Cholesterol-Lowering Drugs as Potential Antivirals: A Repurposing Approach against Flavivirus Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture, Virus, and Reagents
2.2. Flavivirus Infection and Treatment
2.3. Flow Cytometry
2.4. Cell Viability Assay, CC50, IC50, and Selectivity Index (SI) Determination
2.5. Drug Combination Assay and Synergism
2.6. Treatment and Survival Assays in the AG129 Mouse Model
2.7. Statistics Analysis
2.8. Ethical Statements
3. Results
3.1. Effect of Cholesterol-Lowering Drugs Atorvastatin and Ezetimibe in Monotherapy on In Vitro DENV, ZIKV, and YFV Infection
3.2. Effect of Cholesterol-Lowering Drugs in Combination on In Vitro DENV, ZIKV, and YFV Infection
3.3. Combination and Synergism
3.4. The Effect of Monotherapy and Combination Therapy with Ezetimibe and Atorvastatin on DENV 2-Infected AG129 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suwanmanee, S.; Luplertlop, N. Dengue and Zika Viruses: Lessons Learned from the Similarities between These Aedes Mosquito-Vectored Arboviruses. J. Microbiol. 2017, 55, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Chippaux, J.-P.; Chippaux, A. Yellow Fever in Africa and the Americas: A Historical and Epidemiological Perspective. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente, C.R.; da Silva, T.C.C.; Pereira, L.D.; Miranda, A.E. Impact of Concurrent Epidemics of Dengue, Chikungunya, Zika, and COVID-19. Rev. Soc. Bras. Med. Trop. 2021, 54, e08372020. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P.; Vasconcelos, P.F.C. Yellow Fever. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2015, 64, 160–173. [Google Scholar] [CrossRef]
- Osuna-Ramos, J.F.; Reyes-Ruiz, J.M.; del Ángel, R.M. The Role of Host Cholesterol During Flavivirus Infection. Front. Cell. Infect. Microbiol. 2018, 8, 388. [Google Scholar] [CrossRef] [Green Version]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Reyes-Ruiz, J.M.; Hurtado-Monzón, A.M.; Osuna-Ramos, J.F.; González-González, A.M.; De Jesús-González, L.A.; Palacios-Rápalo, S.N.; del Ángel, R.M. Anti-Flavivirus Properties of Lipid-Lowering Drugs. Front. Physiol. 2021, 12, 749770. [Google Scholar] [CrossRef]
- Soto-Acosta, R.; Mosso, C.; Cervantes-Salazar, M.; Puerta-Guardo, H.; Medina, F.; Favari, L.; Ludert, J.E.; del Angel, R.M. The Increase in Cholesterol Levels at Early Stages after Dengue Virus Infection Correlates with an Augment in LDL Particle Uptake and HMG-CoA Reductase Activity. Virology 2013, 442, 132–147. [Google Scholar] [CrossRef] [Green Version]
- Soto-Acosta, R.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Angel-Ambrocio, A.H.; Del Angel, R.M. DENV Up-Regulates the HMG-CoA Reductase Activity through the Impairment of AMPK Phosphorylation: A Potential Antiviral Target. PLoS Pathog. 2017, 13, e1006257. [Google Scholar] [CrossRef] [Green Version]
- Osuna-Ramos, J.F.; Reyes-Ruiz, J.M.; Bautista-Carbajal, P.; Cervantes-Salazar, M.; Farfan-Morales, C.N.; De Jesús-González, L.A.; Hurtado-Monzon, A.M.; del Ángel, R.M. Ezetimibe Inhibits Dengue Virus Infection in Huh-7 Cells by Blocking the Cholesterol Transporter Niemann–Pick C1-like 1 Receptor. Antivir. Res. 2018, 160, 151–164. [Google Scholar] [CrossRef]
- Rothwell, C.; LeBreton, A.; Young Ng, C.; Lim, J.Y.H.; Liu, W.; Vasudevan, S.; Labow, M.; Gu, F.; Gaither, L.A. Cholesterol Biosynthesis Modulation Regulates Dengue Viral Replication. Virology 2009, 389, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carro, A.C.; Damonte, E.B. Requirement of Cholesterol in the Viral Envelope for Dengue Virus Infection. Virus Res. 2013, 174, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug Repurposing: A Promising Tool to Accelerate the Drug Discovery Process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug Repurposing: Progress, Challenges and Recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Botta, L.; Rivara, M.; Zuliani, V.; Radi, M. Drug Repurposing Approaches to Fight Dengue Virus Infection and Related Diseases. Front. Biosci. (Landmark Ed.) 2018, 23, 997–1019. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, J.; Mohan, M.; Byrareddy, S.N. Drug Repurposing Approaches to Combating Viral Infections. J. Clin. Med. 2020, 9, 3777. [Google Scholar] [CrossRef]
- Españo, E.; Nam, J.-H.; Song, E.-J.; Song, D.; Lee, C.-K.; Kim, J.-K. Lipophilic Statins Inhibit Zika Virus Production in Vero Cells. Sci. Rep. 2019, 9, 11461. [Google Scholar] [CrossRef] [Green Version]
- Lucifora, J.; Esser, K.; Protzer, U. Ezetimibe Blocks Hepatitis B Virus Infection after Virus Uptake into Hepatocytes. Antivir. Res. 2013, 97, 195. [Google Scholar] [CrossRef]
- Shrivastava, S.; Trivedi, J.; Mitra, D. Gene Expression Profiling Reveals Nef Induced Deregulation of Lipid Metabolism in HIV-1 Infected T Cells. Biochem. Biophys. Res. Commun. 2016, 472, 169–174. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Gutierrez, M.; Correa-Londoño, L.A.; Castellanos, J.E.; Gallego-Gómez, J.C.; Osorio, J.E. Lovastatin Delays Infection and Increases Survival Rates in AG129 Mice Infected with Dengue Virus Serotype 2. PLoS ONE 2014, 9, e87412. [Google Scholar] [CrossRef]
- Sainz, B.; Barretto, N.; Martin, D.N.; Hiraga, N.; Imamura, M.; Hussain, S.; Marsh, K.A.; Yu, X.; Chayama, K.; Alrefai, W.A.; et al. Identification of the Niemann-Pick C1-like 1 Cholesterol Absorption Receptor as a New Hepatitis C Virus Entry Factor. Nat. Med. 2012, 18, 281–285. [Google Scholar] [CrossRef]
- Farfan-Morales, C.N.; Cordero-Rivera, C.D.; Osuna-Ramos, J.F.; Monroy-Muñoz, I.E.; De Jesús-González, L.A.; Muñoz-Medina, J.E.; Hurtado-Monzón, A.M.; Reyes-Ruiz, J.M.; Del Ángel, R.M. The Antiviral Effect of Metformin on Zika and Dengue Virus Infection. Sci. Rep. 2021, 11, 8743. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 2.0: Visual Analytics of Multi-Drug Combination Synergies. Nucleic Acids Res. 2020, 48, W488–W493. [Google Scholar] [CrossRef]
- Orozco, S.; Schmid, M.A.; Parameswaran, P.; Lachica, R.; Henn, M.R.; Beatty, R.; Harris, E. Characterization of a Model of Lethal Dengue Virus 2 Infection in C57BL/6 Mice Deficient in the Alpha/Beta Interferon Receptor. J. Gen. Virol. 2012, 93, 2152–2157. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; Giri, A.K.; Gautam, P.; Kononov, A.; Potdar, S.; Saarela, J.; Wennerberg, K.; Aittokallio, T. Prediction of Drug Combination Effects with a Minimal Set of Experiments. Nat. Mach. Intell. 2019, 1, 568–577. [Google Scholar] [CrossRef]
- Holbrook, M.R. Historical Perspectives on Flavivirus Research. Viruses 2017, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirtori, C.R. The Pharmacology of Statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef]
- Banach, M.; Penson, P.E. Lipid-Lowering Therapies: Better Together. Atherosclerosis 2021, 320, 86–88. [Google Scholar] [CrossRef]
- Castiglione, V.; Chiriacò, M.; Emdin, M.; Taddei, S.; Vergaro, G. Statin Therapy in COVID-19 Infection. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 258–259. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Bianconi, V.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Pirro, M.; Sahebkar, A. Antiviral Effects of Statins. Prog. Lipid Res. 2020, 79, 101054. [Google Scholar] [CrossRef]
- Wani, M.A.; Mukherjee, S.; Mallick, S.; Akbar, I.; Basu, A. Atorvastatin Ameliorates Viral Burden and Neural Stem/ Progenitor Cell (NSPC) Death in an Experimental Model of Japanese Encephalitis. J. Biosci. 2020, 45, 77. [Google Scholar] [CrossRef] [PubMed]
- Gower, T.L.; Graham, B.S. Antiviral Activity of Lovastatin against Respiratory Syncytial Virus In Vivo and In Vitro. Antimicrob. Agents Chemother. 2001, 45, 1231–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San-Juan-Vergara, H.; Sampayo-Escobar, V.; Reyes, N.; Cha, B.; Pacheco-Lugo, L.; Wong, T.; Peeples, M.E.; Collins, P.L.; Castaño, M.E.; Mohapatra, S.S. Cholesterol-Rich Microdomains as Docking Platforms for Respiratory Syncytial Virus in Normal Human Bronchial Epithelial Cells. J. Virol. 2012, 86, 1832–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajimaya, S.; Frankl, T.; Hayashi, T.; Takimoto, T. Cholesterol Is Required for Stability and Infectivity of Influenza A and Respiratory Syncytial Viruses. Virology 2017, 510, 234–241. [Google Scholar] [CrossRef]
- Mañes, S.; del Real, G.; Lacalle, R.A.; Lucas, P.; Gómez-Moutón, C.; Sánchez-Palomino, S.; Delgado, R.; Alcamí, J.; Mira, E.; Martínez-A, C. Membrane Raft Microdomains Mediate Lateral Assemblies Required for HIV-1 Infection. EMBO Rep. 2000, 1, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Liao, Z.; Graham, D.R.; Hildreth, J.E.K. Lipid Rafts and HIV Pathogenesis: Virion-Associated Cholesterol Is Required for Fusion and Infection of Susceptible Cells. AIDS Res. Hum. Retrovir. 2003, 19, 675–687. [Google Scholar] [CrossRef]
- Bley, H.; Schöbel, A.; Herker, E. Whole Lotta Lipids-from HCV RNA Replication to the Mature Viral Particle. Int. J. Mol. Sci. 2020, 21, 2888. [Google Scholar] [CrossRef] [Green Version]
- Ge, L.; Wang, J.; Qi, W.; Miao, H.-H.; Cao, J.; Qu, Y.-X.; Li, B.-L.; Song, B.-L. The Cholesterol Absorption Inhibitor Ezetimibe Acts by Blocking the Sterol-Induced Internalization of NPC1L1. Cell Metab. 2008, 7, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.-Y.; Chang, C. Ezetimibe Blocks Internalization of the NPC1L1/Cholesterol Complex. Cell Metab. 2008, 7, 469–471. [Google Scholar] [CrossRef] [Green Version]
- Carette, J.E.; Raaben, M.; Wong, A.C.; Herbert, A.S.; Obernosterer, G.; Mulherkar, N.; Kuehne, A.I.; Kranzusch, P.J.; Griffin, A.M.; Ruthel, G.; et al. Ebola Virus Entry Requires the Cholesterol Transporter Niemann-Pick C1. Nature 2011, 477, 340–343. [Google Scholar] [CrossRef] [Green Version]
- Herbert, A.S.; Davidson, C.; Kuehne, A.I.; Bakken, R.; Braigen, S.Z.; Gunn, K.E.; Whelan, S.P.; Brummelkamp, T.R.; Twenhafel, N.A.; Chandran, K.; et al. Niemann-Pick C1 Is Essential for Ebolavirus Replication and Pathogenesis In Vivo. mBio 2015, 6, e00565-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Españo, E.; Kim, J.K. Effects of Statin Combinations on Zika Virus Infection in Vero Cells. Pharmaceutics 2022, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Bryan-Marrugo, O.L.; Arellanos-Soto, D.; Rojas-Martinez, A.; Barrera-Saldaña, H.; Ramos-Jimenez, J.; Vidaltamayo, R.; Rivas-Estilla, A.M. The Anti-dengue Virus Properties of Statins May Be Associated with Alterations in the Cellular Antiviral Profile Expression. Mol. Med. Rep. 2016, 14, 2155–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Gutierrez, M.; Castellanos, J.E.; Gallego-Gómez, J.C. Statins Reduce Dengue Virus Production via Decreased Virion Assembly. Intervirology 2011, 54, 202–216. [Google Scholar] [CrossRef]
- Vicente, C.R.; Herbinger, K.-H.; Fröschl, G.; Malta Romano, C.; de Souza Areias Cabidelle, A.; Cerutti Junior, C. Serotype Influences on Dengue Severity: A Cross-Sectional Study on 485 Confirmed Dengue Cases in Vitória, Brazil. BMC Infect. Dis. 2016, 16, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino-Ramos, T.; Vázquez-Calvo, Á.; Casas, J.; Sobrino, F.; Saiz, J.C.; Martín-Acebes, M.A. Modification of the Host Cell Lipid Metabolism Induced by Hypolipidemic Drugs Targeting the Acetyl Coenzyme A Carboxylase Impairs West Nile Virus Replication. Antimicrob. Agents Chemother. 2015, 60, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gao, W.; Li, J.; Wu, W.; Jiu, Y. The Role of Host Cytoskeleton in Flavivirus Infection. Virol. Sin. 2019, 34, 30–41. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The Continued Threat of Emerging Flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Charlier, N.; Davidson, A.; Dallmeier, K.; Molenkamp, R.; De Clercq, E.; Neyts, J. Replication of Not-Known-Vector Flaviviruses in Mosquito Cells Is Restricted by Intracellular Host Factors Rather than by the Viral Envelope Proteins. J. Gen. Virol. 2010, 91, 1693–1697. [Google Scholar] [CrossRef]
- Altmann, S.W.; Davis, H.R.; Zhu, L.J.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.P.N.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 Protein Is Critical for Intestinal Cholesterol Absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [Green Version]
- Davis, H.R.; Zhu, L.J.; Hoos, L.M.; Tetzloff, G.; Maguire, M.; Liu, J.; Yao, X.; Iyer, S.P.N.; Lam, M.H.; Lund, E.G.; et al. Niemann-Pick C1 Like 1 (NPC1L1) Is the Intestinal Phytosterol and Cholesterol Transporter and a Key Modulator of Whole-Body Cholesterol Homeostasis. J. Biol. Chem. 2004, 279, 33586–33592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sané, A.T.; Sinnett, D.; Delvin, E.; Bendayan, M.; Marcil, V.; Ménard, D.; Beaulieu, J.F.; Levy, E. Localization and Role of NPC1L1 in Cholesterol Absorption in Human Intestine. J. Lipid Res. 2006, 47, 2112–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]

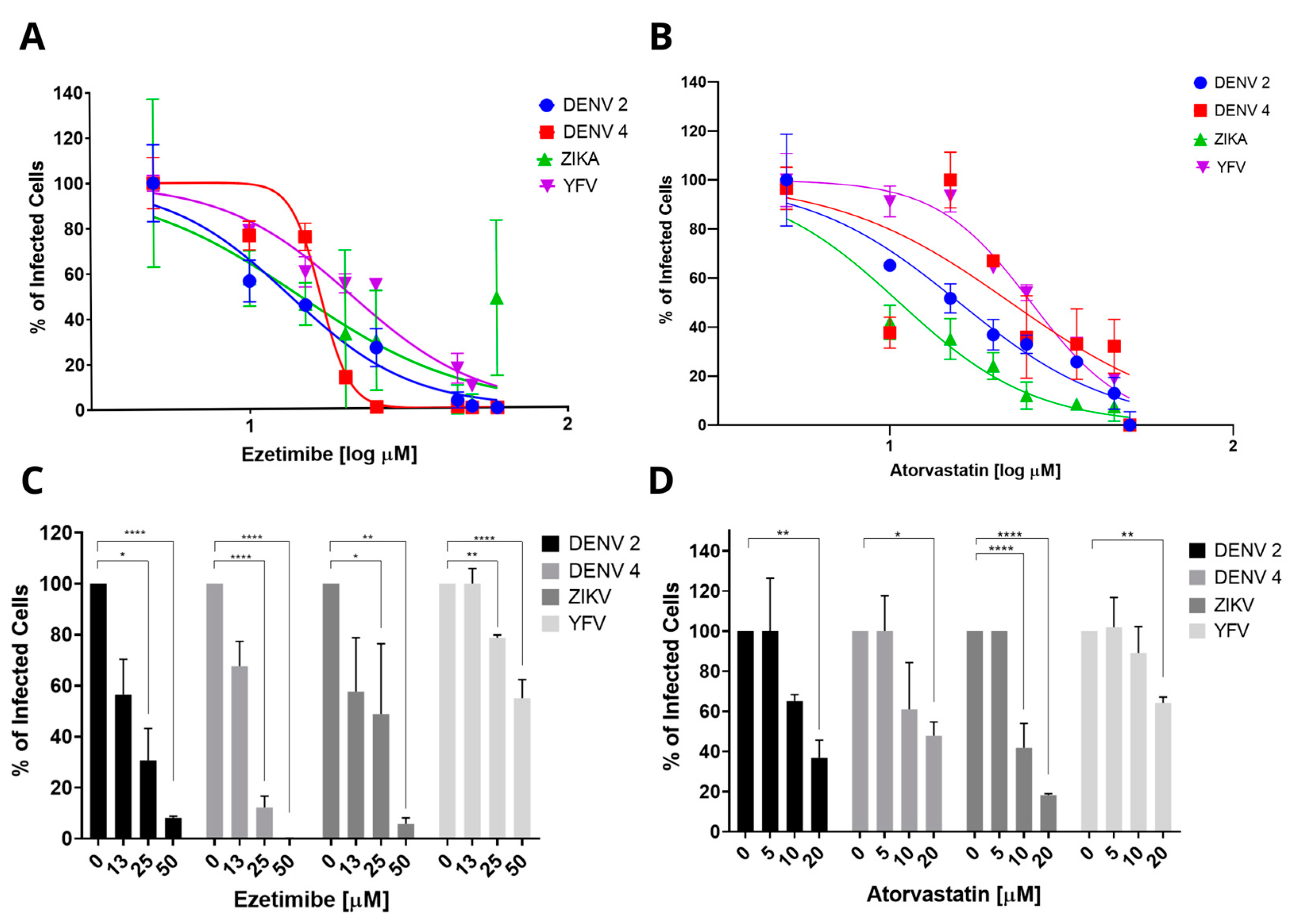
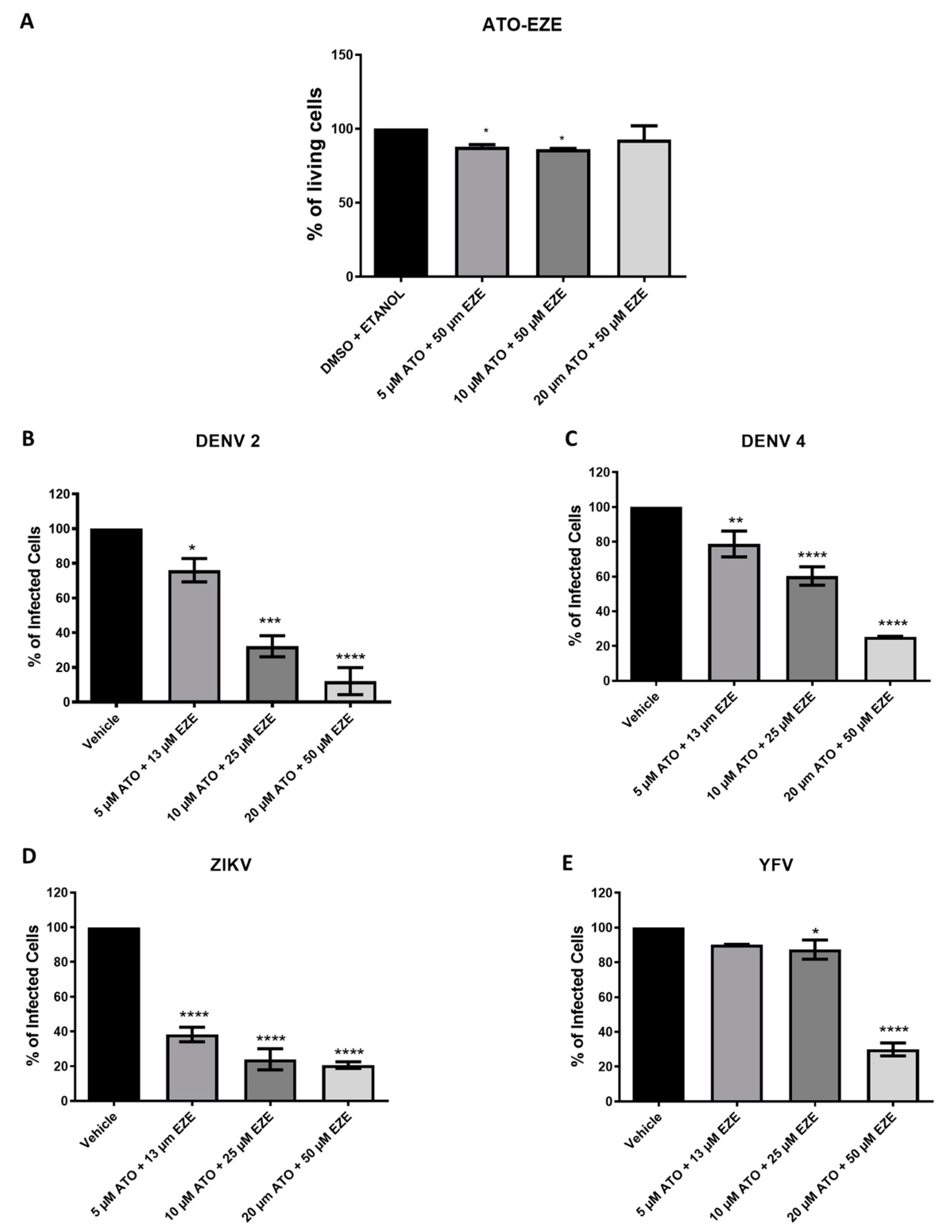
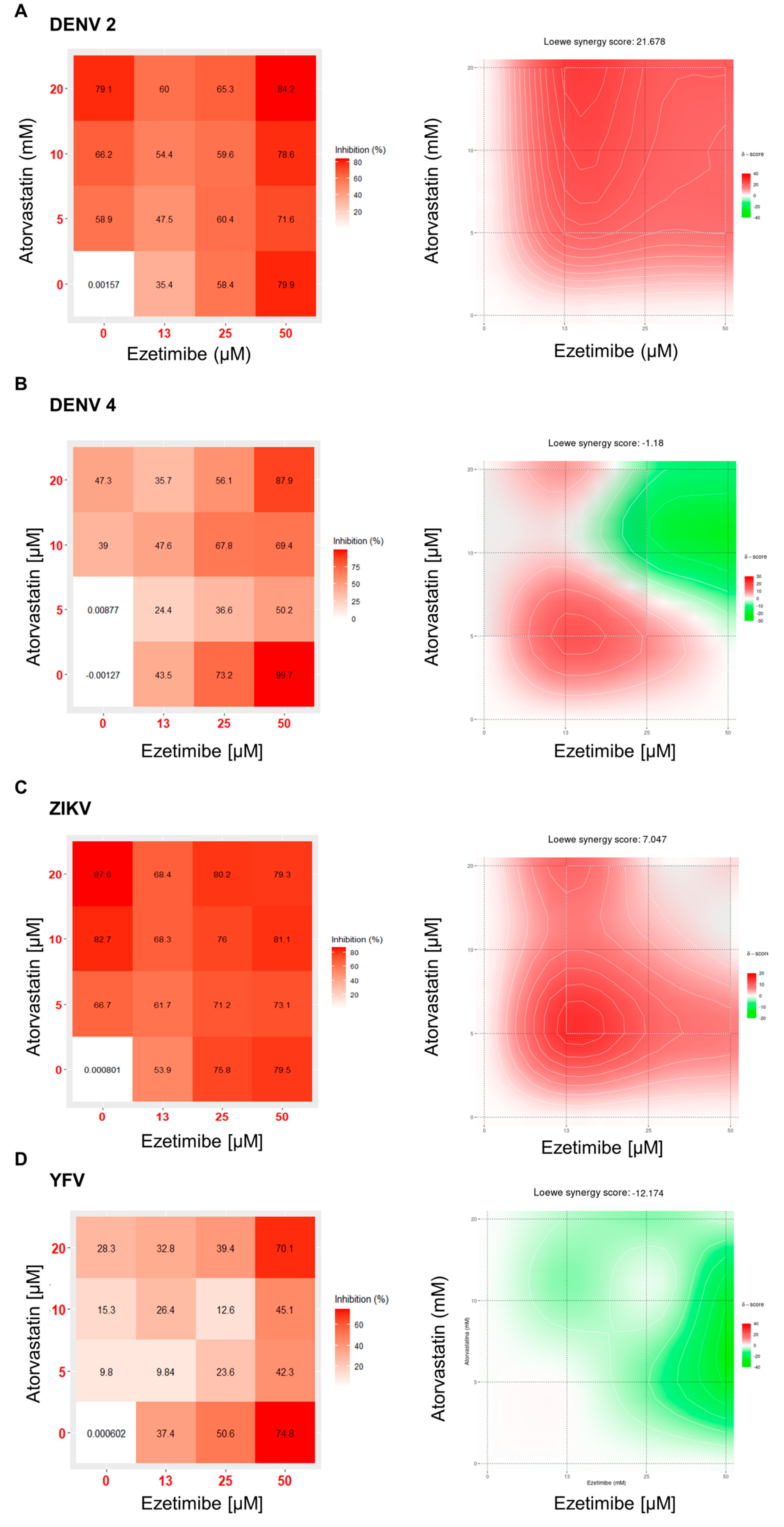

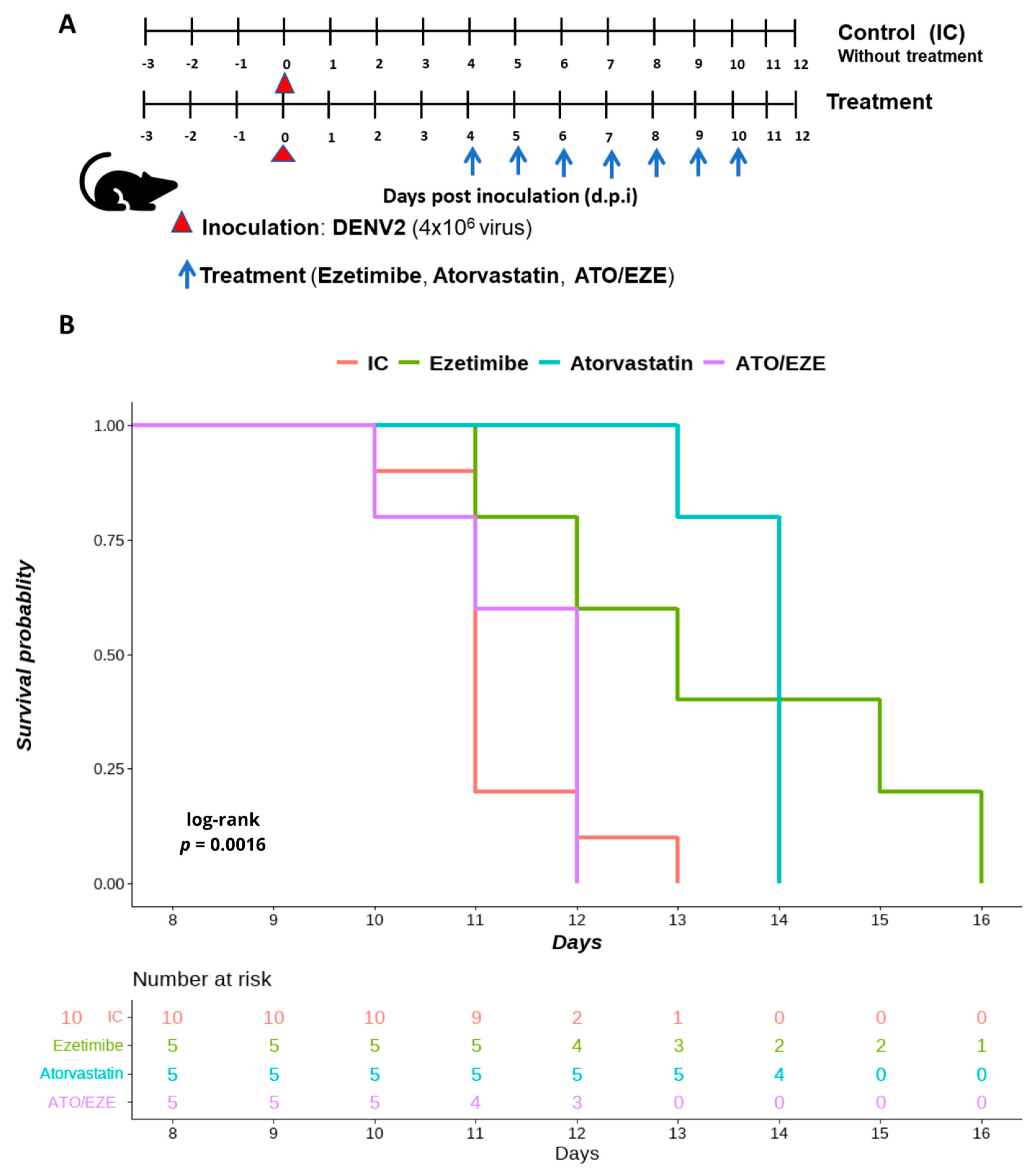
| Virus | Ezetimibe | Atorvastatin |
|---|---|---|
| DENV 2 | 2.93 * | 2.35 |
| DENV 4 | 2.34 * | 1.67 |
| ZIKV | 2.73 | 3.67 |
| YFV | 1.87 | 1.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osuna-Ramos, J.F.; Farfan-Morales, C.N.; Cordero-Rivera, C.D.; De Jesús-González, L.A.; Reyes-Ruiz, J.M.; Hurtado-Monzón, A.M.; Palacios-Rápalo, S.N.; Jiménez-Camacho, R.; Meraz-Ríos, M.A.; Del Ángel, R.M. Cholesterol-Lowering Drugs as Potential Antivirals: A Repurposing Approach against Flavivirus Infections. Viruses 2023, 15, 1465. https://doi.org/10.3390/v15071465
Osuna-Ramos JF, Farfan-Morales CN, Cordero-Rivera CD, De Jesús-González LA, Reyes-Ruiz JM, Hurtado-Monzón AM, Palacios-Rápalo SN, Jiménez-Camacho R, Meraz-Ríos MA, Del Ángel RM. Cholesterol-Lowering Drugs as Potential Antivirals: A Repurposing Approach against Flavivirus Infections. Viruses. 2023; 15(7):1465. https://doi.org/10.3390/v15071465
Chicago/Turabian StyleOsuna-Ramos, Juan Fidel, Carlos Noe Farfan-Morales, Carlos Daniel Cordero-Rivera, Luis Adrián De Jesús-González, José Manuel Reyes-Ruiz, Arianna M. Hurtado-Monzón, Selvin Noé Palacios-Rápalo, Ricardo Jiménez-Camacho, Marco Antonio Meraz-Ríos, and Rosa María Del Ángel. 2023. "Cholesterol-Lowering Drugs as Potential Antivirals: A Repurposing Approach against Flavivirus Infections" Viruses 15, no. 7: 1465. https://doi.org/10.3390/v15071465








