Reduced Humoral and Cellular Immune Response to Primary COVID-19 mRNA Vaccination in Kidney Transplanted Children Aged 5–11 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Cohort and Ethics
2.2. Sample Processing
2.3. Serum Antibody Measurements
2.4. PBMC Peptide Stimulation
2.5. Flow Cytometry
2.6. Multiplex Detection of Cytokines
2.7. Data Analysis and Statistics
3. Results
3.1. Cohort Characteristics
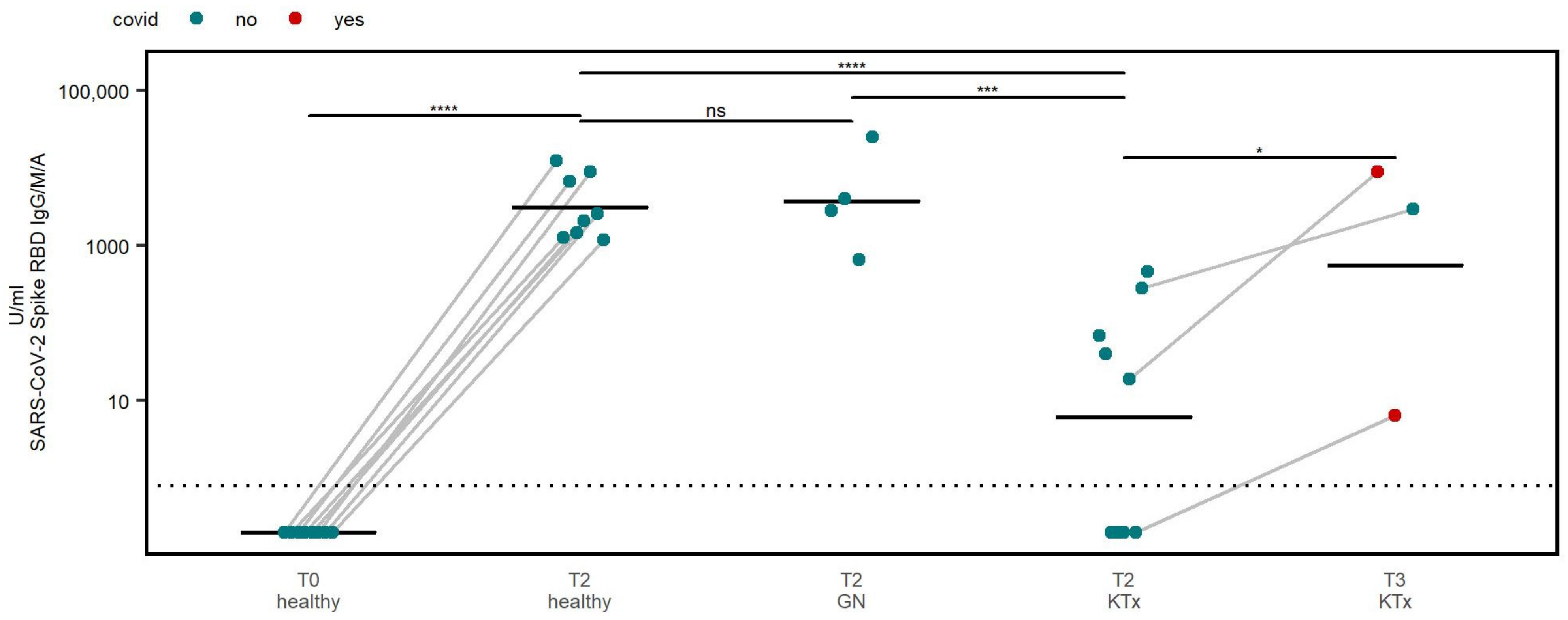
3.2. Humoral Immune Response
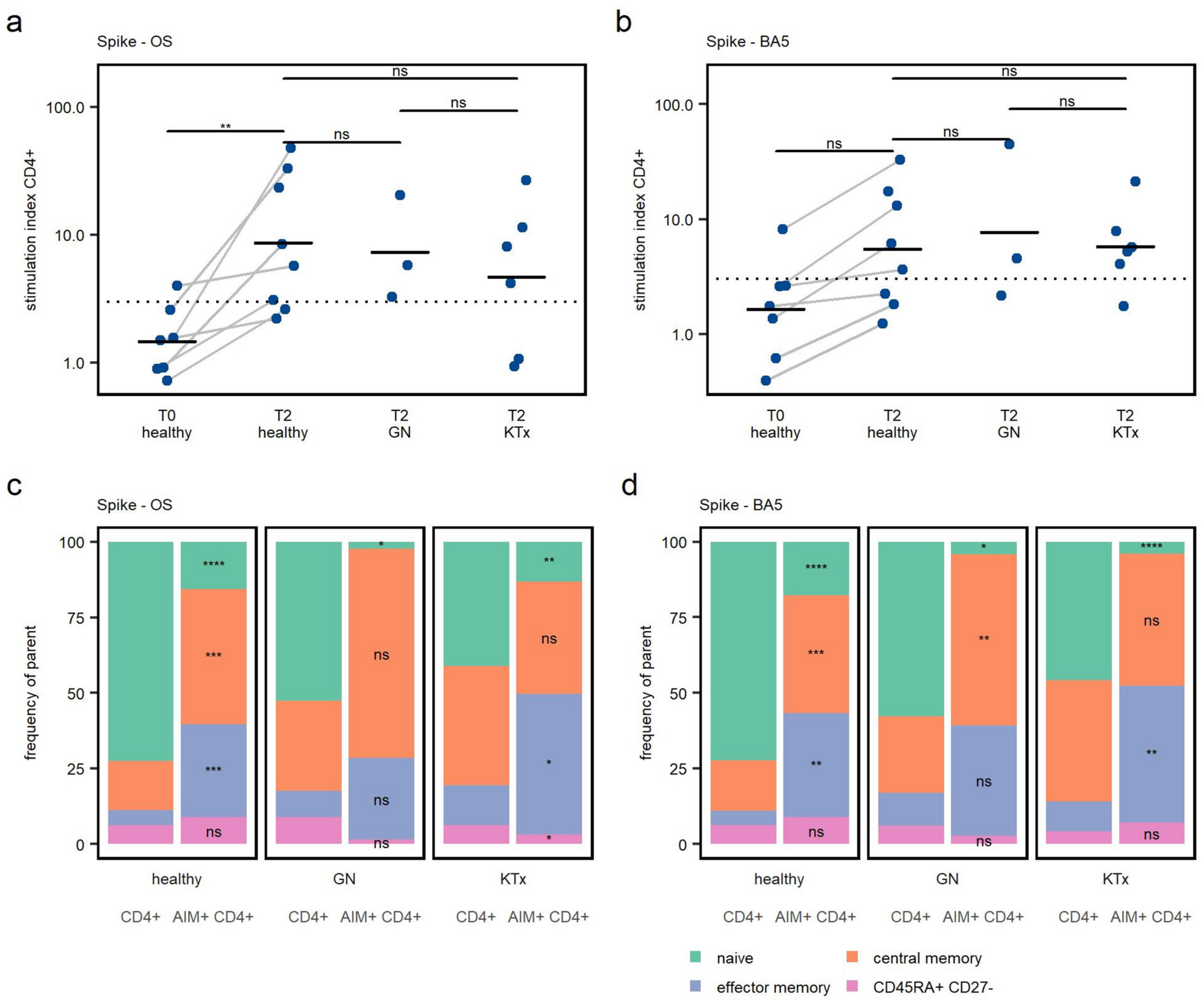
3.3. CD4+ T Cell Response
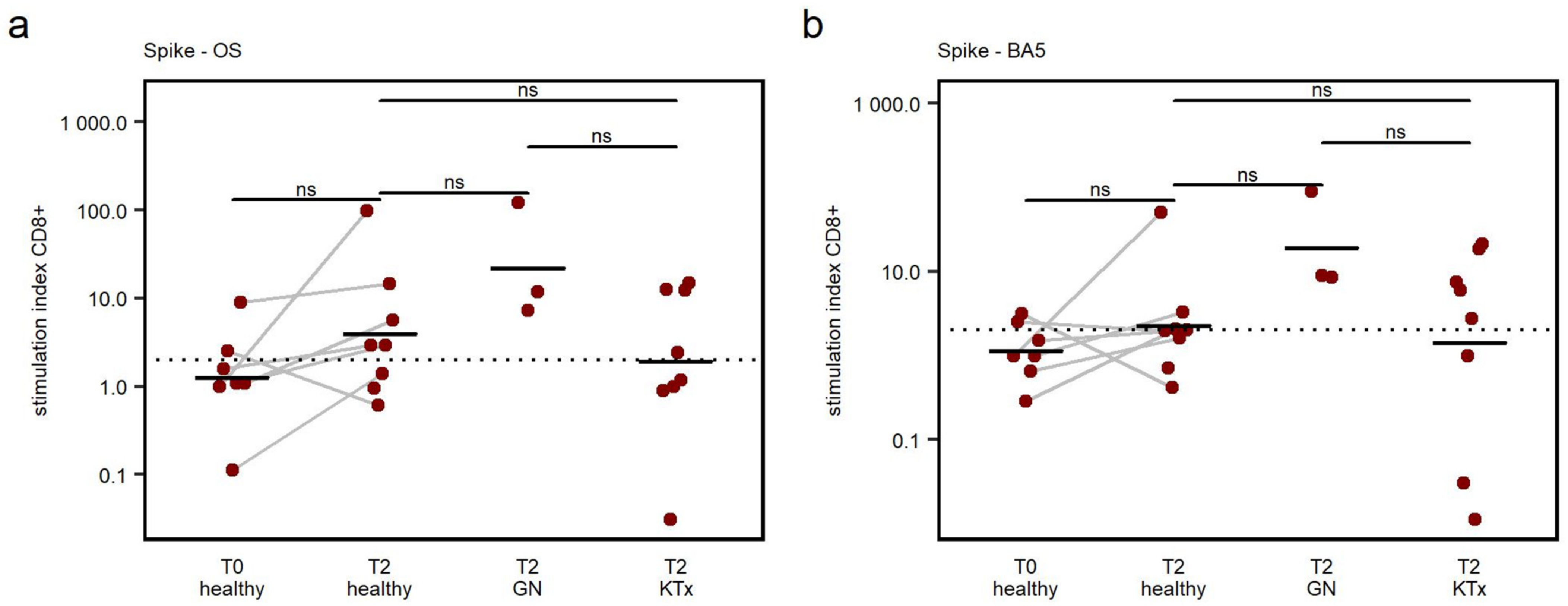
3.4. CD8+ T Cell Response
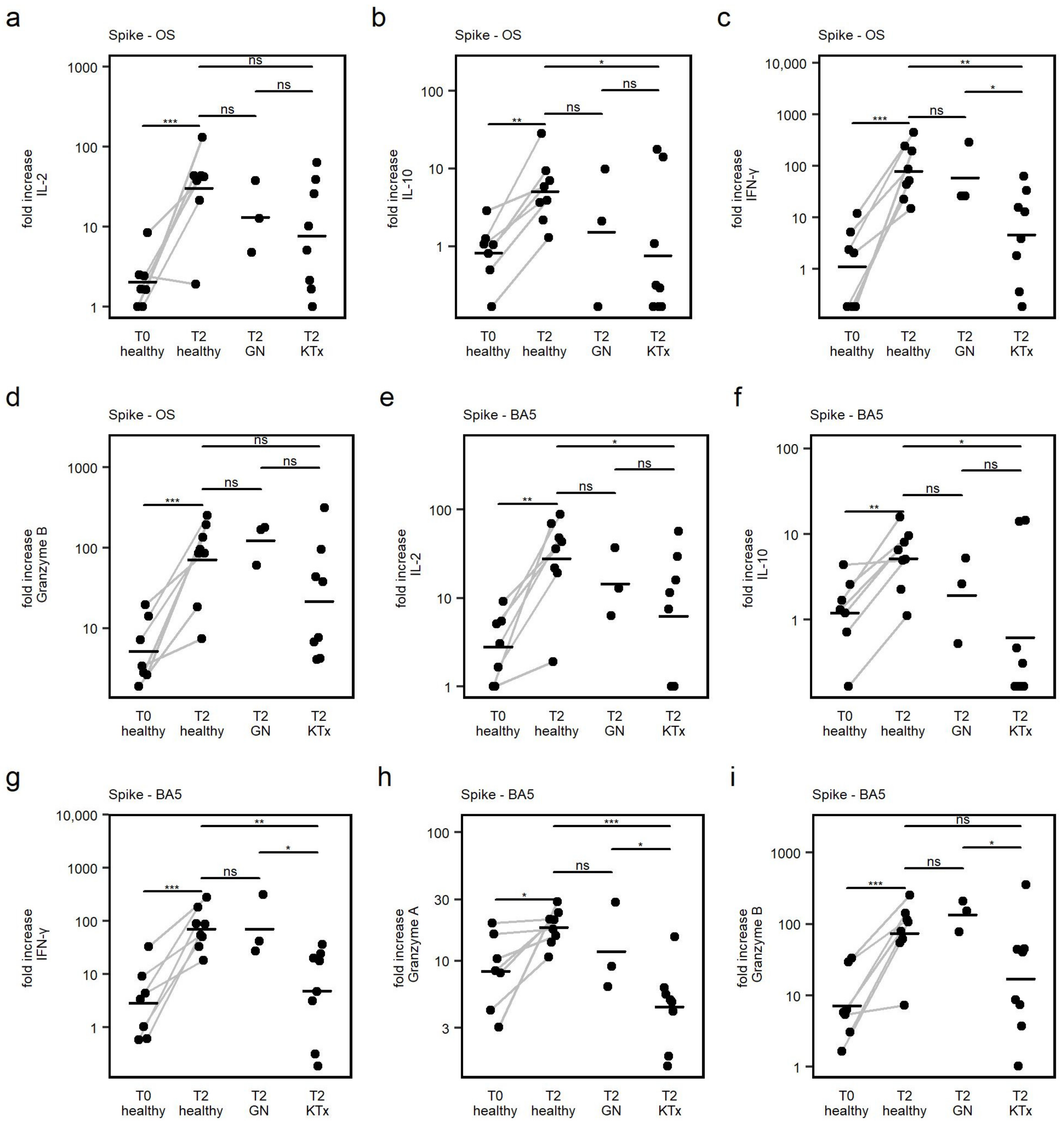
3.5. Cytokine Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Cinicola, B.L.; Mortari, E.P.; Zicari, A.M.; Agrati, C.; Bordoni, V.; Albano, C.; Fedele, G.; Schiavoni, I.; Leone, P.; Fiore, S.; et al. The BNT162b2 vaccine induces humoral and cellular immune memory to SARS-CoV-2 Wuhan strain and the Omicron variant in children 5 to 11 years of age. Front. Immunol. 2022, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vygen-Bonnet, S.; Schlaberg, J.; Koch, J. Role, working methods and recommendations of the Standing Committee on Vaccinations (STIKO) in the context of the COVID-19 pandemic. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2022, 65, 1251–1261. [Google Scholar] [CrossRef]
- COVID-19 Vaccines for People Who Are Moderately or Severely Immunocompromised|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html (accessed on 19 March 2023).
- Morgans, H.A.; Bradley, T.; Flebbe-Rehwaldt, L.; Selvarangan, R.; Bagherian, A.; Barnes, A.P.; Bass, J.; Cooper, A.M.; Fischer, R.; Kleiboeker, S.; et al. Humoral and cellular response to the COVID-19 vaccine in immunocompromised children. Pediatr. Res. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stich, M.; Di Cristanziano, V.; Tönshoff, B.; Weber, L.T.; Dötsch, J.; Rammer, M.T.; Rieger, S.; Heger, E.; Garbade, S.F.; Burgmaier, K.; et al. Humoral immune response and live-virus neutralization of the SARS-CoV-2 omicron (BA.1) variant after COVID-19 mRNA vaccination in children and young adults with chronic kidney disease. Pediatr. Nephrol. 2022, 38, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, I.; Perales-Fraile, I.; González-García, A.; Muñoz-Blanco, A.; Manzano, L.; Fabregate, M.; Díez-Manglano, J.; Aizpuru, E.F.; Fernández, F.A.; García, A.G.; et al. In-hospital mortality among immunosuppressed patients with COVID-19: Analysis from a national cohort in Spain. PLoS ONE 2021, 16, e0255524. [Google Scholar] [CrossRef]
- Malahe, S.R.K.; Hoek, R.A.S.; Dalm, V.A.S.H.; Broers, A.E.C.; Hoed, C.M.D.; Manintveld, O.C.; Baan, C.C.; van Deuzen, C.M.; Papageorgiou, G.; I Bax, H.; et al. Clinical Characteristics and Outcomes of Immunocompromised Patients With Coronavirus Disease 2019 Caused by the Omicron Variant: A Prospective, Observational Study. Clin. Infect. Dis. 2022, 76, e172–e178. [Google Scholar] [CrossRef]
- Bertran, M.; Amin-Chowdhury, Z.; Davies, H.G.; Allen, H.; Clare, T.; Davison, C.; Sinnathamby, M.; Seghezzo, G.; Kall, M.; Williams, H.; et al. COVID-19 deaths in children and young people in England, March 2020 to December 2021: An active prospective national surveillance study. PLoS Med. 2022, 19, e1004118. [Google Scholar] [CrossRef]
- Varnell, C.J.; Harshman, L.A.; Smith, L.; Liu, C.; Chen, S.; Al-Akash, S.; Barletta, G.M.; Belsha, C.; Brakeman, P.; Chaudhuri, A.; et al. COVID-19 in pediatric kidney transplantation: The Improving Renal Outcomes Collaborative. Am. J. Transplant. 2021, 21, 2740–2748. [Google Scholar] [CrossRef]
- Singer, P.S.; Sethna, C.; Molmenti, E.; Fahmy, A.; Grodstein, E.; Castellanos-Reyes, L.; Fassano, J.; Teperman, L. COVID-19 infection in a pediatric kidney transplant population: A single–center experience. Pediatr. Transplant. 2021, 25, e14018. [Google Scholar] [CrossRef]
- Bansal, N.; Ovchinsky, N.; Foca, M.; Lamour, J.M.; Kogan-Liberman, D.; Hsu, D.T.; Beddows, K.; Abraham, L.; Coburn, M.; Cunningham, R.; et al. COVID-19 infection in pediatric solid organ transplant patients. Pediatr. Transplant. 2022, 26, e14156. [Google Scholar] [CrossRef]
- Yuksel, M.; Akturk, H.; Mizikoglu, O.; Toroslu, E.; Arikan, C. A single-center report of COVID-19 disease course and management in liver transplanted pediatric patients. Pediatr. Transplant. 2021, 25, e14061. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, N.; Yıldırım, Z.Y.; Yıldız, N.; Taşdemir, M.; Göknar, N.; Evrengül, H.; Gülmez, R.; Aksu, B.; Dursun, H.; Özçelik, G.; et al. COVID-19 in pediatric patients undergoing chronic dialysis and kidney transplantation. Eur. J. Pediatr. 2022, 181, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Abrams, J.Y.; E Oster, M.; E Godfred-Cato, S.; Bryant, B.; Datta, S.D.; Campbell, A.P.; Leung, J.W.; A Tsang, C.; Pierce, T.J.; Kennedy, J.L.; et al. Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child Adolesc. Health 2021, 5, 323–331. [Google Scholar] [CrossRef]
- Rhedin, S.; Lundholm, C.; Horne, A.; Smew, A.I.; Osvald, E.C.; Haddadi, A.; Alfvén, T.; Kahn, R.; Król, P.; Brew, B.H.; et al. Risk factors for multisystem inflammatory syndrome in children—A population-based cohort study of over 2 million children. Lancet Reg. Health-Eur. 2022, 19, 100443. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; O Orzel, A.; Young, C.C.; A Boom, J.; Sahni, L.C.; Maddux, A.B.; et al. BNT162b2 mRNA Vaccination Against COVID-19 is Associated with a Decreased Likelihood of Multisystem Inflammatory Syndrome in Children Aged 5–18 Years—United States, July 2021–April 2022. Clin. Infect. Dis. 2022, 76, e90–e100. [Google Scholar] [CrossRef]
- Paul, K.; Sibbertsen, F.; Weiskopf, D.; Lütgehetmann, M.; Barroso, M.; Danecka, M.K.; Glau, L.; Hecher, L.; Hermann, K.; Kohl, A.; et al. Specific CD4+ T Cell Responses to Ancestral SARS-CoV-2 in Children Increase with Age and Show Cross-Reactivity to Beta Variant. Front. Immunol. 2022, 13, 867577. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; Van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; Van Gorp, E.C.M.; et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489.e15–1501.e15. [Google Scholar] [CrossRef]
- Thieme, C.J.; Anft, M.; Paniskaki, K.; Blazquez-Navarro, A.; Doevelaar, A.; Seibert, F.S.; Hoelzer, B.; Konik, M.J.; Berger, M.M.; Brenner, T.; et al. Robust T Cell Response Toward Spike, Membrane, and Nucleocapsid SARS-CoV-2 Proteins Is Not Associated with Recovery in Critical COVID-19 Patients. Cell Rep. Med. 2020, 1, 100092. [Google Scholar] [CrossRef]
- Mahnke, Y.D.; Brodie, T.M.; Sallusto, F.; Roederer, M.; Lugli, E. The who’s who of T-cell differentiation: Human memory T-cell subsets. Eur. J. Immunol. 2013, 43, 2797–2809. [Google Scholar] [CrossRef]
- Joseph, G.; Klein, E.; Lustig, Y.; Weiss-Ottolenghi, Y.; Asraf, K.; Indenbaum, V.; Amit, S.; Kriger, O.; Gilboa, M.; Levy, Y.; et al. Real-World Immunogenicity and Reactogenicity of Two Doses of Pfizer-BioNTech COVID-19 Vaccination in Children Aged 5–11 Years. Vaccines 2022, 10, 1954. [Google Scholar] [CrossRef]
- Sanders, J.-S.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients With Chronic Kidney Disease, on Dialysis, or Living with a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Rincon-Arevalo, H.; Choi, M.; Stefanski, A.-L.; Halleck, F.; Weber, U.; Szelinski, F.; Jahrsdörfer, B.; Schrezenmeier, H.; Ludwig, C.; Sattler, A.; et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci. Immunol. 2021, 6, eabj1031. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.X.; Auerbach, S.R.; Charnaya, O.; Danziger-Isakov, L.A.; Ebel, N.H.; Feldman, A.G.; Hsu, E.K.; McAteer, J.; Mohammad, S.; Perito, E.R.; et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccination in pediatric solid organ transplant recipients. Am. J. Transplant. 2022, 22, 669–672. [Google Scholar] [CrossRef]
- Caillard, S.; Thaunat, O. COVID-19 vaccination in kidney transplant recipients. Nat. Rev. Nephrol. 2021, 17, 785–787. [Google Scholar] [CrossRef]
- Rabinowich, L.; Grupper, A.; Baruch, R.; Ben-Yehoyada, M.; Halperin, T.; Turner, D.; Katchman, E.; Levi, S.; Houri, I.; Lubezky, N.; et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J. Hepatol. 2021, 75, 435–438. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ierullo, M.; Ku, T.; Marinelli, T.; Majchrzak-Kita, B.; Yousuf, A.; Kulasingam, V.; Humar, A.; Kumar, D. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeier, E.; Rincon-Arevalo, H.; Stefanski, A.-L.; Potekhin, A.; Staub-Hohenbleicher, H.; Choi, M.; Bachmann, F.; Proβ, V.; Hammett, C.; Schrezenmeier, H.; et al. B and t cell responses after a third dose of SARS-CoV-2 vaccine in kidney transplant recipients. J. Am. Soc. Nephrol. 2021, 32, 3027–3033. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three Doses of an mRNA COVID-19 Vaccine in Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Kingstad-Bakke, B.; Lee, W.; Chandrasekar, S.S.; Gasper, D.J.; Salas-Quinchucua, C.; Cleven, T.; Sullivan, J.A.; Talaat, A.; Osorio, J.E.; Suresh, M. Vaccine-induced systemic and mucosal T cell immunity to SARS-CoV-2 viral variants. Proc. Natl. Acad. Sci. USA 2022, 119, e2118312119. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.A.; Li, A.P.Y.; Hachim, A.; Hui, D.S.C.; Kwan, M.Y.W.; Tsang, O.T.Y.; Chiu, S.S.; Chan, W.H.; Yau, Y.S.; Kavian, N.; et al. SARS-CoV-2 specific T cell responses are lower in children and increase with age and time after infection. Nat. Commun. 2021, 12, 4678. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Ruether, D.F.; Schaub, G.M.; Duengelhoef, P.M.; Haag, F.; Brehm, T.T.; Fathi, A.; Wehmeyer, M.; Jahnke-Triankowski, J.; Mayer, L.; Hoffmann, A.; et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin. Gastroenterol. Hepatol. 2022, 20, 162.e9–172.e9. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847.e11–859.e11. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 variants on the total CD4+ and CD8+ T cell reactivity in infected or vaccinated individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Proß, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Luo, J.; Han, X.; Wei, Y.; Wei, X. mRNA vaccine: A potential therapeutic strategy. Mol. Cancer 2021, 20, 33. [Google Scholar] [CrossRef]
- Sacco, C.; Del Manso, M.; Mateo-Urdiales, A.; Rota, M.C.; Petrone, D.; Riccardo, F.; Bella, A.; Siddu, A.; Battilomo, S.; Proietti, V.; et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: A retrospective analysis of January–April 2022. Lancet 2022, 400, 97–103. [Google Scholar] [CrossRef]
- Tso, W.W.; Wong, R.S.; Tung, K.T.; Rao, N.; Fu, K.W.; Yam, J.C.; Chua, G.T.; Chen, E.Y.; Lee, T.M.; Chan, S.K.; et al. Vulnerability and resilience in children during the COVID-19 pandemic. Eur. Child Adolesc. Psychiatry 2022, 31, 161–176. [Google Scholar] [CrossRef]
- Singh, S.; Roy, D.; Sinha, K.; Parveen, S.; Sharma, G.; Joshi, G. Impact of COVID-19 and lockdown on mental health of children and adolescents: A narrative review with recommendations. Psychiatry Res. 2020, 293, 113429. [Google Scholar] [CrossRef] [PubMed]
- Meherali, S.; Punjani, N.; Louie-Poon, S.; Abdul Rahim, K.; Das, J.K.; Salam, R.A.; Lassi, Z.S. Mental health of children and adolescents amidst COVID-19 and past pandemics: A rapid systematic review. Int. J. Env. Res. Public Health 2021, 18, 3432. [Google Scholar] [CrossRef] [PubMed]
- Sahn, B.; Lu, Y.; Hui-Yuen, J.S.; Fishbein, J.; Gottlieb, B.S.; Eberhard, B.A.; Walters, H.M. The safety of COVID-19 vaccination in immunocompromised children and young adults with immune-mediated inflammatory disease. Acta Paediatr. Int. J. Paediatr. 2022, 112, 794–801. [Google Scholar] [CrossRef] [PubMed]

| Healthy | GN | KTx | |
|---|---|---|---|
| Age, mean (range in years) | 7.625 (5–11) | 9.25 (7–11) | 7.7 (5–11) |
| Sex (female, in %) | 5 (62.5%) | 2 (50%) | 4 (44%) |
| Medication | |||
| CNI | 4 | 1 | |
| CNI + MMF | 7 | ||
| CNI + mTOR Inhibitor | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalia, J.K.; Schild, R.; Lütgehetmann, M.; Dunay, G.A.; Kallinich, T.; Kobbe, R.; Massoud, M.; Oh, J.; Pietzsch, L.; Schulze-Sturm, U.; et al. Reduced Humoral and Cellular Immune Response to Primary COVID-19 mRNA Vaccination in Kidney Transplanted Children Aged 5–11 Years. Viruses 2023, 15, 1553. https://doi.org/10.3390/v15071553
Lalia JK, Schild R, Lütgehetmann M, Dunay GA, Kallinich T, Kobbe R, Massoud M, Oh J, Pietzsch L, Schulze-Sturm U, et al. Reduced Humoral and Cellular Immune Response to Primary COVID-19 mRNA Vaccination in Kidney Transplanted Children Aged 5–11 Years. Viruses. 2023; 15(7):1553. https://doi.org/10.3390/v15071553
Chicago/Turabian StyleLalia, Jasmin K., Raphael Schild, Marc Lütgehetmann, Gabor A. Dunay, Tilmann Kallinich, Robin Kobbe, Mona Massoud, Jun Oh, Leonora Pietzsch, Ulf Schulze-Sturm, and et al. 2023. "Reduced Humoral and Cellular Immune Response to Primary COVID-19 mRNA Vaccination in Kidney Transplanted Children Aged 5–11 Years" Viruses 15, no. 7: 1553. https://doi.org/10.3390/v15071553
APA StyleLalia, J. K., Schild, R., Lütgehetmann, M., Dunay, G. A., Kallinich, T., Kobbe, R., Massoud, M., Oh, J., Pietzsch, L., Schulze-Sturm, U., Schuetz, C., Sibbertsen, F., Speth, F., Thieme, S., Witkowski, M., Berner, R., Muntau, A. C., Gersting, S. W., Toepfner, N., ... Paul, K. (2023). Reduced Humoral and Cellular Immune Response to Primary COVID-19 mRNA Vaccination in Kidney Transplanted Children Aged 5–11 Years. Viruses, 15(7), 1553. https://doi.org/10.3390/v15071553






