Effect of Pre-Treatment with a Recombinant Chicken Interleukin-17A on Vaccine Induced Immunity against a Very Virulent Marek’s Disease Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmid and Cloning
2.2. Cell Culture
2.3. Virus Preparation
2.4. Experimental Design and Sampling
2.5. Spleen Mononuclear Cell Preparation and Stimulation
2.6. RNA Extraction and Reverse Transcription
2.7. Real-Time Polymerase Chain Reaction (RT-PCR)
2.8. Flow Cytometry
2.9. Confocal Microscopy
2.10. ELISA
2.11. Statistical Analysis
3. Results
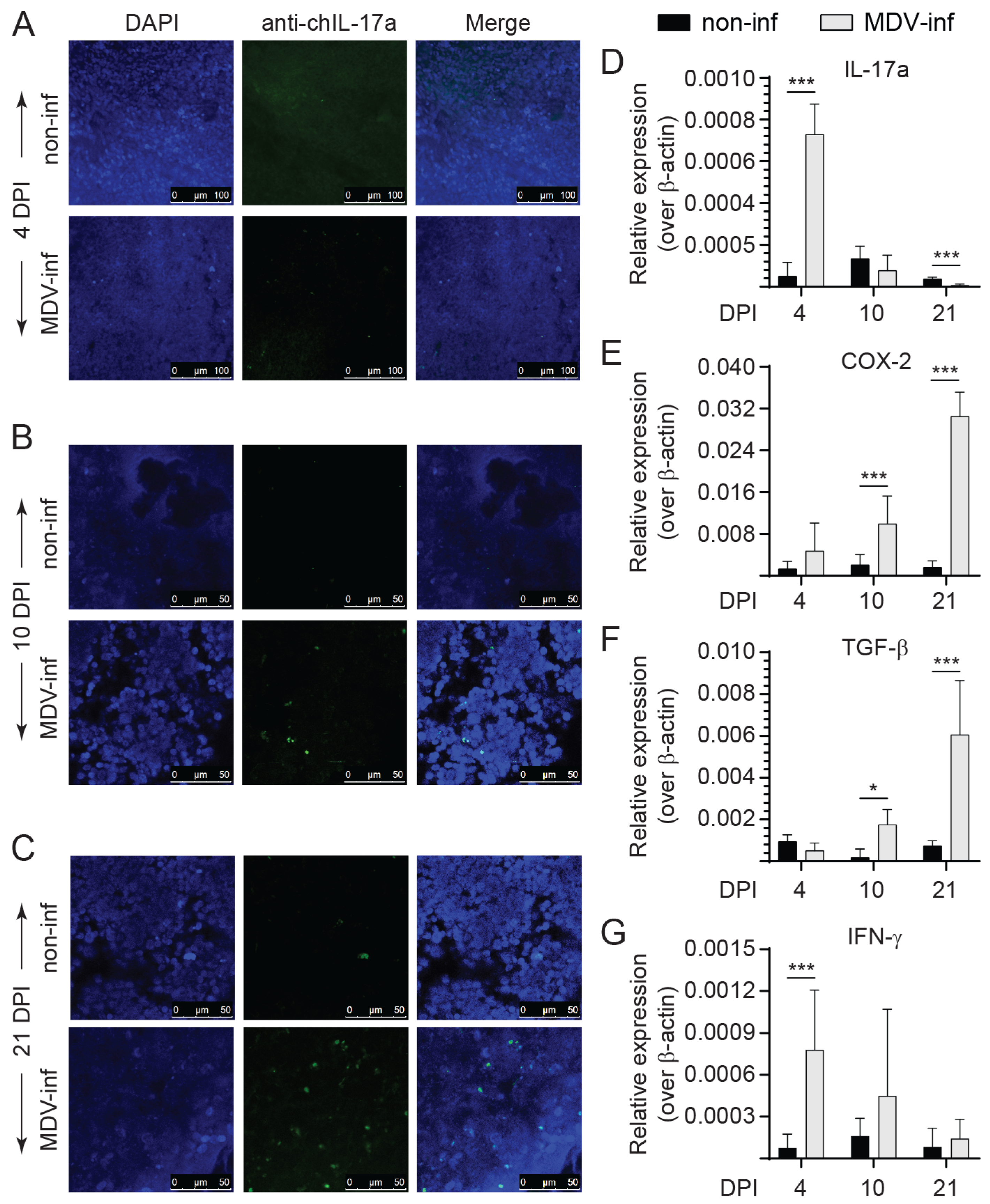
3.1. Expression of IL-17A, COX-2, TGF-β, and IFN-γ in vvMDV-RB1B Infected Chickens
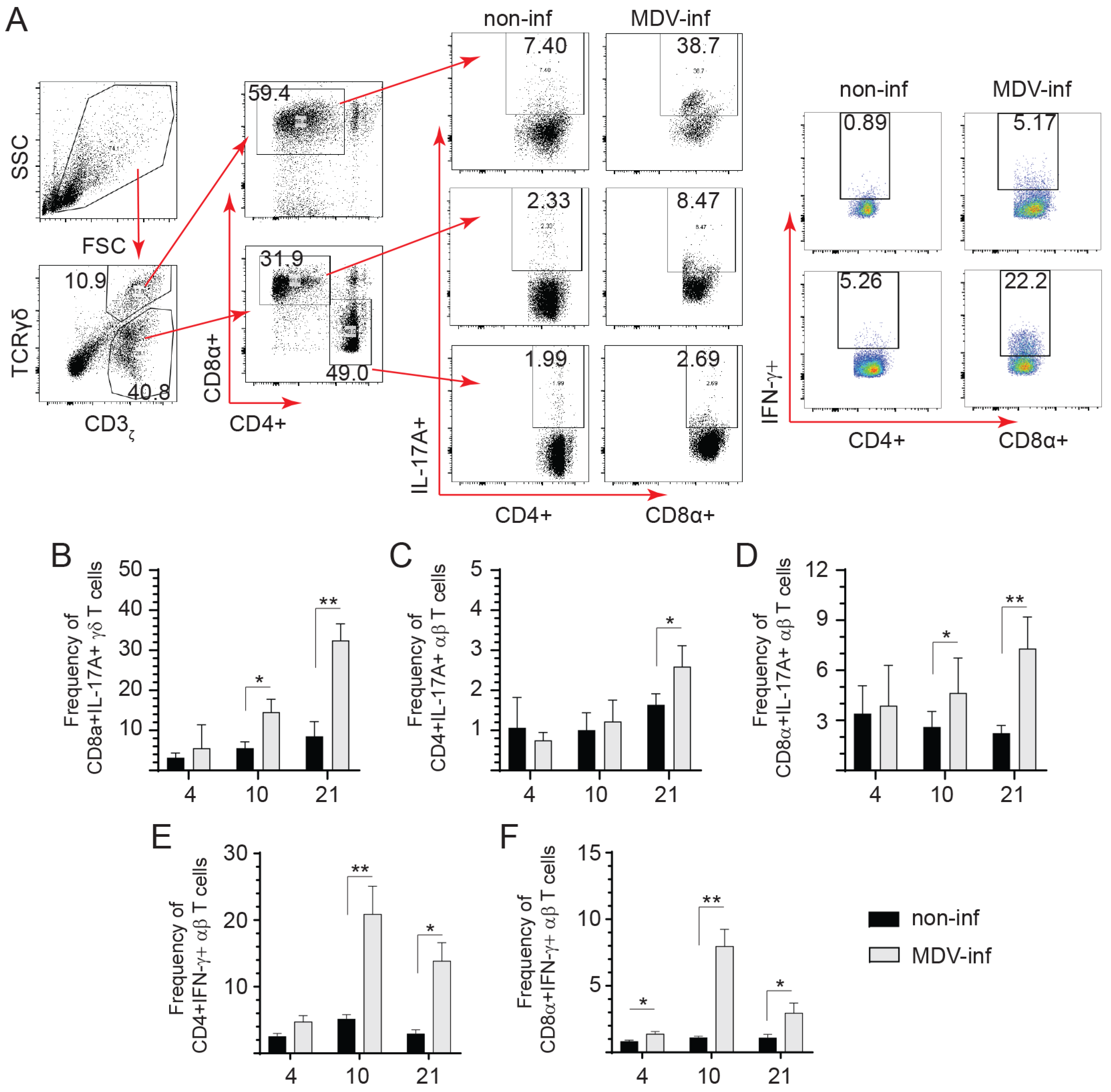
3.2. Detection of IL-17A+ and IFN-γ+ T Cells Post vvMDV Infection
3.3. Cloning, Expression and Evaluation of the rchIL-17A Bioactivity Based on Receptor Binding
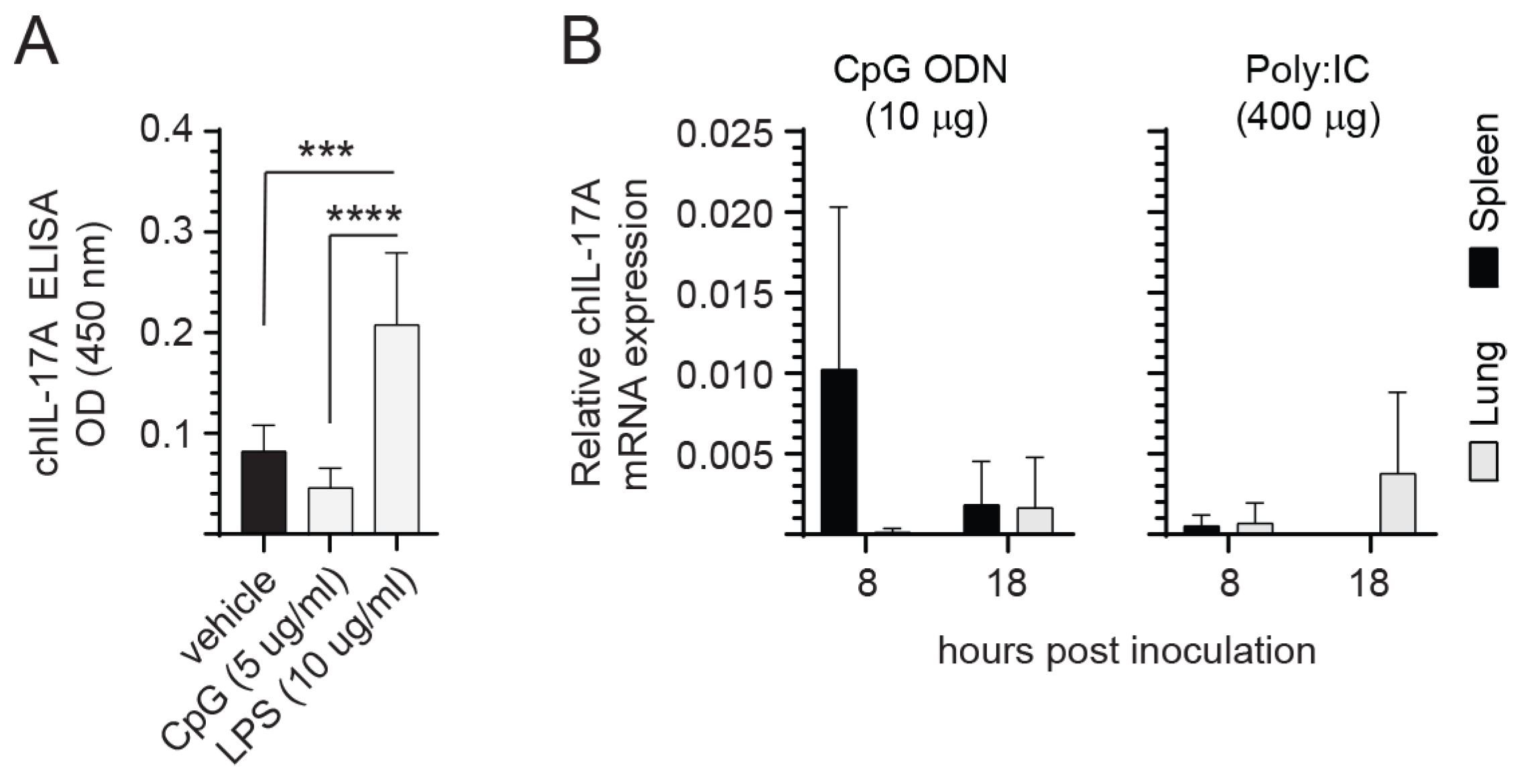
3.4. Neither CpG ODN 2007 Nor Poly:IC Treatment Induce IL-17A Expression
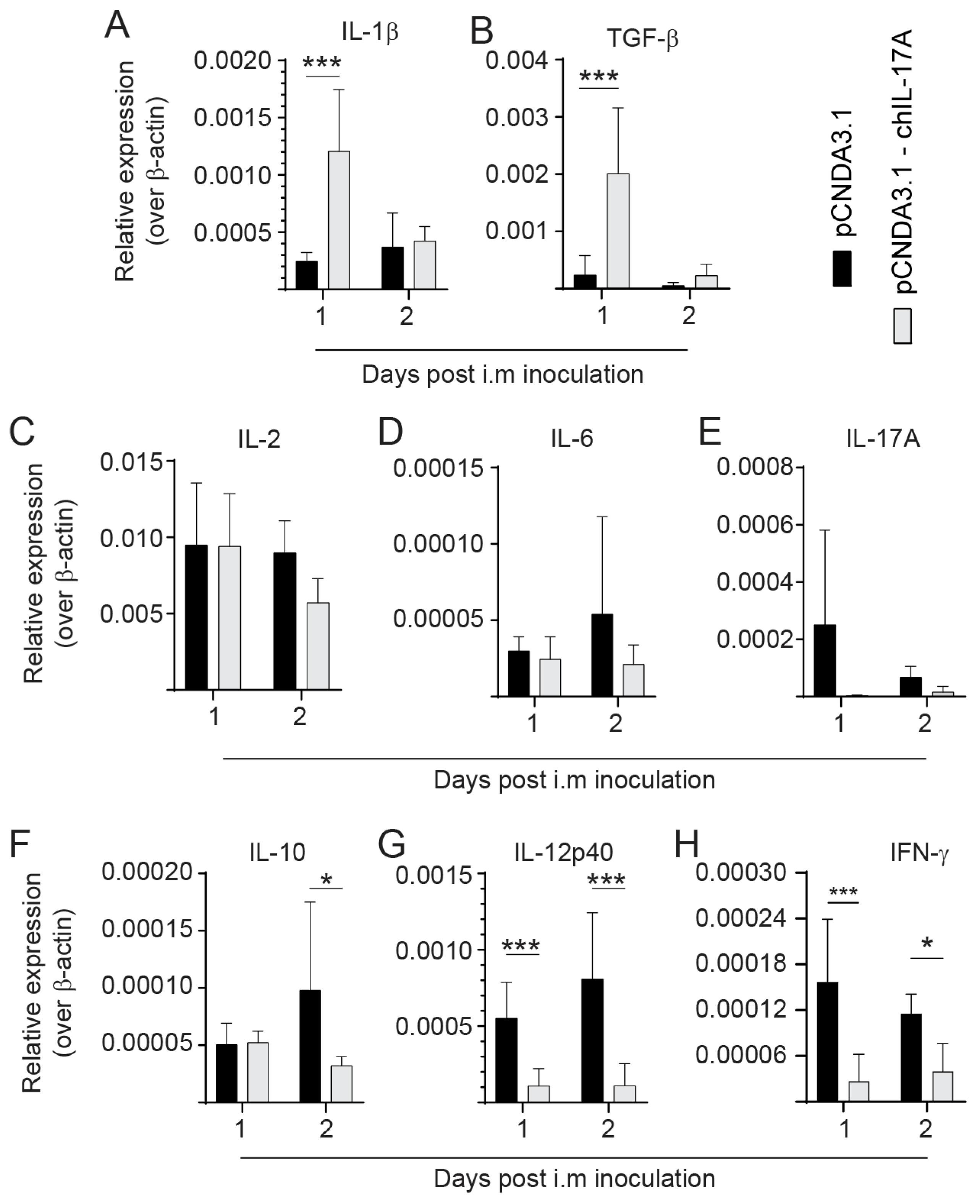
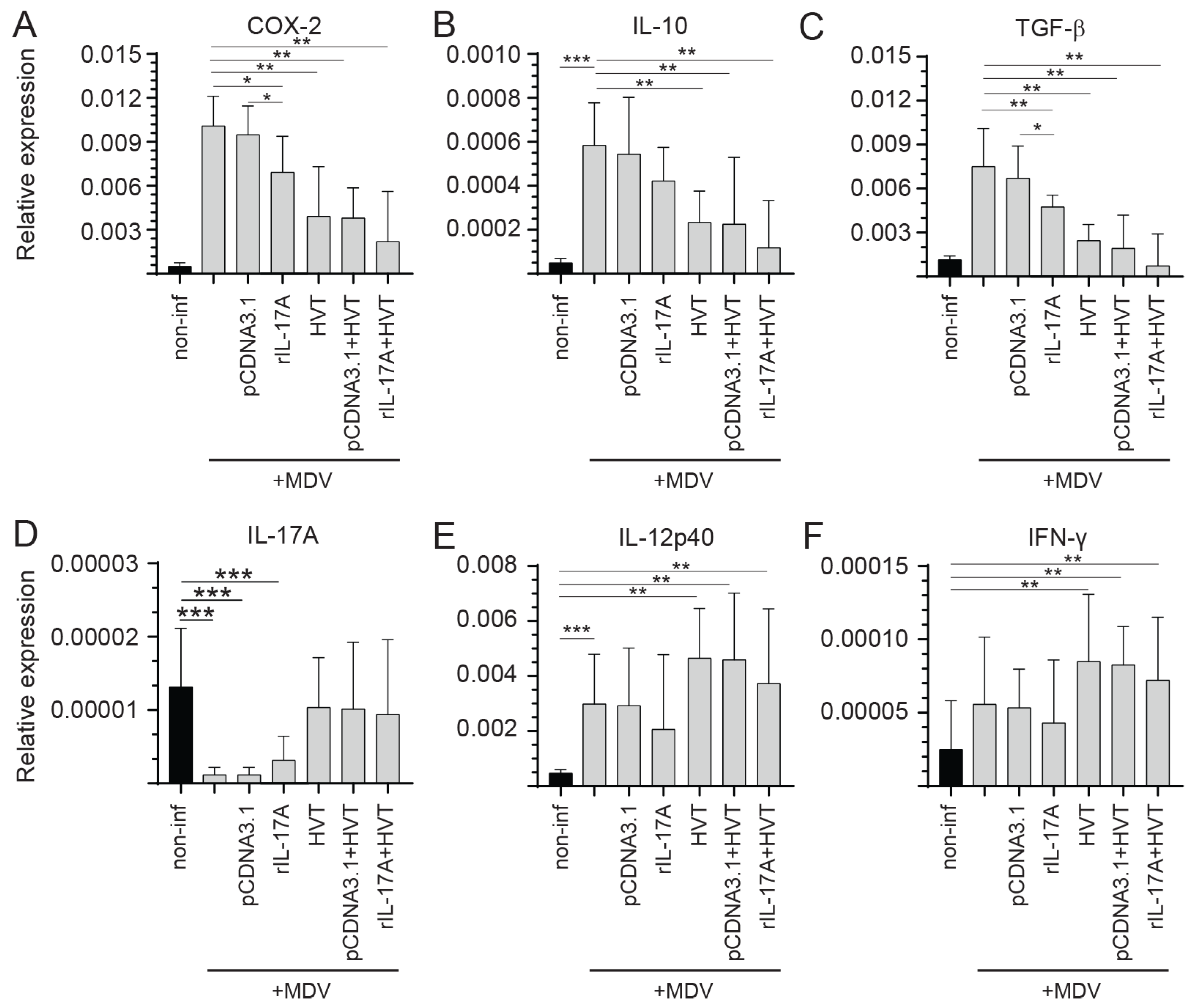
3.5. Modulation of Cytokine Expression following rchIL-17A Inoculation
- MD Tumor Incidence in rchIL-17A-Treated and HVT-Vaccinated Chickens
- Differential Cytokine Expression in Spleen of vvMDV-RB1B Infected Chickens Pre-Treated with the rchIL-17A
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moseley, T.; Haudenschild, D.; Rose, L.; Reddi, A. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003, 14, 155–174. [Google Scholar] [CrossRef]
- Veldhoen, M.; Hocking, R.J.; Atkins, C.J.; Locksley, R.M.; Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006, 24, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, P.; Poh, T.Y.; Rothwell, L.; Avery, S.; Balu, S.; Pathania, U.S.; Hughes, S.; Goodchild, M.; Morrell, S.; Watson, M.; et al. A Genomic Analysis of Chicken Cytokines and Chemokines. J. Interf. Cytokine Res. 2005, 25, 467–484. [Google Scholar] [CrossRef]
- Veldhoen, M. Interleukin 17 is a chief orchestrator of immunity. Nat. Immunol. 2017, 18, 612–621. [Google Scholar] [CrossRef]
- Walliser, I.; Göbel, T.W. Generation of glycosylphosphatidylinositol linked chicken IL-17 to generate specific monoclonal antibodies applicable for intracellular cytokine staining. Dev. Comp. Immunol. 2017, 73, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Walliser, I.; Göbel, T.W. Chicken IL-17A is expressed in αβ and γδ T cell subsets and binds to a receptor present on macrophages, and T cells. Dev. Comp. Immunol. 2018, 81, 44–53. [Google Scholar] [CrossRef]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17–producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Patera, A.C.; Pesnicak, L.; Bertin, J.; Cohen, J.I. Interleukin 17 Modulates the Immune Response to Vaccinia Virus Infection. Virology 2002, 299, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.; Chanthaphavong, R.S.; Sun, S.; Trigilio, J.A.; Phasouk, K.; Jin, L.; Layton, E.D.; Li, A.Z.; Correnti, C.E.; De van der Schueren, W.; et al. Keratinocytes produce IL-17c to protect peripheral nervous systems during human HSV-2 reactivation. J. Exp. Med. 2017, 214, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Bagri, P.; Anipindi, V.C.; Nguyen, P.V.; Vitali, D.; Stampfli, M.; Kaushic, C. Novel Role for Interleukin-17 in Enhancing Type 1 Helper T Cell Immunity in the Female Genital Tract following Mucosal Herpes Simplex Virus 2 Vaccination. J. Virol. 2017, 91, e01234-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahiri, A.; Sharif, S.; Mallick, A.I. Intragastric delivery of recombinant Lactococcus lactis displaying ectodomain of influenza matrix protein 2 (M2e) and neuraminidase (NA) induced focused mucosal and systemic immune responses in chickens. Mol. Immunol. 2019, 114, 497–512. [Google Scholar] [CrossRef]
- Boodhoo, N.; Gurung, A.; Sharif, S.; Behboudi, S. Marek’s disease in chickens: A review with focus on immunology. Veter. Res. 2016, 47, 119. [Google Scholar] [CrossRef] [Green Version]
- Calnek, B.W. Pathogenesis of Marek’s Disease Virus Infection. In Marek’s Disease; Current Topics in Microbiology and Immunology; Hirai, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 255, pp. 25–55. [Google Scholar] [CrossRef]
- Boodhoo, N.; Kamble, N.; Kaufer, B.B.; Behboudi, S. Replication of Marek’s Disease Virus Is Dependent on Synthesis of De Novo Fatty Acid and Prostaglandin E2. J. Virol. 2019, 93, e00352-19. [Google Scholar] [CrossRef] [Green Version]
- Schat, K.; Calnek, B.; Fabricant, J. Characterisation of two highly oncogenic strains of Marek’s disease virus. Avian Pathol. 1982, 11, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Boodhoo, N.; Behboudi, S. Differential Virus-Specific IFN-Gamma Producing T Cell Responses to Marek’s Disease Virus in Chickens with B19 and B21 MHC Haplotypes. Front. Immunol. 2022, 12, 784359. [Google Scholar] [CrossRef]
- Haq, K.; Elawadli, I.; Parvizi, P.; Mallick, A.I.; Behboudi, S.; Sharif, S. Interferon-γ influences immunity elicited by vaccines against very virulent Marek’s disease virus. Antivir. Res. 2011, 90, 218–226. [Google Scholar] [CrossRef]
- Lee, Y.K.; Turner, H.; Maynard, C.L.; Oliver, J.R.; Chen, D.; Elson, C.O.; Weaver, C.T. Late Developmental Plasticity in the T Helper 17 Lineage. Immunity 2009, 30, 92–107. [Google Scholar] [CrossRef] [Green Version]
- Gurung, A.; Kamble, N.; Kaufer, B.B.; Pathan, A.; Behboudi, S. Association of Marek’s Disease induced immunosuppression with activation of a novel regulatory T cells in chickens. PLoS Pathog. 2017, 13, e1006745. [Google Scholar] [CrossRef] [Green Version]
- Kamble, N.; Gurung, A.; Kaufer, B.B.; Pathan, A.A.; Behboudi, S. Marek’s Disease Virus Modulates T Cell Proliferation via Activation of Cyclooxygenase 2-Dependent Prostaglandin E2. Front. Immunol. 2021, 12, 801781. [Google Scholar] [CrossRef]
- Abdul-Careem, M.F.; Read, L.R.; Parvizi, P.; Thanthrige-Don, N.; Sharif, S. Marek’s disease virus-induced expression of cytokine genes in feathers of genetically defined chickens. Dev. Comp. Immunol. 2009, 33, 618–623. [Google Scholar] [CrossRef]
- Boodhoo, N.; Sharif, S.; Behboudi, S. 1α,25(OH)2 Vitamin D3 Modulates Avian T Lymphocyte Functions without Inducing CTL Unresponsiveness. PLoS ONE 2016, 11, e0150134. [Google Scholar] [CrossRef] [Green Version]
- Bavananthasivam, J.; Alizadeh, M.; Astill, J.; Alqazlan, N.; Matsuyama-Kato, A.; Shojadoost, B.; Taha-Abdelaziz, K.; Sharif, S. Effects of administration of probiotic lactobacilli on immunity conferred by the herpesvirus of turkeys vaccine against challenge with a very virulent Marek’s disease virus in chickens. Vaccine 2021, 39, 2424–2433. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of Lactobacilli on Cytokine Expression by Chicken Spleen and Cecal Tonsil Cells. Clin. Vaccine Immunol. 2010, 17, 1337–1343. [Google Scholar] [CrossRef] [Green Version]
- Yitbarek, A.; Rodriguez-Lecompte, J.C.; Echeverry, H.M.; Munyaka, P.; Barjesteh, N.; Sharif, S.; Camelo-Jaimes, G. Performance, histomorphology, and Toll-like receptor, chemokine, and cytokine profile locally and systemically in broiler chickens fed diets supplemented with yeast-derived macromolecules. Poult. Sci. 2013, 92, 2299–2310. [Google Scholar] [CrossRef]
- Paul, M.S.; Paolucci, S.; Barjesteh, N.; Wood, R.D.; Schat, K.A.; Sharif, S. Characterization of Chicken Thrombocyte Responses to Toll-Like Receptor Ligands. PLoS ONE 2012, 7, e43381. [Google Scholar] [CrossRef]
- Taha-Abdelaziz, K.; Alkie, T.N.; Hodgins, D.C.; Yitbarek, A.; Shojadoost, B.; Sharif, S. Gene expression profiling of chicken cecal tonsils and ileum following oral exposure to soluble and PLGA-encapsulated CpG ODN, and lysate of Campylobacter jejuni. Vet. Microbiol. 2017, 212, 67–74. [Google Scholar] [CrossRef]
- Crhanova, M.; Hradecka, H.; Faldynova, M.; Matulova, M.; Havlickova, H.; Sisak, F.; Rychlik, I. Immune Response of Chicken Gut to Natural Colonization by Gut Microflora and to Salmonella enterica Serovar Enteritidis Infection. Infect. Immun. 2011, 79, 2755–2763. [Google Scholar] [CrossRef] [Green Version]
- Brisbin, J.T.; Zhou, H.; Gong, J.; Sabour, P.; Akbari, M.R.; Haghighi, H.R.; Yu, H.; Clarke, A.; Sarson, A.J.; Sharif, S. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev. Comp. Immunol. 2008, 32, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama-Kato, A.; Iseki, H.; Boodhoo, N.; Bavananthasivam, J.; Alqazlan, N.; Abdul-Careem, M.F.; Plattner, B.L.; Behboudi, S.; Sharif, S. Phenotypic characterization of gamma delta (γδ) T cells in chickens infected with or vaccinated against Marek’s disease virus. Virology 2022, 568, 115–125. [Google Scholar] [CrossRef]
- Xu, S.; Cao, X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010, 7, 164–174. [Google Scholar] [CrossRef] [Green Version]
- Haq, K.; Wootton, S.K.; Barjesteh, N.; Golovan, S.; Bendall, A.; Sharif, S. Effects of interferon-γ knockdown on vaccine-induced immunity against Marek’s disease in chickens. Can. J. Vet. Res. 2015, 79, 7. [Google Scholar]
- Bertzbach, L.D.; Harlin, O.; Härtle, S.; Fehler, F.; Vychodil, T.; Kaufer, B.B.; Kaspers, B. IFNα and IFNγ Impede Marek’s Disease Progression. Viruses 2019, 11, 1103. [Google Scholar] [CrossRef] [Green Version]
- Bertzbach, L.D.; van Haarlem, D.A.; Härtle, S.; Kaufer, B.B.; Jansen, C.A. Marek’s Disease Virus Infection of Natural Killer Cells. Microorganisms 2019, 7, 588. [Google Scholar] [CrossRef] [Green Version]
- Haas, J.D.; Ravens, S.; Düber, S.; Sandrock, I.; Oberdörfer, L.; Kashani, E.; Chennupati, V.; Föhse, L.; Naumann, R.; Weiss, S.; et al. Development of Interleukin-17-Producing γδ T Cells Is Restricted to a Functional Embryonic Wave. Immunity 2012, 37, 48–59. [Google Scholar] [CrossRef]
- Ribot, J.C.; Debarros, A.; Pang, D.J.; Neves, J.F.; Peperzak, V.; Roberts, S.J.; Girardi, M.; Borst, J.; Hayday, A.C.; Pennington, D.J.; et al. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17–producing γδ T cell subsets. Nat. Immunol. 2009, 10, 427–436. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.-Y.; Huang, F.; Chen, Y.; Thai, P.; Wachi, S.; Kim, C.; Tam, L.; Wu, R. Up-Regulation of CC Chemokine Ligand 20 Expression in Human Airway Epithelium by IL-17 through a JAK-Independent but MEK/NF-κB-Dependent Signaling Pathway. J. Immunol. 2005, 175, 6676–6685. [Google Scholar] [CrossRef] [Green Version]
- Teng, M.; Zhu, Z.-J.; Yao, Y.; Nair, V.; Zhang, G.-P.; Luo, J. Critical roles of non-coding RNAs in lifecycle and biology of Marek’s disease herpesvirus. Sci. China Life Sci. 2023, 66, 251–268. [Google Scholar] [CrossRef]
- Hao, X.; Li, S.; Li, J.; Yang, Y.; Qin, A.; Shang, S. An Anti-Tumor Vaccine Against Marek’s Disease Virus Induces Differential Activation and Memory Response of γδ T Cells and CD8 T Cells in Chickens. Front. Immunol. 2021, 12, 645426. [Google Scholar] [CrossRef]
- Jondle, C.N.; Johnson, K.E.; Aurubin, C.; Sylvester, P.; Xin, G.; Cui, W.; Huppler, A.R.; Tarakanova, V.L. Gammaherpesvirus Usurps Host IL-17 Signaling to Support the Establishment of Chronic Infection. mBio 2021, 12, e00566-21. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, M.; Lin, Q.-W.; Cao, A.-L.; Yu, X.; Dong, J.-H.; Wang, J.-P.; Zhang, J.-H.; Wang, M.; Guo, H.-P.; et al. Th17 Cells Contribute to Viral Replication in Coxsackievirus B3-Induced Acute Viral Myocarditis. J. Immunol. 2010, 185, 4004–4010. [Google Scholar] [CrossRef] [Green Version]
- Papotto, P.H.; Ribot, J.C.; Silva-Santos, B. IL-17 + Γδ T Cells as Kick-Starters of Inflammation. Nat. Immunol. 2017, 18, 604–611. [Google Scholar]
- Sciammas, R.; Kodukula, P.; Tang, Q.; Hendricks, R.; Bluestone, J.; Persson, K.; Mörgelin, M.; Lindbom, L.; Alm, P.; Björck, L.; et al. T Cell Receptor–γ/δ Cells Protect Mice from Herpes Simplex Virus Type 1–induced Lethal Encephalitis. J. Exp. Med. 1997, 185, 1969–1975. [Google Scholar] [CrossRef]
- Shiromizu, C.M.; Jancic, C.C. γδ T Lymphocytes: An Effector Cell in Autoimmunity and Infection. Front. Immunol. 2018, 9, 2389. [Google Scholar]
- Fletcher, O.J.; Tan, X.; Cortes, L.; Gimeno, I. Cost effective and time efficient measurement of CD4, CD8, major histocompatibility complex Class II, and macrophage antigen expression in the lungs of chickens. Vet. Immunol. Immunopathol. 2012, 146, 225–236. [Google Scholar] [CrossRef]
- Ma, Y.; Aymeric, L.; Locher, C.; Mattarollo, S.R.; Delahaye, N.F.; Pereira, P.; Boucontet, L.; Apetoh, L.; Ghiringhelli, F.; Casares, N.; et al. Contribution of IL-17–producing γδ T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 2011, 208, 491–503. [Google Scholar] [CrossRef]
- Yang, X.O.; Chang, S.H.; Park, H.; Nurieva, R.; Shah, B.; Acero, L.; Wang, Y.-H.; Schluns, K.S.; Broaddus, R.R.; Zhu, Z.; et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008, 205, 1063–1075. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2019, 217, e20190297. [Google Scholar] [CrossRef]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef]
- Lupiani, B.; Lee, L.F.; Cui, X.; Gimeno, I.; Anderson, A.; Morgan, R.W.; Silva, R.F.; Witter, R.L.; Kung, H.-J.; Reddy, S.M. Marek’s disease virus-encoded Meq gene is involved in transformation of lymphocytes but is dispensable for replication. Proc. Natl. Acad. Sci. USA 2004, 101, 11815–11820. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; He, M.; Luo, Q.; Liu, F.; Chen, R. Marek’s Disease Virus Activates the PI3K/Akt Pathway through Interaction of Its Protein Meq with the P85 Subunit of PI3K to Promote Viral Replication. Front. Microbiol. 2018, 9, 2547. [Google Scholar] [CrossRef] [Green Version]
- Kurebayashi, Y.; Nagai, S.; Ikejiri, A.; Ohtani, M.; Ichiyama, K.; Baba, Y.; Yamada, T.; Egami, S.; Hoshii, T.; Hirao, A.; et al. PI3K-Akt-mTORC1-S6K1/2 Axis Controls Th17 Differentiation by Regulating Gfi1 Expression and Nuclear Translocation of RORγ. Cell Rep. 2012, 1, 360–373. [Google Scholar] [CrossRef] [Green Version]
| Target | Primer Sequences | Accession No. | |
|---|---|---|---|
| Full length chIL-17A | FWD | AAGCTTATGTCTCCGATCCCTTG | NM_204460.2 |
| REV | GATATCAGCCTGGTGCTGGATCAGTGGG | ||
| Target | Primer Sequences | Reference | |
|---|---|---|---|
| IL-1β | FWD | GTGAGGCTCAACATTGCGCTGTA | [23] |
| REV | TGTCCAGGCGGTAGAAGATGAAG | ||
| TGF-β | FWD | CGGCCGACGATGAGTGGCTC | [26] |
| REV | CGGGGCCCATCTCACAGGGA | ||
| COX-2 | FWD | CTGCTCCCTCCCATGTCAGA | [16] |
| REV | CACGTGAAGAATTCCGGTGTT | ||
| IL-2 | FWD | TGCAGTGTTACCTGGGAGAAGTGGT | [27] |
| REV | ACTTCCGGTGTGATTTAGACCCGT | ||
| IL-6 | FWD | CGTGTGCGAGAACAGCATGGAGA | [28] |
| REV | TCAGGCATTTCTCCTCGTCGAAGC | ||
| IL-10 | FWD | TTTGGCTGCCAGTCTGTGTC | [29] |
| REV | CTCATCCATCTTCTCGAACGTC | ||
| IL-12p40 | FWD | CCAAGACCTGGAGCACACCGAAG | [28] |
| REV | CGATCCCTGGCCTGCACAGAGA | ||
| IL-17A | FWD | TATCAGCAAACGCTCACTGG | [30] |
| REV | AGTTCACGCACCTGGAATG | ||
| IFN-γ | FWD | ACACTGACAAGTCAAAGCCGCACA | [26] |
| REV | AGTCGTTCATCGGGAGCTTGGC | ||
| MDV-Meq | FWD | GTCCCCCCTCGATCTTTCTC | [23] |
| REV | CGTCTGCTTCCTGCGTCTTC | ||
| β-actin | FWD | CAACACAGTGCTGTCTGGTGGTA | [31] |
| REV | ATCGTACTCCTGCTTGCTGATCC | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boodhoo, N.; Matsuyama-Kato, A.; Raj, S.; Fazel, F.; St-Denis, M.; Sharif, S. Effect of Pre-Treatment with a Recombinant Chicken Interleukin-17A on Vaccine Induced Immunity against a Very Virulent Marek’s Disease Virus. Viruses 2023, 15, 1633. https://doi.org/10.3390/v15081633
Boodhoo N, Matsuyama-Kato A, Raj S, Fazel F, St-Denis M, Sharif S. Effect of Pre-Treatment with a Recombinant Chicken Interleukin-17A on Vaccine Induced Immunity against a Very Virulent Marek’s Disease Virus. Viruses. 2023; 15(8):1633. https://doi.org/10.3390/v15081633
Chicago/Turabian StyleBoodhoo, Nitish, Ayumi Matsuyama-Kato, Sugandha Raj, Fatemeh Fazel, Myles St-Denis, and Shayan Sharif. 2023. "Effect of Pre-Treatment with a Recombinant Chicken Interleukin-17A on Vaccine Induced Immunity against a Very Virulent Marek’s Disease Virus" Viruses 15, no. 8: 1633. https://doi.org/10.3390/v15081633
APA StyleBoodhoo, N., Matsuyama-Kato, A., Raj, S., Fazel, F., St-Denis, M., & Sharif, S. (2023). Effect of Pre-Treatment with a Recombinant Chicken Interleukin-17A on Vaccine Induced Immunity against a Very Virulent Marek’s Disease Virus. Viruses, 15(8), 1633. https://doi.org/10.3390/v15081633






