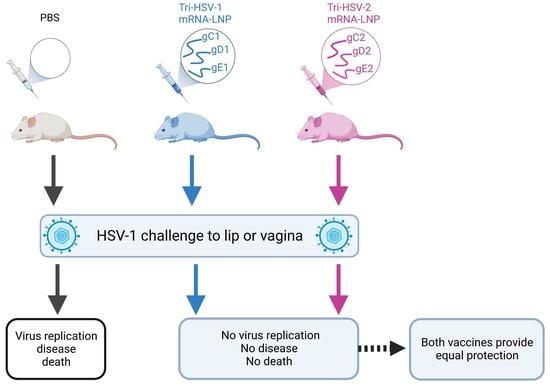
A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tri-HSV-1 and Tri-HSV-2 mRNA Immunogens
2.2. Immunization of Mice
2.3. Immunology Assays
2.4. Mouse Challenge Models
2.4.1. HSV-1 Lip Infection
2.4.2. HSV-1 Vaginal Infection
2.5. Statistical Analysis
3. Results
3.1. IgG ELISA and Neutralizing Antibody Responses to Tri-HSV-1 and Tri-HSV-2 Vaccines
3.2. CD4+ and CD8+ T-Cell Responses Produced by Tri-HSV-1 and Tri-HSV-2 Immunization
3.3. HSV-1 Challenge Infection Using the Mouse Lip Infection Model
3.4. HSV-1 Challenge Infection Using the Mouse Genital Infection Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, C.; Harfouche, M.; Welton, N.J.; Turner, K.M.; Abu-Raddad, L.J.; Gottlieb, S.L.; Looker, K.J. Herpes simplex virus: Global infection prevalence and incidence estimates, 2016. Bull. World Health Organ. 2020, 98, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Labib, B.A.; Chigbu, D.I. Clinical Management of Herpes Simplex Virus Keratitis. Diagnostics 2022, 12, 2368. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.; Beckham, J.D.; Piquet, A.L.; Tyler, K.L.; Chauhan, L.; Pastula, D.M. Herpesvirus-Associated Encephalitis: An Update. Curr. Trop. Med. Rep. 2022, 9, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [Green Version]
- Engelberg, R.; Carrell, D.; Krantz, E.; Corey, L.; Wald, A. Natural history of genital herpes simplex virus type 1 infection. Sex. Transm. Dis. 2003, 30, 174–177. [Google Scholar] [CrossRef]
- Johnston, C.; Magaret, A.; Son, H.; Stern, M.; Rathbun, M.; Renner, D.; Szpara, M.; Gunby, S.; Ott, M.; Jing, L.; et al. Viral Shedding 1 Year Following First-Episode Genital HSV-1 Infection. JAMA 2022, 328, 1730. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Newman, L.M.; Gottlieb, S.L. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health 2017, 5, e300–e309. [Google Scholar] [CrossRef] [Green Version]
- Prober, C.G.; Sullender, W.M.; Yasukawa, L.L.; Au, D.S.; Yeager, A.S.; Arvin, A.M. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N. Engl. J. Med. 1987, 316, 240–244. [Google Scholar] [CrossRef]
- Langenberg, A.G.; Corey, L.; Ashley, R.L.; Leong, W.P.; Straus, S.E. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. N. Engl. J. Med. 1999, 341, 1432–1438. [Google Scholar] [CrossRef]
- Brown, Z.A.; Selke, S.; Zeh, J.; Kopelman, J.; Maslow, A.; Ashley, R.L.; Watts, D.H.; Berry, S.; Herd, M.; Corey, L. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 1997, 337, 509–515. [Google Scholar] [CrossRef]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belshe, R.B.; Heineman, T.C.; Bernstein, D.I.; Bellamy, A.R.; Ewell, M.; van der Most, R.; Deal, C.D. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J. Infect. Dis. 2014, 209, 828–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, S.; Balliet, J.W.; Flynn, J.A.; Lubinski, J.M.; Shaw, C.E.; DiStefano, D.J.; Cai, M.; Brown, M.; Smith, J.F.; Kowalski, R.; et al. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. J. Virol. 2014, 88, 2000–2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, S.; Hook, L.M.; Pardi, N.; Wang, F.; Myles, A.; Cancro, M.P.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 2019, 4, eaaw7083. [Google Scholar] [CrossRef]
- Egan, K.P.; Hook, L.M.; Naughton, A.; Pardi, N.; Awasthi, S.; Cohen, G.H.; Weissman, D.; Friedman, H.M. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection. PLoS Pathog. 2020, 16, e1008795. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human Papillomavirus Vaccines: An Updated Review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- Chang, Y.J.; Jiang, M.; Lubinski, J.M.; King, R.D.; Friedman, H.M. Implications for herpes simplex virus vaccine strategies based on antibodies produced to herpes simplex virus type 1 glycoprotein gC immune evasion domains. Vaccine 2005, 23, 4658–4665. [Google Scholar] [CrossRef]
- Sisk, W.P.; Bradley, J.D.; Leipold, R.J.; Stoltzfus, A.M.; Ponce de Leon, M.; Hilf, M.; Peng, C.; Cohen, G.H.; Eisenberg, R.J. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 1994, 68, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, S.; Huang, J.; Shaw, C.; Friedman, H.M. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J. Virol. 2014, 88, 8421–8432. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Leng, Q.; Mixson, A.J. Alteration in the IL-2 signal peptide affects secretion of proteins in vitro and in vivo. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2005, 7, 354–365. [Google Scholar]
- Awasthi, S.; Lubinski, J.M.; Shaw, C.E.; Barrett, S.M.; Cai, M.; Wang, F.; Betts, M.; Kingsley, S.; Distefano, D.J.; Balliet, J.W.; et al. Immunization with a Vaccine Combining Herpes Simplex Virus 2 (HSV-2) Glycoprotein C (gC) and gD Subunits Improves the Protection of Dorsal Root Ganglia in Mice and Reduces the Frequency of Recurrent Vaginal Shedding of HSV-2 DNA in Guinea Pigs Compared to Immunization with gD Alone. J. Virol. 2011, 85, 10472–10486. [Google Scholar] [CrossRef] [Green Version]
- Brittle, E.E.; Wang, F.; Lubinski, J.M.; Bunte, R.M.; Friedman, H.M. A replication-competent, neuronal spread-defective, live attenuated herpes simplex virus type 1 vaccine. J. Virol. 2008, 82, 8431–8441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, K.P.; Allen, A.G.; Wigdahl, B.; Jennings, S.R. Modeling the pathology, immune responses, and kinetics of HSV-1 replication in the lip scarification model. Virology 2018, 514, 124–133. [Google Scholar] [CrossRef]
- Betts, M.R.; Brenchley, J.M.; Price, D.A.; De Rosa, S.C.; Douek, D.C.; Roederer, M.; Koup, R.A. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods 2003, 281, 65–78. [Google Scholar] [CrossRef]
- Awasthi, S.; Knox, J.J.; Desmond, A.; Alameh, M.G.; Gaudette, B.T.; Lubinski, J.M.; Naughton, A.; Hook, L.M.; Egan, K.P.; Tam, Y.K.; et al. Trivalent nucleoside-modified mRNA vaccine yields durable memory B cell protection against genital herpes in preclinical models. J. Clin. Investig. 2021, 131, e152310. [Google Scholar] [CrossRef]
- Hook, L.M.; Awasthi, S.; Dubin, J.; Flechtner, J.; Long, D.; Friedman, H.M. A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine 2019, 37, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Kastrukoff, L.; Hamada, T.; Schumacher, U.; Long, C.; Doherty, P.C.; Koprowski, H. Central nervous system infection and immune response in mice inoculated into the lip with herpes simplex virus type 1. J. Neuroimmunol. 1982, 2, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.S.; Ramchandran, R.S.; Hopkins, J.J.; Friedman, H.M. Herpes simplex virus type-1 and -2 pathogenesis is restricted by the epidermal basement membrane. Arch. Virol. 2000, 145, 385–396. [Google Scholar] [CrossRef]
- Hook, L.M.; Awasthi, S.; Cairns, T.M.; Alameh, M.G.; Fowler, B.T.; Egan, K.P.; Sung, M.M.H.; Weissman, D.; Cohen, G.H.; Friedman, H.M. Antibodies to Crucial Epitopes on HSV-2 Glycoprotein D as a Guide to Dosing an mRNA Genital Herpes Vaccine. Viruses 2022, 14, 540. [Google Scholar] [CrossRef]
- Liu, T.; Khanna, K.M.; Chen, X.; Fink, D.J.; Hendricks, R.L. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J. Exp. Med. 2000, 191, 1459–1466. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.M.; Bonneau, R.H.; Kinchington, P.R.; Hendricks, R.L. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity 2003, 18, 593–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knickelbein, J.E.; Khanna, K.M.; Yee, M.B.; Baty, C.J.; Kinchington, P.R.; Hendricks, R.L. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 2008, 322, 268–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, G.N.; Bernstein, D.I.; Bourne, N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 1998, 160, 6093–6100. [Google Scholar] [CrossRef]
- Milligan, G.N.; Bernstein, D.I. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 1997, 229, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernstein, D.I.; Cardin, R.D.; Bravo, F.J.; Awasthi, S.; Lu, P.; Pullum, D.A.; Dixon, D.A.; Iwasaki, A.; Friedman, H.M. Successful application of prime and pull strategy for a therapeutic HSV vaccine. NPJ Vaccines 2019, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.K.; Laycock, K.A.; Umphress, J.A.; Hook, K.K.; Stuart, P.M.; Pepose, J.S. A comparison of recurrent and primary herpes simplex keratitis in NIH inbred mice. Cornea 1996, 15, 497–504. [Google Scholar] [CrossRef]
- Hudson, S.J.; Dix, R.D.; Streilein, J.W. Induction of encephalitis in SJL mice by intranasal infection with herpes simplex virus type 1: A possible model of herpes simplex encephalitis in humans. J. Infect. Dis. 1991, 163, 720–727. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egan, K.P.; Awasthi, S.; Tebaldi, G.; Hook, L.M.; Naughton, A.M.; Fowler, B.T.; Beattie, M.; Alameh, M.-G.; Weissman, D.; Cohen, G.H.; et al. A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1. Viruses 2023, 15, 1483. https://doi.org/10.3390/v15071483
Egan KP, Awasthi S, Tebaldi G, Hook LM, Naughton AM, Fowler BT, Beattie M, Alameh M-G, Weissman D, Cohen GH, et al. A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1. Viruses. 2023; 15(7):1483. https://doi.org/10.3390/v15071483
Chicago/Turabian StyleEgan, Kevin P., Sita Awasthi, Giulia Tebaldi, Lauren M. Hook, Alexis M. Naughton, Bernard T. Fowler, Mitchell Beattie, Mohamad-Gabriel Alameh, Drew Weissman, Gary H. Cohen, and et al. 2023. "A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1" Viruses 15, no. 7: 1483. https://doi.org/10.3390/v15071483
APA StyleEgan, K. P., Awasthi, S., Tebaldi, G., Hook, L. M., Naughton, A. M., Fowler, B. T., Beattie, M., Alameh, M.-G., Weissman, D., Cohen, G. H., & Friedman, H. M. (2023). A Trivalent HSV-2 gC2, gD2, gE2 Nucleoside-Modified mRNA-LNP Vaccine Provides Outstanding Protection in Mice against Genital and Non-Genital HSV-1 Infection, Comparable to the Same Antigens Derived from HSV-1. Viruses, 15(7), 1483. https://doi.org/10.3390/v15071483