How Interactions during Viral–Viral Coinfection Can Shape Infection Kinetics
Abstract
1. Introduction
2. Materials and Methods
2.1. Data for RSV-IAV Coinfection in Ferrets
2.2. Data for IAV, RV, and SARS-CoV-2 Infections in Humans
2.3. Data for IAV Coinfection with RV or SARS-CoV-2 in Mice
2.4. Mathematical Model of Viral Monoinfection
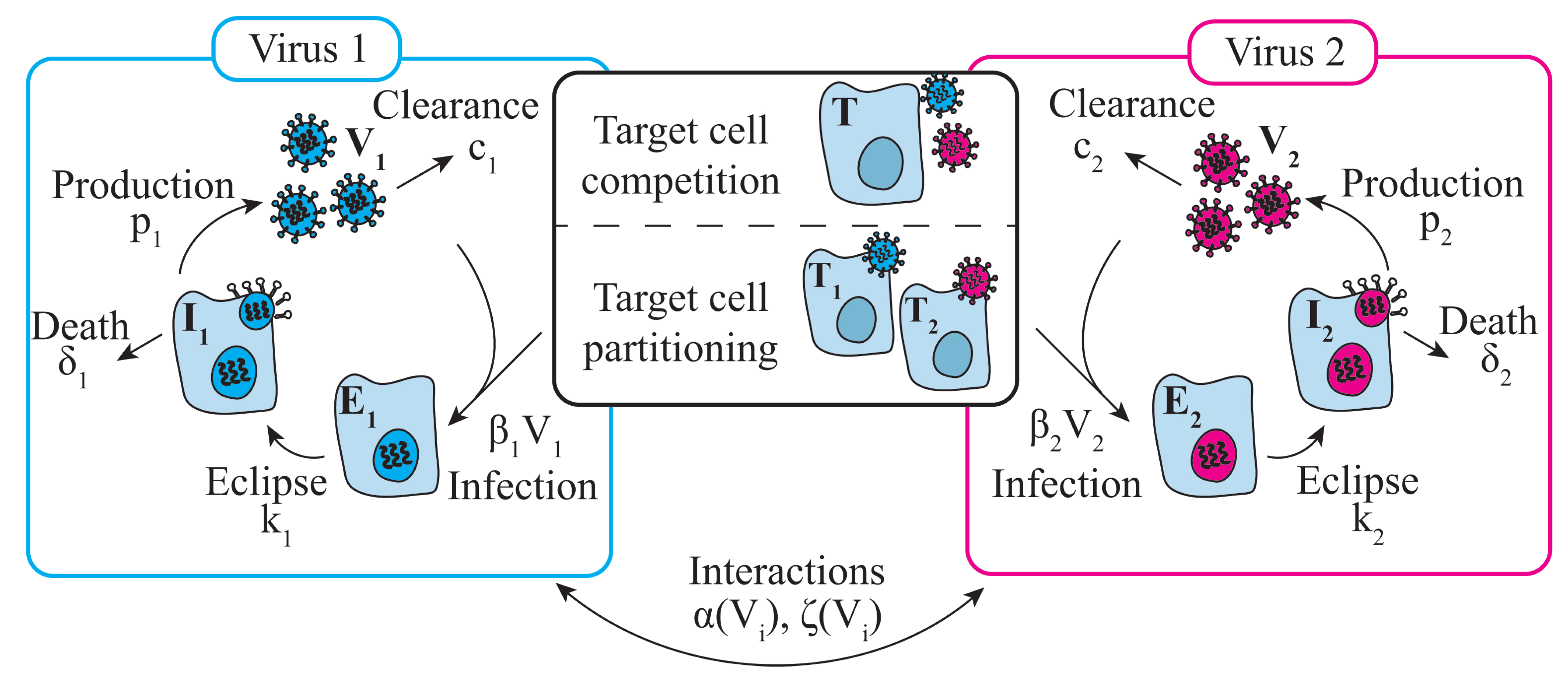
2.5. Mathematical Model of Viral–Viral Coinfection
2.5.1. Target Cell Competition
2.5.2. Target Cell Partitioning
2.5.3. Modeling Viral–Viral Interactions
2.6. Quantifying the Relative Change in Total Virus
2.7. Quantifying Disease Severity
2.8. Parameter Estimation
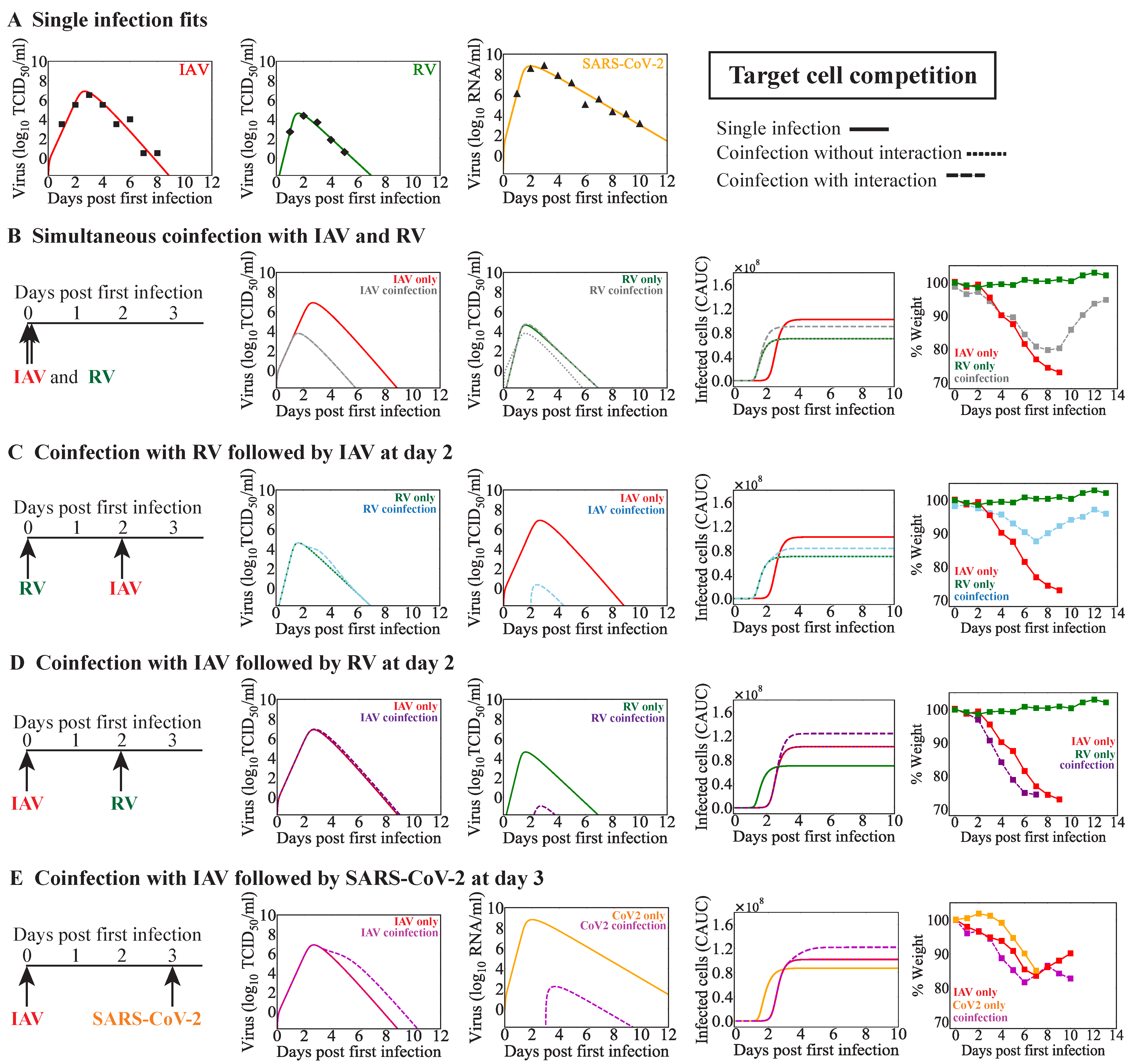
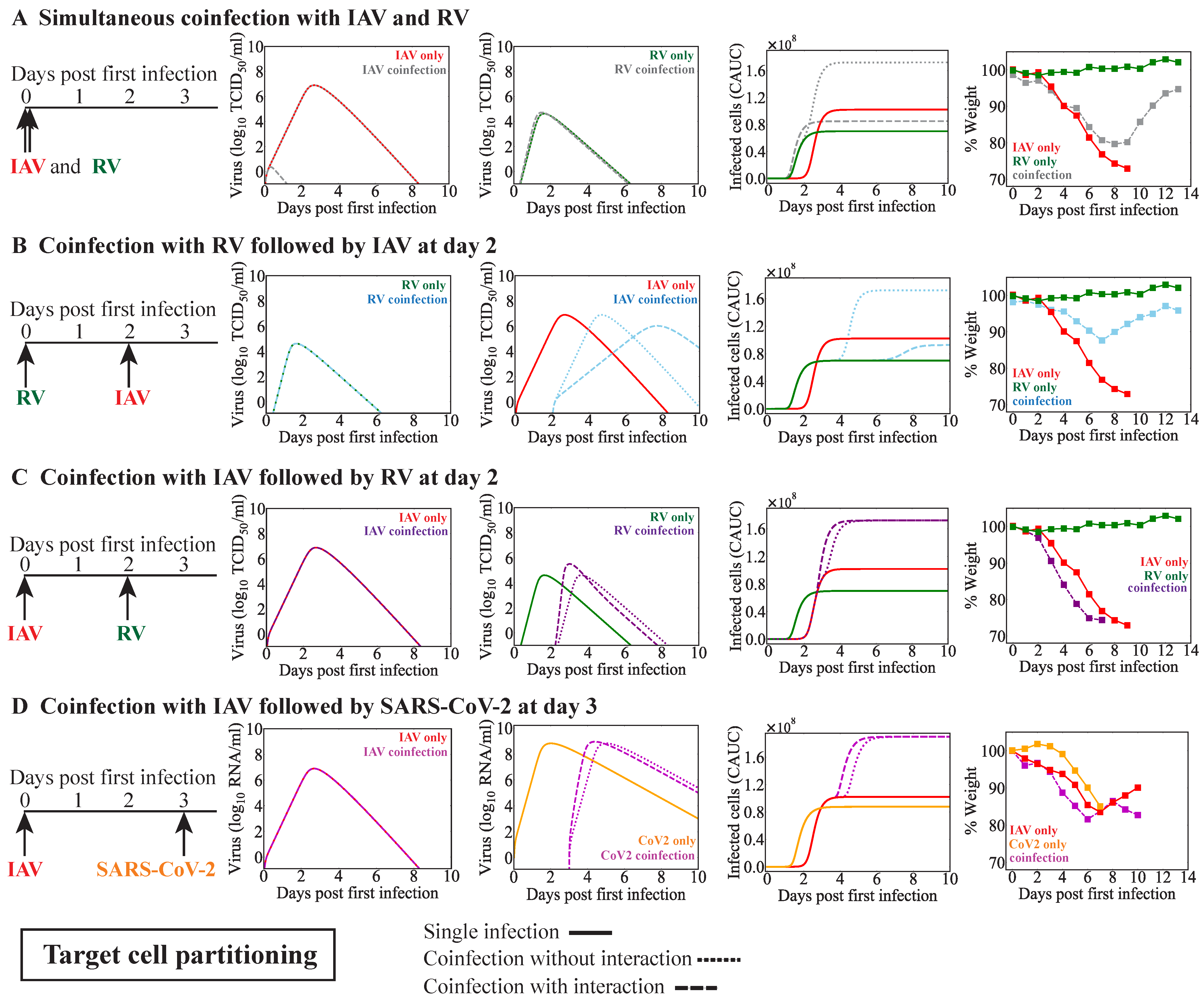
3. Results
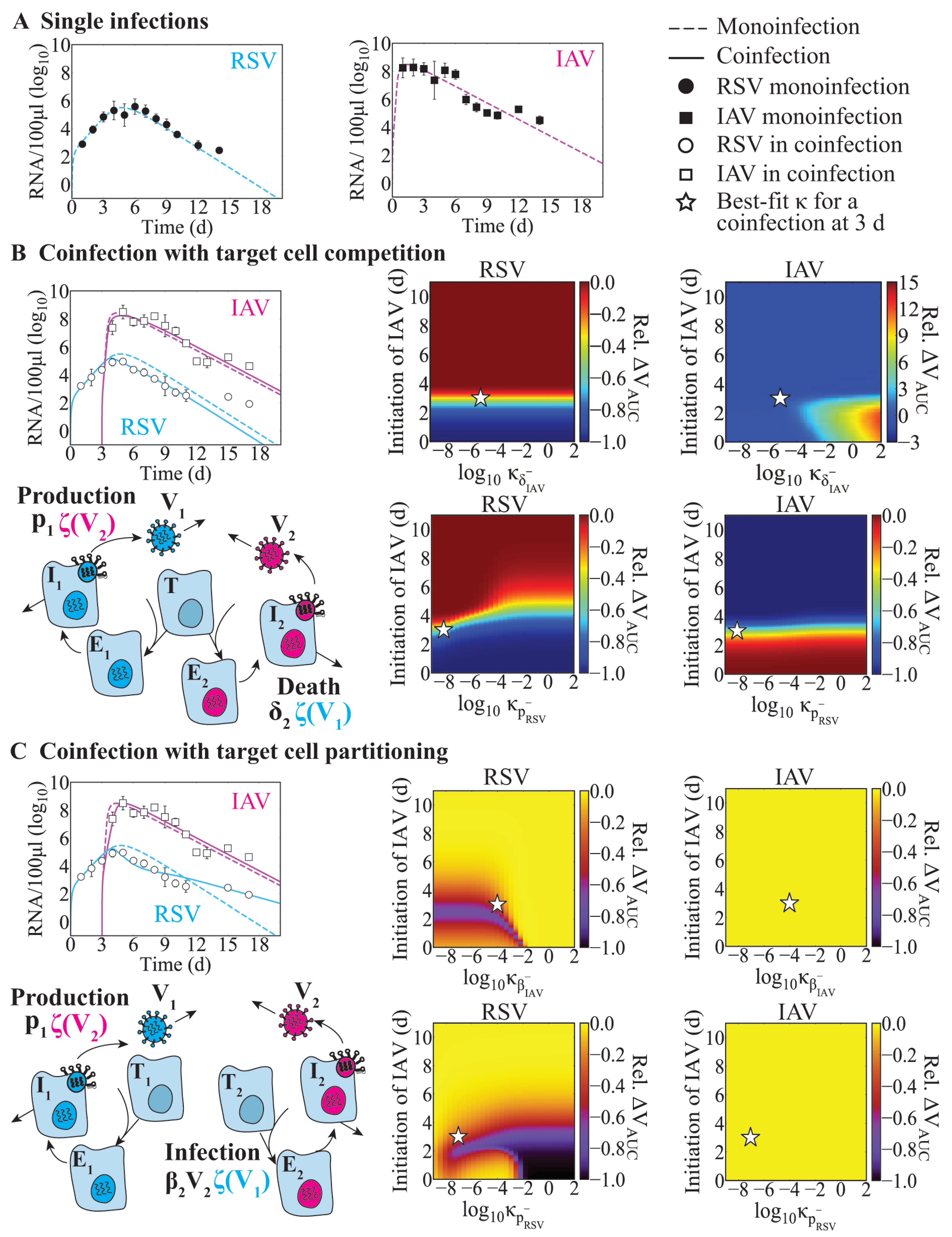
3.1. Model-Predicted Mechanisms of RSV–IAV Coinfection
3.2. Effect of Infection Timing and Interaction Strength in RSV-IAV Coinfection
3.3. IAV Coinfection with RV
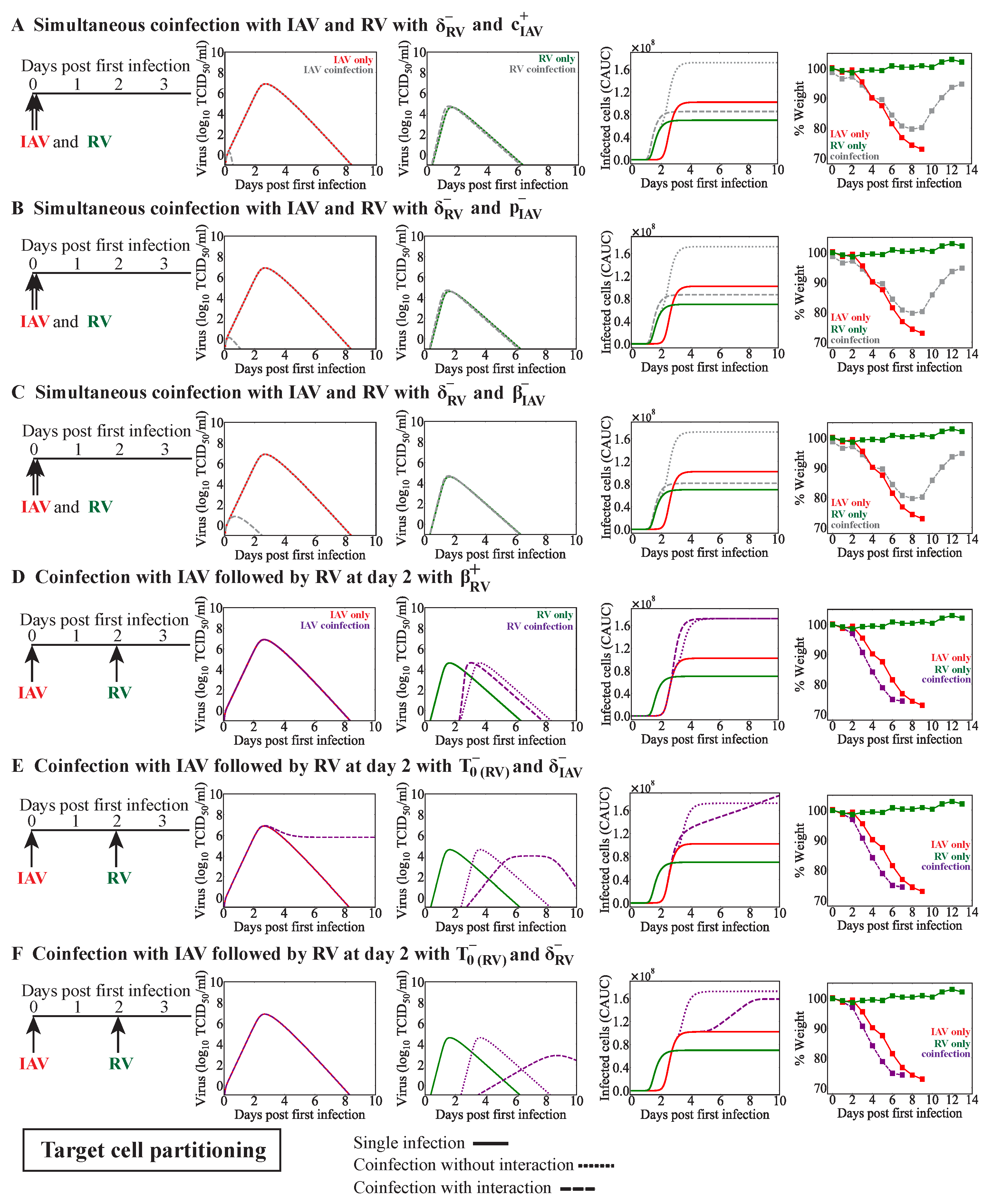
3.3.1. Simultaneous or Sequential RV–IAV Coinfection
3.3.2. IAV-RV Coinfection
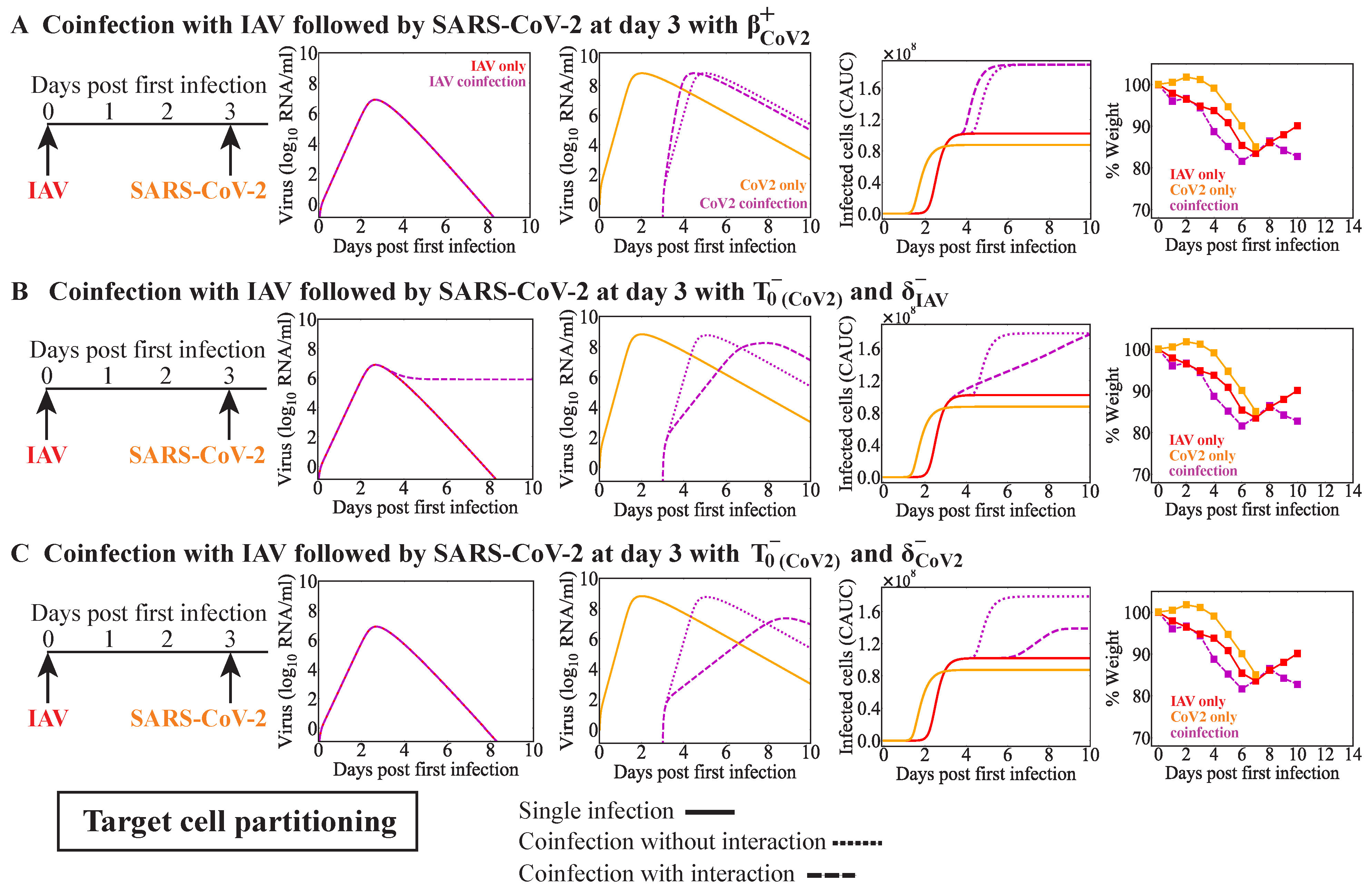
3.4. IAV Coinfection with SARS-CoV-2
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Complete Results from Fitting the Coinfection Models to Data from RSV-IAV Coinfection in Ferrets
| Interaction | IAV | RSV | -2LL | |||||
|---|---|---|---|---|---|---|---|---|
| Strength of | Strength of | Strength of | Strength of | |||||
| Enhancement, | Inhibition, | Enhancement, | Inhibition, | |||||
| RNA | RNA | RNA | RNA | |||||
| No interaction | 0 | 0 | 0 | 0 | 185.0 | 189.0 | 0.79 | 0.81 |
| Single interaction | ||||||||
| (6.5) | – | – | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| (2.5) | – | – | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| (8.5) | – | – | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| (3.5) | – | – | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| – | (6.9) | – | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| – | (5.3) | – | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| – | (0.1) | – | – | 166.2 | 174.2 | 0.79 | 0.65 | |
| – | (0.1) | – | – | 167.8 | 175.8 | 0.79 | 0.66 | |
| – | – | (4.5) | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| – | – | (2.0) | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| – | – | (2.0) | – | 185.0 | 193.0 | 0.79 | 0.81 | |
| – | – | (0.2) | – | 182.5 | 190.5 | 0.76 | 0.81 | |
| – | – | – | (2.3) | 182.0 | 190.0 | 0.81 | 0.76 | |
| – | – | – | (0.4) | 182.5 | 190.5 | 0.76 | 0.80 | |
| – | – | – | (0.9) | 183.7 | 191.7 | 0.77 | 0.81 | |
| – | – | – | (2.2) | 185.0 | 193.0 | 0.79 | 0.81 | |
| Double interactions | ||||||||
| and | – | (0.1) | – | (11.1) | 166.1 | 178.1 | 0.79 | 0.65 |
| and | – | (0.1) | – | (0.2) | 163.7 | 175.7 | 0.76 | 0.65 |
| and | – | (0.1) | (5.3) | – | 166.1 | 178.1 | 0.79 | 0.65 |
| and | – | (0.1) | (0.2) | – | 163.8 | 175.8 | 0.76 | 0.65 |
| and | – | (0.1) | – | (16.6) | 167.7 | 179.7 | 0.79 | 0.66 |
| and | – | (0.1) | – | (0.3) | 165.5 | 177.5 | 0.76 | 0.66 |
| and | – | (0.1) | (5.1) | – | 167.7 | 179.7 | 0.79 | 0.66 |
| and | – | (0.1) | (0.3) | – | 165.7 | 177.7 | 0.77 | 0.66 |
| Interaction | IAV | RSV | -2LL | |||||
|---|---|---|---|---|---|---|---|---|
| Strength of | Strength of | Strength of | Strength of | |||||
| Enhancement, | Inhibition, | Enhancement, | Inhibition, | |||||
| RNA | RNA | RNA | RNA | |||||
| No interaction | 0 | 0 | 0 | 0 | 181.3 | 185.3 | 0.88 | 0.72 |
| Single interaction | ||||||||
| (2.9) | – | – | – | 181.3 | 189.3 | 0.88 | 0.72 | |
| (2.2) | – | – | – | 181.3 | 189.3 | 0.88 | 0.72 | |
| (2.3) | – | – | – | 181.3 | 189.3 | 0.88 | 0.72 | |
| (2.2) | – | – | – | 181.3 | 189.3 | 0.88 | 0.72 | |
| – | (0.90) | – | – | 180.5 | 188.5 | 0.88 | 0.71 | |
| – | (0.06) | – | – | 168.6 | 176.6 | 0.88 | 0.62 | |
| – | (0.2) | – | – | 178.3 | 186.3 | 0.88 | 0.70 | |
| – | (1.9) | – | – | 180.7 | 188.7 | 0.88 | 0.71 | |
| – | – | (1.0) | – | 178.8 | 186.8 | 0.84 | 0.72 | |
| – | – | (12.6) | – | 181.2 | 189.2 | 0.88 | 0.72 | |
| – | – | (0.04) | – | 131.0 | 139.0 | 0.40 | 0.72 | |
| – | – | (0.10) | – | 169.2 | 177.2 | 0.72 | 0.72 | |
| – | – | – | (0.1) | 128.6 | 136.6 | 0.38 | 0.72 | |
| – | – | – | (0.1) | 169.7 | 177.7 | 0.73 | 0.72 | |
| – | – | – | (4.1) | 181.3 | 189.3 | 0.88 | 0.72 | |
| – | – | – | (6.5) | 181.3 | 189.3 | 0.88 | 0.72 | |
| Double interactions | ||||||||
| and | – | (0.3) | – | (0.1) | 125.9 | 137.9 | 0.38 | 0.70 |
| and | – | (0.2) | – | (0.08) | 164.9 | 176.9 | 0.70 | 0.70 |
| and | – | (10.9) | (0.05) | – | 131.0 | 143.0 | 0.40 | 0.72 |
| and | – | (0.1) | (0.1) | – | 164.0 | 176.0 | 0.70 | 0.70 |
| and | – | (7.2) | – | (0.2) | 128.5 | 140.5 | 0.38 | 0.72 |
| and | – | (0.3) | – | (0.1) | 163.9 | 175.9 | 0.66 | 0.72 |
| and | – | (3.0) | (0.03) | – | 130.9 | 142.9 | 0.40 | 0.72 |
| and | – | (0.2) | (0.07) | – | 163.1 | 175.1 | 0.65 | 0.73 |
| and | – | (0.4) | – | (0.1) | 120.2 | 132.2 | 0.40 | 0.62 |
| and | – | (0.04) | – | (0.1) | 127.3 | 139.3 | 0.45 | 0.63 |


Appendix A.2. Alternate Interactions from Simulating the Target Cell Partitioning Model for IAV Coinfection with RV or SARS-CoV-2
References
- Nickbakhsh, S.; Mair, C.; Matthews, L.; Reeve, R.; Johnson, P.C.; Thorburn, F.; Von Wissmann, B.; Reynolds, A.; McMenamin, J.; Gunson, R.N.; et al. Virus–virus interactions impact the population dynamics of influenza and the common cold. Proc. Natl. Acad. Sci. USA 2019, 116, 27142–27150. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.D.; Sordillo, E.M.; Gitman, M.R.; Mondolfi, A.E.P. Coinfection in SARS-CoV-2 infected patients: Where are influenza virus and rhinovirus/enterovirus? J. Med. Virol. 2020, 92, 1699. [Google Scholar] [CrossRef] [PubMed]
- Sanz, I.; Perez, D.; Rojo, S.; Domínguez-Gil, M.; de Lejarazu, R.O.; Eiros, J.M. Coinfections of influenza and other respiratory viruses are associated to children. An. Pediatr. 2022, 96, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chu, S.Y.; Shin, Y.Y.; Ryu, I.K.; Tang, C.L.; Choi, J.; Kim, H.B.; Kim, C.K. Comparison of clinical severity between single-and coinfections of respiratory syncytial virus and influenza virus with common respiratory viruses. Allergy Asthma Respir. Dis. 2019, 7, 86–91. [Google Scholar] [CrossRef]
- Nolan, V.G.; Arnold, S.R.; Bramley, A.M.; Ampofo, K.; Williams, D.J.; Grijalva, C.G.; Self, W.H.; Anderson, E.J.; Wunderink, R.G.; Edwards, K.M.; et al. Etiology and impact of coinfections in children hospitalized with community-acquired pneumonia. J. Infect. Dis. 2018, 218, 179–188. [Google Scholar] [CrossRef]
- Pacheco, G.A.; Gálvez, N.; Soto, J.A.; Andrade, C.A.; Kalergis, A.M. Bacterial and Viral Coinfections with the Human Respiratory Syncytial Virus. Microorganisms 2021, 9, 1293. [Google Scholar] [CrossRef]
- Calcagno, A.; Ghisetti, V.; Burdino, E.; Trunfio, M.; Allice, T.; Boglione, L.; Bonora, S.; Di Perri, G. Co-infection with other respiratory pathogens in COVID-19 patients. Clin. Microbiol. Infect. 2021, 27, 297–298. [Google Scholar] [CrossRef]
- Meskill, S.D.; O’Bryant, S.C. Respiratory virus co-infection in acute respiratory infections in children. Curr. Infect. Dis. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, J.; Wang, H.; Wang, X.; Hu, Z.; Li, H.; Zhang, H.; Liu, X. Co-infection of influenza A virus and SARS-CoV-2: A retrospective cohort study. J. Med. Virol. 2021, 93, 2947–2954. [Google Scholar] [CrossRef]
- Rotzén-Östlund, M.; Eriksson, M.; Tiveljung Lindell, A.; Allander, T.; Zweygberg Wirgart, B.; Grillner, L. Children with multiple viral respiratory infections are older than those with single viruses. Acta Paediatr. 2014, 103, 100–104. [Google Scholar] [CrossRef]
- Fayyadh, T.K.; Ma, F.; Qin, C.; Zhang, X.; Li, W.; Zhang, X.E.; Zhang, Z.; Cui, Z. Simultaneous detection of multiple viruses in their coinfected cells using multicolour imaging with self-assembled quantum dot probes. Microchim. Acta 2017, 184, 2815–2824. [Google Scholar] [CrossRef]
- Choi, S.H.; Chung, J.W.; Kim, H.R. Clinical relevance of multiple respiratory virus detection in adult patients with acute respiratory illness. J. Clin. Microbiol. 2015, 53, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Williams, D.J.; Arnold, S.R.; Ampofo, K.; Bramley, A.M.; Reed, C.; Stockmann, C.; Anderson, E.J.; Grijalva, C.G.; Self, W.H.; et al. Community-acquired pneumonia requiring hospitalization among US children. N. Engl. J. Med. 2015, 372, 835–845. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-acquired pneumonia requiring hospitalization among US adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef]
- Goka, E.A.; Vallely, P.J.; Mutton, K.J.; Klapper, P.E. Single and multiple respiratory virus infections and severity of respiratory disease: A systematic review. Paediatr. Respir. Rev. 2014, 15, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.T.; Kuypers, J.; Wald, A.; Englund, J.A. Multiple vs. single virus respiratory infections: Viral load and clinical disease severity in hospitalized children. Influenza Other Respir. Viruses 2012, 6, 71–77. [Google Scholar] [CrossRef]
- Aberle, J.H.; Aberle, S.W.; Pracher, E.; Hutter, H.P.; Kundi, M.; Popow-Kraupp, T. Single vs. dual respiratory virus infections in hospitalized infants: Impact on clinical course of disease and interferon-γ response. Pediatr. Infect. Dis. J. 2005, 24, 605–610. [Google Scholar] [CrossRef]
- Stefanska, I.; Romanowska, M.; Donevski, S.; Gawryluk, D.; Brydak, L.B. Co-infections with influenza and other respiratory viruses. In Respiratory Regulation—The Molecular Approach; Springer: Berlin/Heidelberg, Germany, 2013; pp. 291–301. [Google Scholar]
- Scotta, M.C.; Chakr, V.C.B.G.; de Moura, A.; Becker, R.G.; de Souza, A.P.D.; Jones, M.H.; Pinto, L.A.; Sarria, E.E.; Pitrez, P.M.; Stein, R.T.; et al. Respiratory viral coinfection and disease severity in children: A systematic review and meta-analysis. J. Clin. Virol. 2016, 80, 45–56. [Google Scholar] [CrossRef]
- Greer, R.M.; McErlean, P.; Arden, K.E.; Faux, C.E.; Nitsche, A.; Lambert, S.B.; Nissen, M.D.; Sloots, T.P.; Mackay, I.M. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J. Clin. Virol. 2009, 45, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Cebey-López, M.; Herberg, J.; Pardo-Seco, J.; Gómez-Carballa, A.; Martinón-Torres, N.; Salas, A.; Martinón-Sánchez, J.M.; Gormley, S.; Sumner, E.; Fink, C.; et al. Viral co-infections in pediatric patients hospitalized with lower tract acute respiratory infections. PLoS ONE 2015, 10, e0136526. [Google Scholar] [CrossRef]
- Zhong, P.; Zhang, H.; Chen, X.; Lv, F. Clinical characteristics of the lower respiratory tract infection caused by a single infection or coinfection of the human parainfluenza virus in children. J. Med. Virol. 2019, 91, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Goka, E.; Vallely, P.; Mutton, K.; Klapper, P. Influenza A viruses dual and multiple infections with other respiratory viruses and risk of hospitalization and mortality. Influenza Other Respir. Viruses 2013, 7, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, Y.; Wang, H.; Zhang, L.; Bao, Y.; Zhou, X. High incidence of multiple viral infections identified in upper respiratory tract infected children under three years of age in Shanghai, China. PLoS ONE 2012, 7, e44568. [Google Scholar] [CrossRef]
- Brand, H.K.; de Groot, R.; Galama, J.M.; Brouwer, M.L.; Teuwen, K.; Hermans, P.W.; Melchers, W.J.; Warris, A. Infection with multiple viruses is not associated with increased disease severity in children with bronchiolitis. Pediatr. Pulmonol. 2012, 47, 393–400. [Google Scholar] [CrossRef]
- Shinjoh, M.; Omoe, K.; Saito, N.; Matsuo, N.; Nerome, K. In vitro growth profiles of respiratory syncytial virus in the presence of influenza virus. Acta Virol. 2000, 44, 91–97. [Google Scholar]
- Chan, K.F.; Carolan, L.A.; Korenkov, D.; Druce, J.; McCaw, J.; Reading, P.C.; Barr, I.G.; Laurie, K.L. Investigating viral interference between influenza A virus and human respiratory syncytial virus in a ferret model of infection. J. Infect. Dis. 2018, 218, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Essaidi-Laziosi, M.; Geiser, J.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C. Interferon-dependent and respiratory virus-specific interference in dual infections of airway epithelia. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Drori, Y.; Jacob-Hirsch, J.; Pando, R.; Glatman-Freedman, A.; Friedman, N.; Mendelson, E.; Mandelboim, M. Influenza A virus inhibits RSV infection via a two-wave expression of IFIT proteins. Viruses 2020, 12, 1171. [Google Scholar] [CrossRef]
- Hartwig, S.M.; Miller, A.M.; Varga, S.M. Respiratory Syncytial Virus Provides Protection against a Subsequent Influenza A Virus Infection. J. Immunol. 2022, 208, 720–731. [Google Scholar] [CrossRef]
- Haney, J.; Vijayakrishnan, S.; Streetley, J.; Dee, K.; Goldfarb, D.M.; Clarke, M.; Mullin, M.; Carter, S.D.; Bhella, D.; Murcia, P.R. Coinfection by influenza A virus and respiratory syncytial virus produces hybrid virus particles. Nat. Microbiol. 2022, 7, 1879–1890. [Google Scholar] [CrossRef]
- Goto, H.; Ihira, H.; Morishita, K.; Tsuchiya, M.; Ohta, K.; Yumine, N.; Tsurudome, M.; Nishio, M. Enhanced growth of influenza A virus by coinfection with human parainfluenza virus type 2. Med. Microbiol. Immunol. 2016, 205, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.J.; Ijezie, E.C.; Balemba, O.B.; Miura, T.A. Attenuation of influenza A virus disease severity by viral coinfection in a mouse model. J. Virol. 2018, 92, e00881-18. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Mihaylova, V.T.; Landry, M.L.; Foxman, E.F. Interference between rhinovirus and influenza A virus: A clinical data analysis and experimental infection study. Lancet Microbe 2020, 1, e254–e262. [Google Scholar] [CrossRef] [PubMed]
- Van Leuven, J.T.; Gonzalez, A.J.; Ijezie, E.C.; Wixom, A.Q.; Clary, J.L.; Naranjo, M.N.; Ridenhour, B.J.; Miller, C.R.; Miura, T.A. Rhinovirus reduces the severity of subsequent respiratory viral infections by interferon-dependent and-independent mechanisms. Msphere 2021, 6, e00479-21. [Google Scholar] [CrossRef]
- Geiser, J.; Boivin, G.; Huang, S.; Constant, S.; Kaiser, L.; Tapparel, C.; Essaidi-Laziosi, M. RSV and HMPV Infections in 3D Tissue Cultures: Mechanisms Involved in Virus-Host and Virus-Virus Interactions. Viruses 2021, 13, 139. [Google Scholar] [CrossRef]
- Clark, J.J.; Penrice-Randal, R.; Sharma, P.; Kipar, A.; Dong, X.; Davidson, A.D.; Williamson, M.K.; Matthews, D.A.; Turtle, L.; Prince, T.; et al. Sequential infection with influenza A virus followed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to more severe disease and encephalitis in a mouse model of COVID-19. bioRxiv 2020, in press. [Google Scholar]
- Huang, Y.; Skarlupka, A.L.; Jang, H.; Blas-Machado, U.; Holladay, N.; Hogan, R.J.; Ross, T.M. SARS-CoV-2 and Influenza A virus Co-infections in Ferrets. J. Virol. 2022, 96, e0179121. [Google Scholar] [CrossRef]
- Zhang, A.J.; Lee, A.C.Y.; Chan, J.F.W.; Liu, F.; Li, C.; Chen, Y.; Chu, H.; Lau, S.Y.; Wang, P.; Chan, C.C.S.; et al. Coinfection by Severe Acute Respiratory Syndrome Coronavirus 2 and Influenza A (H1N1) pdm09 Virus Enhances the Severity of Pneumonia in Golden Syrian Hamsters. Clin. Infect. Dis. 2021, 72, e978–e992. [Google Scholar] [CrossRef]
- Bao, L.; Deng, W.; Qi, F.; Lv, Q.; Song, Z.; Liu, J.; Gao, H.; Wei, Q.; Yu, P.; Xu, Y.; et al. Sequential infection with H1N1 and SARS-CoV-2 aggravated COVID-19 pathogenesis in a mammalian model, and co-vaccination as an effective method of prevention of COVID-19 and influenza. Signal Transduct. Target. Ther. 2021, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhao, Y.; Dong, J.; Liang, S.; Guo, M.; Liu, X.; Wang, X.; Huang, Z.; Sun, X.; Zhang, Z.; et al. Coinfection with influenza A virus enhances SARS-CoV-2 infectivity. Cell Res. 2021, 31, 395–403. [Google Scholar] [CrossRef]
- Achdout, H.; Vitner, E.B.; Politi, B.; Melamed, S.; Yahalom-Ronen, Y.; Tamir, H.; Erez, N.; Avraham, R.; Weiss, S.; Cherry, L.; et al. Increased lethality in influenza and SARS-CoV-2 coinfection is prevented by influenza immunity but not SARS-CoV-2 immunity. Nat. Commun. 2021, 12, 5819. [Google Scholar] [CrossRef] [PubMed]
- Fage, C.; Hénaut, M.; Carbonneau, J.; Piret, J.; Boivin, G. Influenza A (H1N1) pdm09 Virus but Not Respiratory Syncytial Virus Interferes with SARS-CoV-2 Replication during Sequential Infections in Human Nasal Epithelial Cells. Viruses 2022, 14, 395. [Google Scholar] [CrossRef] [PubMed]
- Dee, K.; Goldfarb, D.M.; Haney, J.; Amat, J.A.; Herder, V.; Stewart, M.; Szemiel, A.M.; Baguelin, M.; Murcia, P.R. Human rhinovirus infection blocks severe acute respiratory syndrome coronavirus 2 replication within the respiratory epithelium: Implications for COVID-19 epidemiology. J. Infect. Dis. 2021, 224, 31–38. [Google Scholar] [CrossRef]
- Smith, A.M. Host-pathogen kinetics during influenza infection and coinfection: Insights from predictive modeling. Immunol. Rev. 2018, 285, 97–112. [Google Scholar] [CrossRef]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the respiratory tract: Viral competition for resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.A.; Smith, A.P.; Lane, L.C.; Moquin, D.J.; Vogel, P.; Woolard, S.; Smith, A.M. Dynamically linking influenza virus infection with lung injury to predict disease severity. eLife 2021, 10, e68864. [Google Scholar] [CrossRef]
- Smith, A.P.; Lane, L.C.; Ramirez Zuniga, I.; Moquin, D.M.; Vogel, P.; Smith, A.M. Increased virus dissemination leads to enhanced lung injury but not inflammation during influenza-associated secondary bacterial infection. FEMS Microbes 2022, 3, xtac022. [Google Scholar] [CrossRef]
- Murphy, B.R.; Rennels, M.B.; Douglas Jr, R.G.; Betts, R.F.; Couch, R.B.; Cate Jr, T.R.; Chanock, R.M.; Kendal, A.P.; Maassab, H.F.; Suwanagool, S.; et al. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect. Immun. 1980, 29, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Hendley, J.O.; Gwaltney Jr, J.M. Viral titers in nasal lining fluid compared to viral titers in nasal washes during experimental rhinovirus infection. J. Clin. Virol. 2004, 30, 326–328. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Smith, A.M.; Perelson, A.S. Influenza A virus infection kinetics: Quantitative data and models. Wiley Interdiscip. Rev. Syst. Biol. Med. 2011, 3, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.; Herrler, G.; Klenk, H.D. Sialic acid receptors of viruses. Top. Curr. Chem. 2015, 367, 1–28. [Google Scholar] [PubMed]
- Johansen, M.; Irving, A.; Montagutelli, X.; Tate, M.; Rudloff, I.; Nold, M.; Hansbro, N.; Kim, R.; Donovan, C.; Liu, G.; et al. Animal and translational models of SARS-CoV-2 infection and COVID-19. Mucosal Immunol. 2020, 13, 877–891. [Google Scholar] [CrossRef]
- Kogure, T.; Suzuki, T.; Takahashi, T.; Miyamoto, D.; Hidari, K.I.; Guo, C.T.; Ito, T.; Kawaoka, Y.; Suzuki, Y. Human trachea primary epithelial cells express both sialyl (α2-3) Gal receptor for human parainfluenza virus type 1 and avian influenza viruses, and sialyl (α2-6) Gal receptor for human influenza viruses. Glycoconj. J. 2006, 23, 101–106. [Google Scholar] [CrossRef]
- Bohmwald, K.; Galvez, N.; Canedo-Marroquín, G.; Pizarro-Ortega, M.S.; Andrade-Parra, C.; Gómez-Santander, F.; Kalergis, A.M. Contribution of cytokines to tissue damage during human respiratory syncytial virus infection. Front. Immunol. 2019, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Shinya, K.; Ebina, M.; Yamada, S.; Ono, M.; Kasai, N.; Kawaoka, Y. Influenza virus receptors in the human airway. Nature 2006, 440, 435–436. [Google Scholar] [CrossRef]
- Antony, F. Monolix Version 2019R1. 2020. Available online: http://lixoft.com/products/monolix/ (accessed on 28 May 2023).
- Smith, A.P.; Moquin, D.J.; Bernhauerova, V.; Smith, A.M. Influenza virus infection model with density dependence supports biphasic viral decay. Front. Microbiol. 2018, 9, 1554. [Google Scholar] [CrossRef]
- Pinky, L.; Burke, C.W.; Russell, C.J.; Smith, A.M. Quantifying dose-, strain-, and tissue-specific kinetics of parainfluenza virus infection. PLoS Comput. Biol. 2021, 17, e1009299. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Springer: Berlin/Heidelberg, Germany, 1998; pp. 199–213. [Google Scholar]
- Kenney, L.L.; Cornberg, M.; Chen, A.T.; Emonet, S.; de la Torre, J.C.; Selin, L.K. Increased immune response variability during simultaneous viral coinfection leads to unpredictability in CD8 T cell immunity and pathogenesis. J. Virol. 2015, 89, 10786–10801. [Google Scholar] [CrossRef]
- Smith, A.M.; Adler, F.R.; Perelson, A.S. An accurate two-phase approximate solution to an acute viral infection model. J. Math. Biol. 2010, 60, 711–726. [Google Scholar] [CrossRef]
- Smith, A.M. Validated models of immune response to virus infection. Curr. Opin. Syst. Biol. 2018, 12, 46–52. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Description | Units | IAV () | RSV () |
|---|---|---|---|---|
| Viral infectivity | (RNA/100 L) d | (0.6) | (0.04) | |
| k | Eclipse phase | d | 5.0 | 3.0 |
| Infected cell clearance | d | 0.9 (0.02) | 1.1 (1.8) | |
| p | Viral production | (RNA/100 L) cell d | (0.2) | (0.03) |
| c | Viral clearance | d | 2.0 (0.06) | 1.7 (0.5) |
| Initial target cells | cells | |||
| Initial eclipse cells | cells | |||
| Initial infected cells | cells | 0 | 0 | |
| Initial virus | (RNA/100 L) | 0 | 0 |
| Interaction | Effect on IAV | Effect on RSV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Strength of | Strength of | Strength of | Strength of | -2LL | |||||||
| Enhancement, | Inhibition, | Enhancement, | Inhibition, | ||||||||
| IAV | RSV | ||||||||||
| RNA | RNA | RNA | RNA | ||||||||
| Competition | No interaction | 0 | 0 | 0 | 0 | 185.0 | 189.0 | 0.79 | 0.81 | −0.54 | −0.48 |
| – | (0.1) | – | – | 166.2 | 174.2 | 0.79 | 0.65 | −0.20 | −0.48 | ||
| – | (0.1) | – | – | 167.8 | 175.8 | 0.79 | 0.66 | 0.74 | −0.49 | ||
| and | – | (0.1) | – | (0.2) | 163.7 | 175.7 | 0.76 | 0.65 | −0.18 | −0.66 | |
| and | – | (0.1) | (0.2) | – | 163.8 | 175.8 | 0.76 | 0.65 | −0.19 | −0.63 | |
| Partitioning | No interaction | 0 | 0 | 0 | 0 | 181.3 | 185.3 | 0.88 | 0.72 | 0 | 0 |
| – | – | (0.04) | – | 131.0 | 139.0 | 0.40 | 0.72 | 0 | −0.51 | ||
| and | – | (0.04) | – | (0.1) | 127.3 | 139.3 | 0.45 | 0.63 | 0 | −0.53 | |
| Parameter | Description | Units | IAV | RV | SARS-CoV-2 |
|---|---|---|---|---|---|
| Viral infectivity | [V] d | ||||
| k | Eclipse phase | d | 5.0 | 3.0 | 3.0 |
| Infected cell clearance | d | 3.9 | 5.7 | 4.5 | |
| p | Viral production | [V] cell d | 18.8 | ||
| c | Viral clearance | d | 3.9 | 5.6 | 1.8 |
| Initial target cells | cells | ||||
| Initial eclipse cells | cells | 100 | 100 | 100 | |
| Initial infected cells | cells | 0 | 0 | 0 | |
| Initial virus | [V] | 0 | 0 | 0 | |
| Strength of interaction | [V] | See text | See text | See text |
| Model | First Virus | Second Virus | Infection Interval (Days) | Observed Disease Severity (Ref.) | Potential Mechanisms | Figure |
|---|---|---|---|---|---|---|
| Competition | RSV | IAV | 3 | Decreased [27] | IAV-infected cell clearance reduced by RSV and RSV production reduced by IAV | Figure 2B |
| IAV infected cell clearance reduced by RSV | Figure A1A | |||||
| IAV clearance reduced by RSV | Figure A1A | |||||
| IAV infected cell clearance reduced by RSV and RSV clearance increased by IAV | Figure A1B | |||||
| IAV | RV | 0 | Decreased [33] | RV infected cell clearance reduced by IAV | Figure 3B | |
| RV | IAV | 2 | Decreased [33] | RV infected cell clearance reduced by IAV | Figure 3C | |
| IAV | RV | 2 | Increased [33] | IAV infected cell clearance reduced by RV | Figure 3D | |
| IAV | SARS-CoV-2 | 3 | Increased [37] | IAV infected cell clearance reduced by SARS-CoV-2 | Figure 3E | |
| Partitioning | RSV | IAV | 3 | Decreased [27] | IAV infectivity reduced by RSV and RSV production reduced by IAV | Figure 2C |
| RSV-infected cell clearance increased by IAV | Figure A1C | |||||
| IAV | RV | 0 | Decreased [33] | RV infected cell clearance reduced by IAV and IAV infected cell clearance increased by RV | Figure 4A | |
| RV infected cell clearance reduced by IAV and IAV clearance reduced by RV | Figure A3A | |||||
| RV infected cell clearance reduced by IAV and IAV production reduced by RV | Figure A3B | |||||
| RV infected cell clearance reduced by IAV and IAV infectivity reduced by RV | Figure A3C | |||||
| RV | IAV | 2 | Decreased [33] | Reduced number of target cells for IAV | Figure 4B | |
| IAV | RV | 2 | Increased [33] | RV production increased by IAV | Figure 4C | |
| No interaction | Figure 4C | |||||
| RV infectivity increased by IAV | Figure A3D | |||||
| Reduced number of target cells for RV and IAV infected cell clearance reduced by RV | Figure A3E | |||||
| Reduced number of target cells for RV and RV infected cell clearance reduced by IAV | Figure A3F | |||||
| IAV | SARS-CoV-2 | 3 | Increased [37] | SARS-CoV-2 production increased by IAV | Figure 4D | |
| No interaction | Figure 4D | |||||
| SARS-CoV-2 infectivity increased by IAV | Figure A4A | |||||
| Reduced number of target cells for SARS-CoV-2 and IAV infected cell clearance reduced by SARS-CoV-2 | Figure A4B | |||||
| Reduced number of target cells for SARS-CoV-2 and SARS-CoV-2-infected cell clearance reduced by IAV | Figure A4C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinky, L.; DeAguero, J.R.; Remien, C.H.; Smith, A.M. How Interactions during Viral–Viral Coinfection Can Shape Infection Kinetics. Viruses 2023, 15, 1303. https://doi.org/10.3390/v15061303
Pinky L, DeAguero JR, Remien CH, Smith AM. How Interactions during Viral–Viral Coinfection Can Shape Infection Kinetics. Viruses. 2023; 15(6):1303. https://doi.org/10.3390/v15061303
Chicago/Turabian StylePinky, Lubna, Joseph R. DeAguero, Christopher H. Remien, and Amber M. Smith. 2023. "How Interactions during Viral–Viral Coinfection Can Shape Infection Kinetics" Viruses 15, no. 6: 1303. https://doi.org/10.3390/v15061303
APA StylePinky, L., DeAguero, J. R., Remien, C. H., & Smith, A. M. (2023). How Interactions during Viral–Viral Coinfection Can Shape Infection Kinetics. Viruses, 15(6), 1303. https://doi.org/10.3390/v15061303






