicidae Anopheles braziliensis Anopheles mediopunctatus * Coquillettidia albicosta (as species of Culex) Coquillettidia venezuelensis Culex nigripalpus Culex portesi Culex spissipes Culex zeteki Mansonia pseudotitillans Mansonia titillans Uranotaenia geometrica Wyeomyia melanocephala Wyeomyia occulta Wyeomyia pseudopecten Phlebotominae Lutzomyia sp. | Birds Ardeola ibis Chiroxiphia pareola Elaenia chiriquensis Glyphorynchus spirurus Leucopternis albicollis Myiozetetes cayanensis Nycticorax violacea Oxyura dominica Psarocolius decumanus Ramphocelus carbo Sakesphorus canadenses Sporophila lineola Tachyphonus rufus Tolmomyias poliocephalus Turdus nudigenis Rodents Sentinel mice | ||||
| [47] | 2011 | 1 | Bats Trachops cirrhosus | Isolation of TONV | |||||
| Cabassou virus | [45] | 1972 | 1 | Paramana | Culicidae Culex portesi | (n = 15 from 1974 to 1980) | |||
| [8] | NA | NA | Saint Laurent du Maroni | Bats Chiroptera sp. | |||||
| [8,74,78] | 1973–1980 | 2 (birds) 3 (mammals) | Cité Jean-Marie¤ Gallion La Chaumière Matoury Montagne des chevaux Paramana Tonate Sinnamary Trou-Poissons | Culicidae Anopheles peryassuiCoquillettidia venezuelensis Culex nigripalpus Culex portesi Limatus pseudomethysticus Mansonia titillans Wyeomyia occulta | Birds Tolmomyias poliocephalus Turdus nudigenis Marsupials Didelphis marsupialis Philander oppossum Rodents Sentinel mice Bats Chiroptera sp. | ||||
| [45] | 1974–1975 | 2 | Gallion | Culicidae Culex portesi | |||||
| Una virus | [8,45] | 1973 | 1 | Montjoly | Culicidae Psorophora ferox | (n = 5 from 1973 to 1977) | |||
| [45] | 1973 | 1 | Sinnamary | Culicidae Coquillettidia venezuelensis | |||||
| [45] | 1973 | 1 | Paramana | Culicidae Coquillettidia albicosta | |||||
| [8,45] | 1975 | 2 | Maripasoula | Culicidae Psorophora lutzii | |||||
| [78] | 1977 | NA | Cité Jean-Marie¤ | Culicidae Anopheles nimbus | |||||
| 1973 | 1 | Inini | Birds Campephilus rubricollis | ||||||
| Aura virus | [8,78] | 1973 | 2 | Inini | Culicidae Aedes serratus | ||||
| [45] | NA | Polder Marianne | Culicidae Mansonia titillans | ||||||
| [45] | 1974 | 1 | Cayenne | One woman with high fever and severe jaundice who died quickly after admission. | The definite proof that the virus was the cause of disease is lacking. | ||||
| Flavivirus | Ilheus virus | [8,45] | 1973 | 3 | Iracoubo Maripasoula Montsinéry | Birds Leistes militaris Molothrus bonariensis Momotus momota Piaya minuta | |||
| [78] | 1977 | 1 | Gallion | Culicidae Coquilletidia venezuelensis | |||||
| [45,79] | 1973 | 2 | Macouria | From the blood of a patient suffering a “dengue-like” syndrome, with fever, headaches, chills and co-infection with Plasmodium falciparum | |||||
| Saint-Louis Encephalitis virus | [8] | 1967 | 1 | NA | Culicidae Culex sp. | ||||
| [8] | 1977 | 1 | NA | Birds Anhinga anhinga | |||||
| Dengue virus | [11,80] | 2006 | 5 | Saint Georges de l’Oyapock | Rodents Proechimys cuvieri | 5/72 (7%), DENV3 3/72 and DENV4 2/72 | |||
| [11,80] | 2001–2007 | 16 (rodents) 39 (Marsupials) 19 (bats) | Camp du Tigre | Rodents Mesomys hispidus (1/5 DENV3) Oecomys spp. (13/28 DENV1 and DENV2) Oryzomys megacephalus (2/8 DENV1 and DENV2) Proechymys cayennensis (7/45 DENV and 2 and 3) Marsupials Caluromys philander (1/14 DENV1) Didelphis marsupialis (12/88 DENV2, 3 and 4) Marmosops parvidens (3/7 DENV3) Marmosa murina (16/96 DENV1, 2 and 3) Micoureus demerarae (4/45 DENV1, 2, 3 and 4) Philander opossum (3/37 DENV1 and 2) Bats Artibeus planirostris (14/42 DENV1, 2 and 3) Carollia perspicillata (5/63 DENV1 and 3) | 16% (87/543) of the animals were infected on the site Distribution of DENV-1, -2, -3, and -4 was 41% (36/87), 20%, 33%, and 6%, respectively Chiroptera 4% (19/543), Rodentia 5%, and Marsupialia 7% | ||||
| Orthobunyavirus (Serogroup C) | Murutucu virus | [8] | 1972–1975 | NA | Cayenne Gallion | Culicidae Anopheles peryassui Culex portesi | |||
| [78] | 1980 | 2 | Montagne des Chevaux | Culicidae Culex portesi | |||||
| [8,78] | 1973 | 3 | Cayenne | Three patients with fever, headache and myalgia. | |||||
| Oriboca virus | [8] | 1975 | 1 | Gallion | Culicidae Culex portesi | ||||
| Caraparu virus | [8] | 1975 | 1 | La Chaumière | Culicidae Culex portesi | ||||
| [78] | 1975 | 1 | Gallion | Culicidae Culex spissipes | |||||
| [78] | 1974 | 1 | Tonate | Culicidae Mansonia titillans | |||||
| [8] | NA | NA | NA | Limatus durhami | |||||
| Group C undifferentiated (Muturucu, Oriboca, Caraparu) | [78] | 1973–1980 | 38 | Gallion La Chaumière Matoury Montagne des chevaux Paramana Tonate Sinnamary Trou-Poissons | Culicidae Aedes arborealis Anopheles peryassui Coquillettidia albicosta Coquillettidia venezuelensis Culex portesi Culex spissipes Mansonia titillans Trichoprosopon digitatum Trichoprosopon sp. Wyeomyia occulta | Marsupials Philander oppossum Rodents Sentinel mice | |||
| Orthobunyavirus (Bunyamwera group) | Guaroa virus | [8,78] | 1973 | 1 | Gallion | Culicidae Anopheles peryassui | |||
| Maguari virus | [8,78] | 1978 | 3 | Cité Jean-Marie Matoury Paramana | Culicidae Wyeomyia sp. Culex portesi Wyeomyia aphobema Wyeomyia occulta | ||||
| [78] | 1979 | NA | Gallion | Culicidae Anopheles nimbus | |||||
| Wyeomyia virus | [8,78] | 1975 | 1 | Pont des Cascades | Culicidae Wyeomyia occulta | ||||
| [78] | 1977 | 1 | Cabassou | Culicidae Coquillettidia albicosta | |||||
| [8] | NA | NA | NA | Culicidae Aedes taeniorhynchus Culex portesi Johnbelkinia longipes ** | |||||
| [78] | 1979 | 6 | Paramana Gallion | Culicidae Anopheles nimbus | |||||
| Orthobunyavirus (Guama group) | Guama group undifferentiated (Bimiti, Catu, Guama.) | [8,78] | 1972–1980 | 107 (mosquitoes) | Cité jean-Marie Gallion La Chaumière Matoury Montagne aux chevaux Paramana Rochambeau Sinnamary Tonate Trou-Poissons | Culicidae Coquillettidia venezuelensis Culex portesi Anopheles braziliensis Anopheles darlingi Culex spissipes Culex taeniopus $ Culex sp. Mansonia titillans Wyeomyia splendida £ Wyeomyia occulta Psorophora ferox Sabethes undosus Trichoprosopon digitatum ** Trichoprosopon longipes Johnbelkinia longipes Phlebotominae Lutzomyia sp. | Rodents Sentinel mice | ||
| [78] | 1976–1978 | 19 | Cogneau Inini La Chaumière Paramana Tonate Trou Poisson | Birds Attila cinnamoneus Columba plumbea Elaenia chiriquensis Galbula dea Galbula galbula Myiarchus ferox Pitangus sulphuratus Platirynchus sp. Querula purpurata Ramphocelus carbo Tamnomanes ardesiacus Thruapis episcopus Thraupis palmarum Turdus fumigatus | |||||
| [78] | 1976 | 5 | La Chaumière Inini Paramana | Marsupials Didelphis Marsupialis Rodents Proechimys guyanensis/ cuvieri | |||||
| [78] | 1977 | 1 | NA | Strain isolated from patient serum | |||||
| Guama virus (Guama group) | [8] | 1974–1975 | 3 | Gallion La Chaumière Paramana | Culicidae Culex portesi Johnbelkinia longipes ** | ||||
| [78] | 1975–1976 | 2 | Matoury | Marsupials Didelphis marsupialìs | |||||
| Bimiti virus (Guama group) | [8,78] | 1972–1976 | 12 | Gallion Iracoubo Matoury Paramana Trou Poisson | Culicidae Culex sp. Culex portesi Culex taeniopus $ Coquillettidia venezuelensis | ||||
| [8,78] | 1972 | 1 | Matoury | Birds Galbula dea | |||||
| Catu virus (Guama group) | [8,78] | 1973–1976 | 4 | Gallion Paramana Pont des Cascades | Culicidae Culex portesi Culex spissipes | ||||
| [78] | 1975 | 1 | La Chaumière | Marsupials Didelphis marsupialìs | |||||
| Inini virus (Simbu group) | [45] | 1973 | 1 | Maripasoula | Birds Pteroglossus aracari | It shows some antigenic relationships with Mermet and Ingwavuma viruses | |||
| Bunyamwera like | Itaporanga virus (Phlebotomus group) | [8,45] | 1977–1978 | 2 | Cité Jean-Marie¤Paramana | Culicidae Culex albinensis Culex spissipes | |||
| [78] | 1977 | 1 | Iracoubo | Birds Nycticorax violacea | |||||
| Ungrouped | Aruac virus | [8] | NA | NA | NA | Culicidae Coquillettidia albicosta Coquillettidia venezuelensis Culex sp. | |||
| Rochambeau virus/Paramana virus | [8,78] | 1973 | 1 | Paramana | Culicidae Coquillettidia albicosta | ||||
| NA | 1 | Paramana | Culicidae Culex portesi | ||||||
| [78] | 1974 | 1 | Paramana | Birds Tyrannus dominicensis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonifay, T.; Le Turnier, P.; Epelboin, Y.; Carvalho, L.; De Thoisy, B.; Djossou, F.; Duchemin, J.-B.; Dussart, P.; Enfissi, A.; Lavergne, A.; et al. Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America. Viruses 2023, 15, 1268. https://doi.org/10.3390/v15061268
Bonifay T, Le Turnier P, Epelboin Y, Carvalho L, De Thoisy B, Djossou F, Duchemin J-B, Dussart P, Enfissi A, Lavergne A, et al. Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America. Viruses. 2023; 15(6):1268. https://doi.org/10.3390/v15061268
Chicago/Turabian StyleBonifay, Timothee, Paul Le Turnier, Yanouk Epelboin, Luisiane Carvalho, Benoit De Thoisy, Félix Djossou, Jean-Bernard Duchemin, Philippe Dussart, Antoine Enfissi, Anne Lavergne, and et al. 2023. "Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America" Viruses 15, no. 6: 1268. https://doi.org/10.3390/v15061268
APA StyleBonifay, T., Le Turnier, P., Epelboin, Y., Carvalho, L., De Thoisy, B., Djossou, F., Duchemin, J. -B., Dussart, P., Enfissi, A., Lavergne, A., Mutricy, R., Nacher, M., Rabier, S., Talaga, S., Talarmin, A., Rousset, D., & Epelboin, L. (2023). Review on Main Arboviruses Circulating on French Guiana, An Ultra-Peripheric European Region in South America. Viruses, 15(6), 1268. https://doi.org/10.3390/v15061268






