Mechanistic Understanding of the Antiviral Properties of Pistachios and Zeaxanthin against HSV-1
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Methods
2.2. Polyphenolic Analysis of Pistachio Extracts
2.3. Cell Culture and Virus
2.4. Cell Viability Assay
2.5. Plaque Reduction Assay
2.6. Binding Assay
2.7. The Time-of-Addition Assay
2.8. Viral Inactivation Assay
2.9. Western Blot Analysis
2.10. Viral DNA Extraction and Real-Time PCR
2.11. Statistical Analysis
3. Results
3.1. RP-HPLC-DAD Identification and Quantification of Phenolic Compounds
3.2. Evaluation of the Cytotoxicity Effect Following Treatment with Pistachio Extracts
3.3. Impact of NRRE and RURE with or without n-Hexane on HSV-1 Replication in VERO Cells
3.4. Mechanisms Involved in the Anti-HSV-1 Exerted by NRRE and RURE n-Hexane
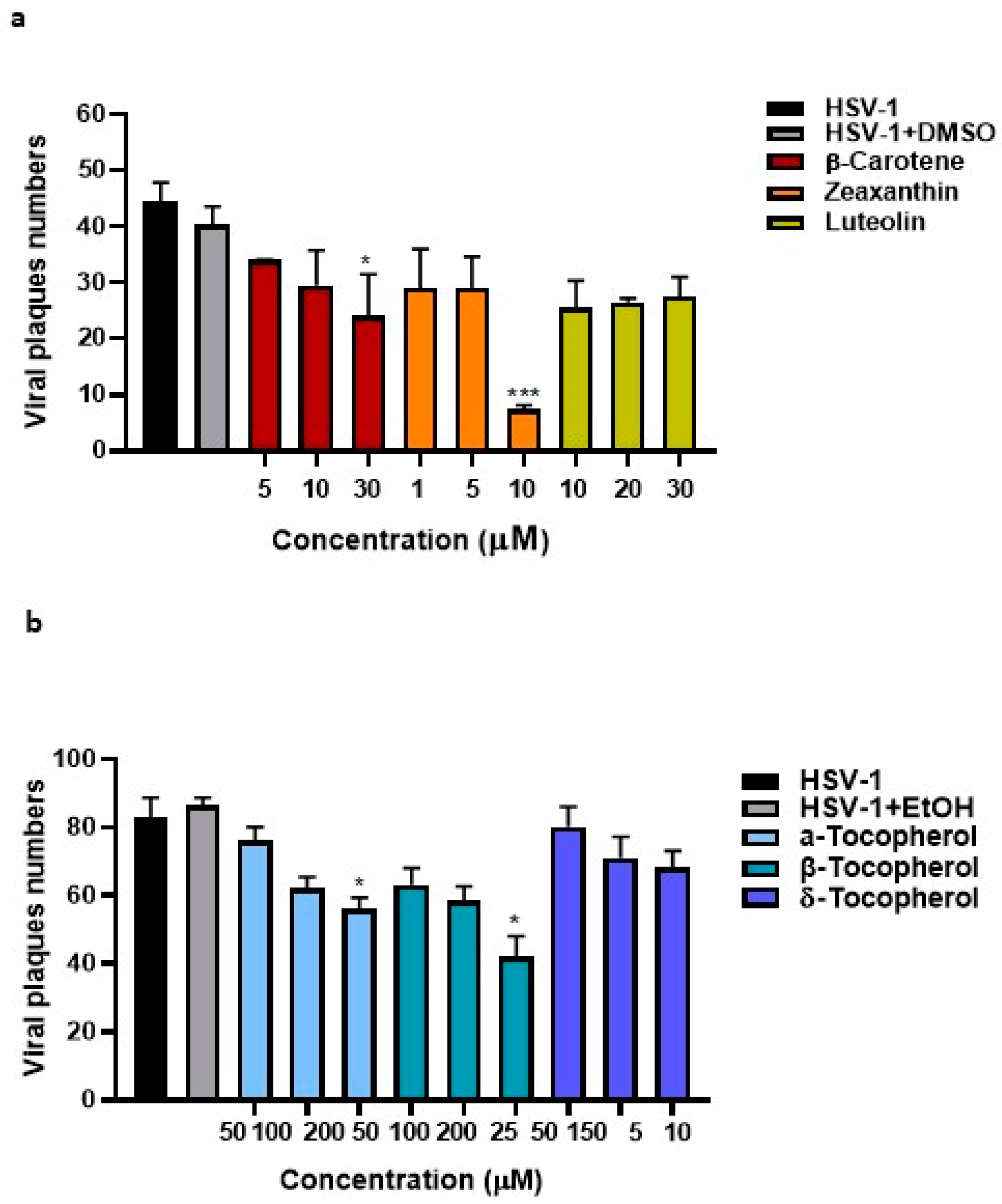
3.5. Determination of Anti-HSV Activity Properties of Tocopherols, Carotenoids, and Xanthophylls Exclusively Present in n-Hexane Pistachios Extraction Method
3.6. Zeaxanthin Inhibits the HSV-1 Replication Acting on Both Viral Internalization and Replication
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, B.J.; Fraefel, C.; Cunningham, A.L.; Diefenbach, R.J. Functional roles of the tegument proteins of herpes simplex virus type 1. Virus Res. 2009, 145, 173–186. [Google Scholar] [PubMed]
- Mody, P.H.; Pathak, S.; Hanson, L.K.; Spencer, J.V. Herpes Simplex Virus: A Versatile Tool for Insights into Evolution, Gene Delivery, and Tumor Immunotherapy. Virology 2020, 11, 1178122X20913274. [Google Scholar]
- Beilstein, F.; Cohen, G.H.; Eisenberg, R.J.; Nicolas, V.; Esclatine, A.; Pasdeloup, D. Dynamic organization of Herpesvirus glycoproteins on the viral envelope revealed by super-resolution microscopy. PLoS Pathog. 2019, 15, e1008209. [Google Scholar] [CrossRef] [PubMed]
- Honess, R.W.; Roizman, B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 1974, 14, 8–19. [Google Scholar] [PubMed]
- Richter, E.R.; Dias, J.K.; Gilbert, J.E., 2nd; Atherton, S.S. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J. Infect. Dis. 2009, 200, 1901–1906. [Google Scholar] [CrossRef]
- Bagga, B.; Kate, A.; Joseph, J.; Dave, V.P. Herpes simplex infection of the eye: An introduction. Community Eye Health 2020, 33, 68–70. [Google Scholar]
- Zandi, S.; Bodaghi, B.; Garweg, J.G. Review for Disease of the Year: Treatment of Viral Anterior Uveitis: A Perspective. Ocul. Immunol. Inflamm. 2018, 26, 1135–1142. [Google Scholar]
- Carter, S.B.; Cohen, E.J. Development of Herpes Simplex Virus Infectious Epithelial Keratitis During Oral Acyclovir Therapy and Response to Topical Antivirals. Cornea 2016, 35, 692–695. [Google Scholar] [CrossRef]
- Duan, R.; de Vries, R.D.; Osterhaus, A.D.; Remeijer, L.; Verjans, G.M. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J. Infect. Dis. 2008, 198, 659–663. [Google Scholar] [CrossRef]
- Castro-Castaneda, C.R.; Altamirano-Lamarque, F.; Ortega-Macías, A.G.; Santa Cruz-Pavlovich, F.J.; Gonzalez-De la Rosa, A.; Armendariz-Borunda, J.; Santos, A.; Navarro-Partida, J. Nutraceuticals: A Promising Therapeutic Approach in Ophthalmology. Nutrients 2022, 14, 5014. [Google Scholar]
- Huang, R.; Shi, F.; Lei, T.; Song, Y.; Hughes, C.L.; Liu, G. Effect of the isoflavone genistein against galactose-induced cataracts in rats. Exp. Biol. Med. 2007, 232, 118–125. [Google Scholar]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar]
- Lem, D.W.; Davey, P.G.; Gierhart, D.L.; Rosen, R.B. A Systematic Review of Carotenoids in the Management of Age-Related Macular Degeneration. Antioxidants 2021, 10, 1255. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Barreca, D.; Gervasi, T.; Roussell, M.A.; Klein, B.; Feeney, M.J.; Carughi, A. Pistachio Nuts (Pistacia vera L.): Production, Nutrients, Bioactives and Novel Health Effects. Plants 2022, 11, 18. [Google Scholar]
- Toti, E.; Chen, C.-Y.O.; Palmery, M.; Villaño Valencia, D.; Peluso, I. Non-Provitamin A and Provitamin A Carotenoids as Immunomodulators: Recommended Dietary Allowance, Therapeutic Index, or Personalized Nutrition? Oxidative Med. Cell. Longev. 2018, 2018, 4637861. [Google Scholar]
- Fazal, Y.; Fatima, S.N.; Shahid, S.M.; Mahboob, T. Nephroprotective effects of b-carotene on ACE gene expression, oxidative stress and antioxidant status in thioacetamide induced renal toxicity in rats. Pak. J. Pharm. Sci. 2016, 29, 1139–1144. [Google Scholar]
- Khalil, A.; Tazeddinova, D.; Aljoumaa, K.; Kazhmukhanbetkyzy, Z.A.; Orazov, A.; Toshev, A.D. Carotenoids: Therapeutic Strategy in the Battle against Viral Emerging Diseases, COVID-19: An Overview. Prev. Nutr. Food Sci. 2021, 26, 241–261. [Google Scholar] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Zivkovic, A.M.; Yiu, G.; Hackman, R.M. Potential roles of dietary zeaxanthin and lutein in macular health and function. Nutr. Rev. 2023, 81, 670–683. [Google Scholar]
- Arunkumar, R.; Gorusupudi, A.; Bernstein, P.S. The macular carotenoids: A biochemical overview. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158617. [Google Scholar]
- Gervasi, T.; D’Arrigo, M.; Rando, R.; Sciortino, M.T.; Carughi, A.; Barreca, D.; Mandalari, G. The Antimicrobial Potential of Hexane Oils and Polyphenols-Rich Extracts from Pistacia vera L. Appl. Sci. 2022, 12, 4389. [Google Scholar]
- Mandalari, G.; Bisignano, C.; Filocamo, A.; Chessa, S.; Sarò, M.; Torre, G.; Faulks, R.M.; Dugo, P. Bioaccessibility of pistachio polyphenols, xanthophylls, and tocopherols during simulated human digestion. Nutrition 2013, 29, 338–344. [Google Scholar]
- Fanali, C.; Dugo, L.; Cacciola, F.; Beccaria, M.; Grasso, S.; Dachà, M.; Dugo, P.; Mondello, L. Chemical characterization of Sacha Inchi (Plukenetia volubilis L.) oil. J. Agric. Food Chem. 2011, 59, 13043–13049. [Google Scholar] [CrossRef]
- Bisignano, C.; Mandalari, G.; Smeriglio, A.; Trombetta, D.; Pizzo, M.M.; Pennisi, R.; Sciortino, M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses 2017, 9, 178. [Google Scholar]
- Lin, L.T.; Chen, T.Y.; Chung, C.Y.; Noyce, R.S.; Grindley, T.B.; McCormick, C.; Lin, T.C.; Wang, G.H.; Lin, C.C.; Richardson, C.D. Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J. Virol. 2011, 85, 4386–4398. [Google Scholar]
- Pennisi, R.; Ben Amor, I.; Gargouri, B.; Attia, H.; Zaabi, R.; Chira, A.B.; Saoudi, M.; Piperno, A.; Trischitta, P.; Tamburello, M.P.; et al. Analysis of Antioxidant and Antiviral Effects of Olive (Olea europaea L.) Leaf Extracts and Pure Compound Using Cancer Cell Model. Biomolecules 2023, 13, 238. [Google Scholar] [CrossRef]
- Saleh, D.; Yarrarapu, S.N.S.; Sharma, S. Herpes Simplex Type 1; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- De Clercq, E.; Andrei, G.; Snoeck, R.; De Bolle, L.; Naesens, L.; Degrève, B.; Balzarini, J.; Zhang, Y.; Schols, D.; Leyssen, P. Acyclic/carbocyclic guanosine analogues as anti-herpesvirus agents. Nucleosides Nucleotides Nucleic Acids 2001, 20, 271–285. [Google Scholar]
- Morfin, F.; Thouvenot, D. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 2003, 26, 29–37. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of Tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar]
- Ballistreri, G.; Arena, E.; Fallico, B. Influence of ripeness and drying process on the polyphenols and tocopherols of Pistacia vera L. Molecules 2009, 14, 4358–4369. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, C.; Filocamo, A.; Faulks, R.M.; Mandalari, G. In vitro antimicrobial activity of pistachio (Pistacia vera L.) polyphenols. FEMS Microbiol. Lett. 2013, 341, 62–67. [Google Scholar] [CrossRef]
- La Camera, E.; Bisignano, C.; Crisafi, G.; Smeriglio, A.; Denaro, M.; Trombetta, D.; Mandalari, G. Biochemical Characterization of Clinical Strains of Staphylococcus spp. and Their Sensitivity to Polyphenols-Rich Extracts from Pistachio (Pistacia vera L.). Pathogens 2018, 7, 82. [Google Scholar] [PubMed]
- Bernstein, P.S.; Delori, F.C.; Richer, S.; van Kuijk, F.J.; Wenzel, A.J. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vis. Res. 2010, 50, 716–728. [Google Scholar]
- Naithani, R.; Huma, L.C.; Holland, L.E.; Shukla, D.; McCormick, D.L.; Mehta, R.G.; Moriarty, R.M. Antiviral activity of phytochemicals: A comprehensive review. Mini Rev. Med. Chem. 2008, 8, 1106–1133. [Google Scholar] [CrossRef]
- Cho, K.S.; Shin, M.; Kim, S.; Lee, S.B. Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018, 2018, 4120458. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Burrows, T.L.; Williams, R.; Rollo, M.; Wood, L.; Garg, M.L.; Jensen, M.; Collins, C.E. Plasma carotenoid levels as biomarkers of dietary carotenoid consumption: A systematic review of the validation studies. J. Nutr. Intermed. Metab. 2015, 2, 15–64. [Google Scholar]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Yeum, K.J.; Taylor, A.; Tang, G.; Russell, R.M. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Investig. Ophthalmol. Vis. Sci. 1995, 36, 2756–2761. [Google Scholar]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef]
- Gopinath, B. The Role of Nutrition in Age-Related Eye Diseases. In Molecular Basis of Nutrition and Aging; Academic Press: Cambridge, MA, USA, 2016; pp. 433–446. [Google Scholar]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, O.; Yeum, K.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2 induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar]
- Kowluru, R.A.; Zhong, Q.; Santos, J.M.; Thandampallayam, M.; Putt, D.; Gierhart, D.L. Beneficial effects of the nutritional supplements on the development of diabetic retinopathy. Nutr. Metab. 2014, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Labetoulle, M.; Boutolleau, D.; Burrel, S.; Haigh, O.; Rousseau, A. Herpes simplex virus, varicella-zoster virus and cytomegalovirus keratitis: Facts for the clinician. Ocul. Surf. 2021; in press. [Google Scholar]
- Barker, N.H. Ocular herpes simplex. BMJ Clin. Evid. 2008, 2008, 0707. [Google Scholar] [PubMed]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Smeriglio, A.; Mandalari, G.; Sciortino, M.T. In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.). Plants 2020, 9, 267. [Google Scholar]
- Tomaino, A.; Martorana, M.; Arcoraci, T.; Monteleone, D.; Giovinazzo, C.; Saija, A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 2010, 92, 1115–1122. [Google Scholar]
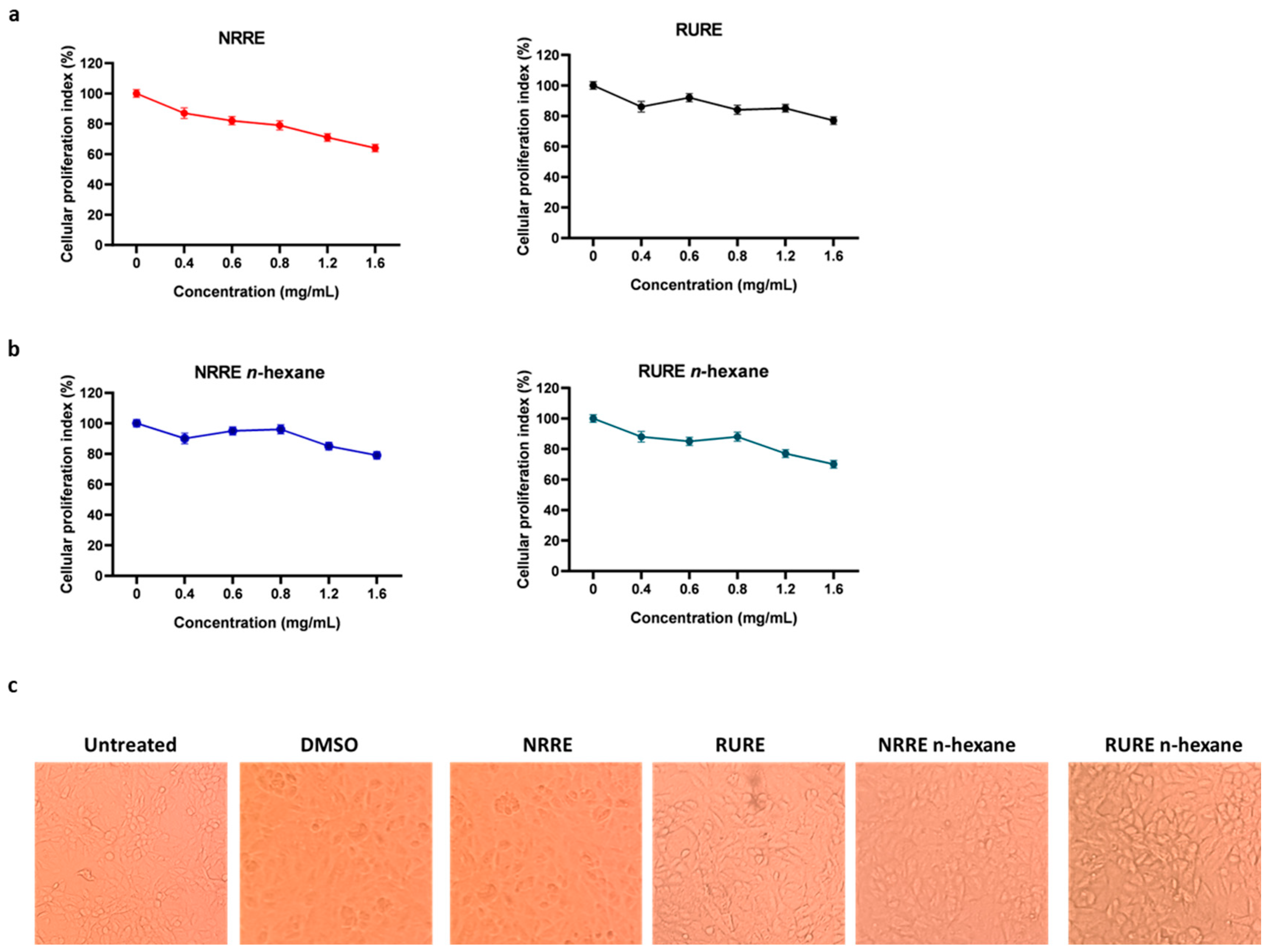
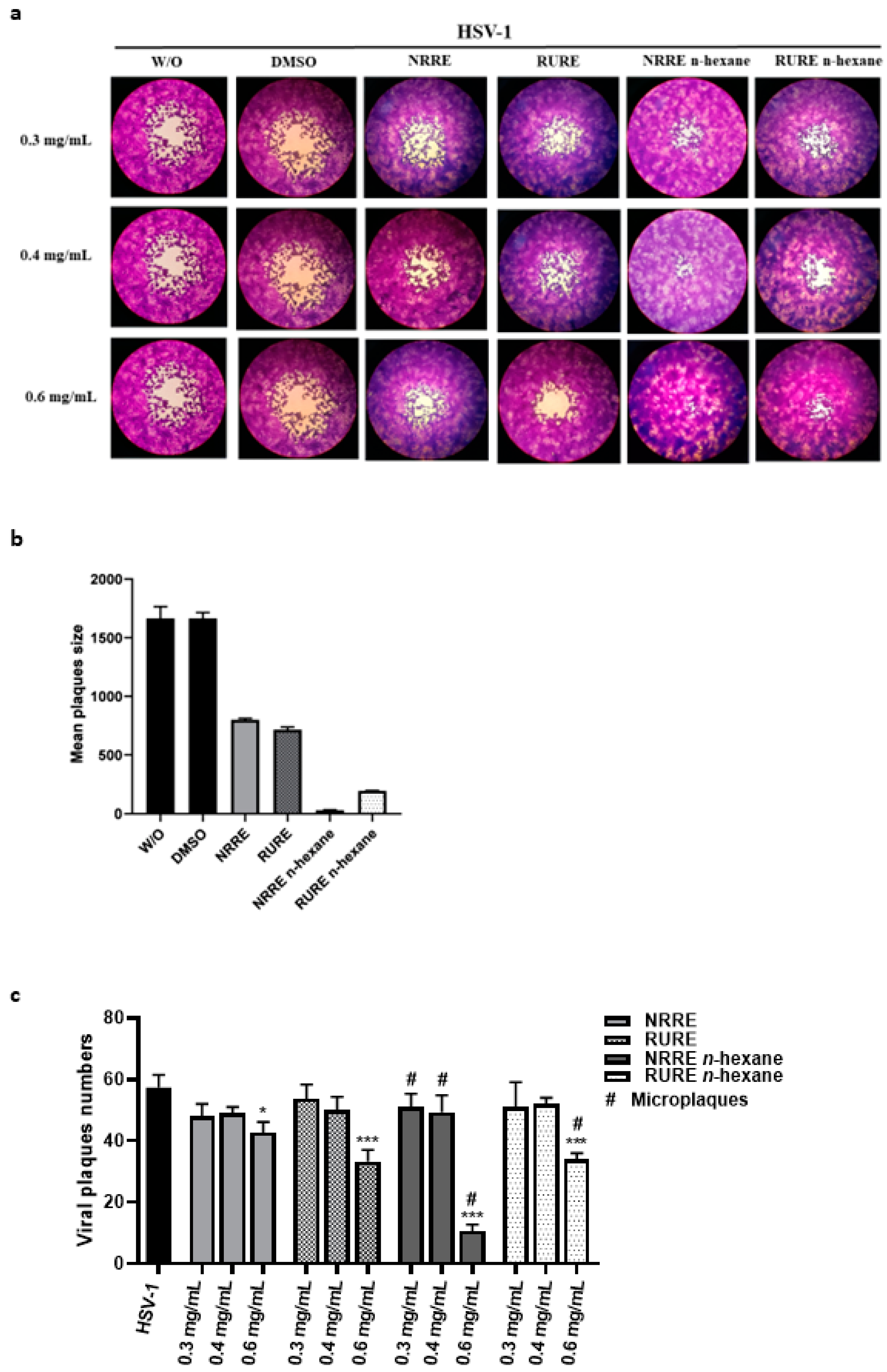
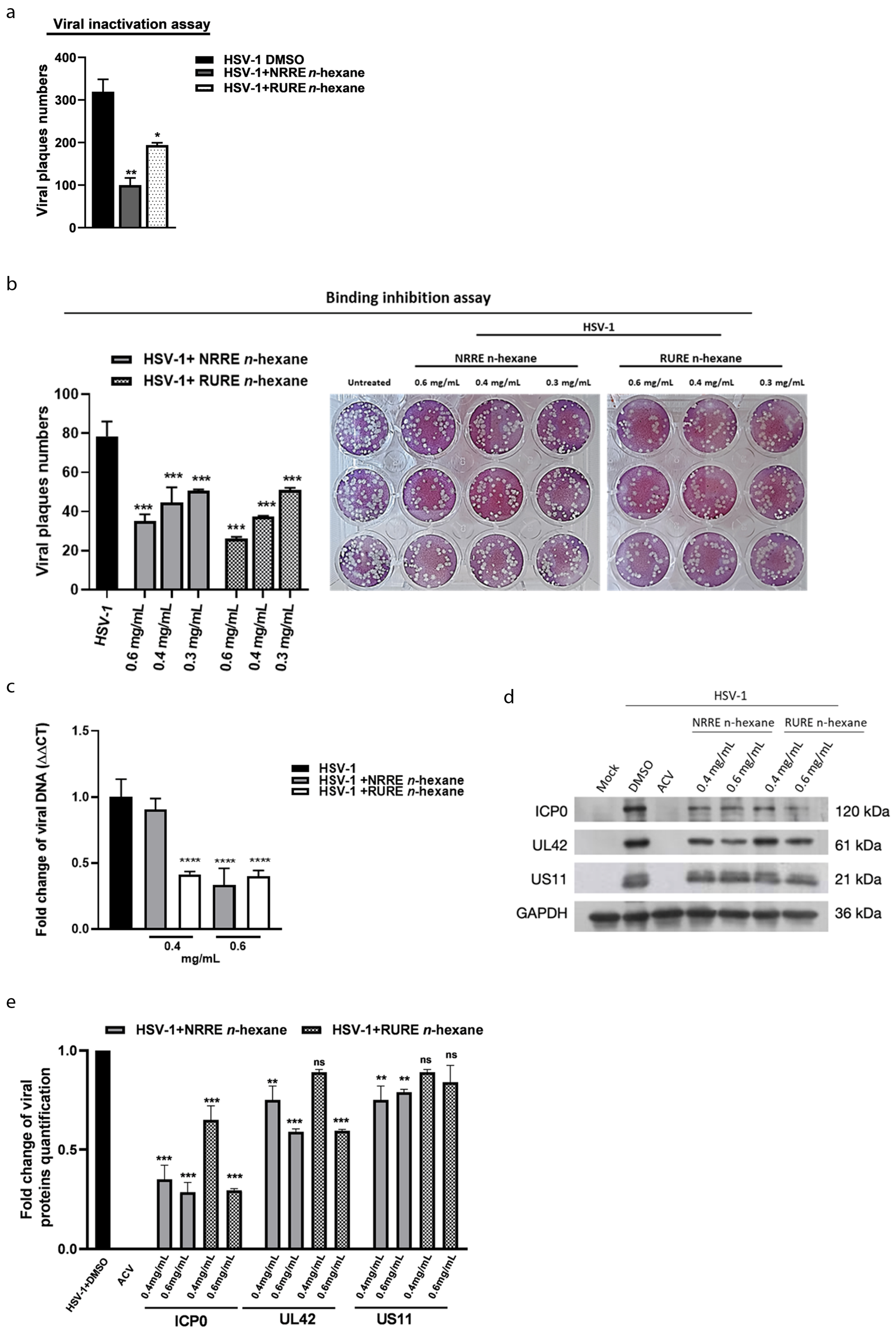
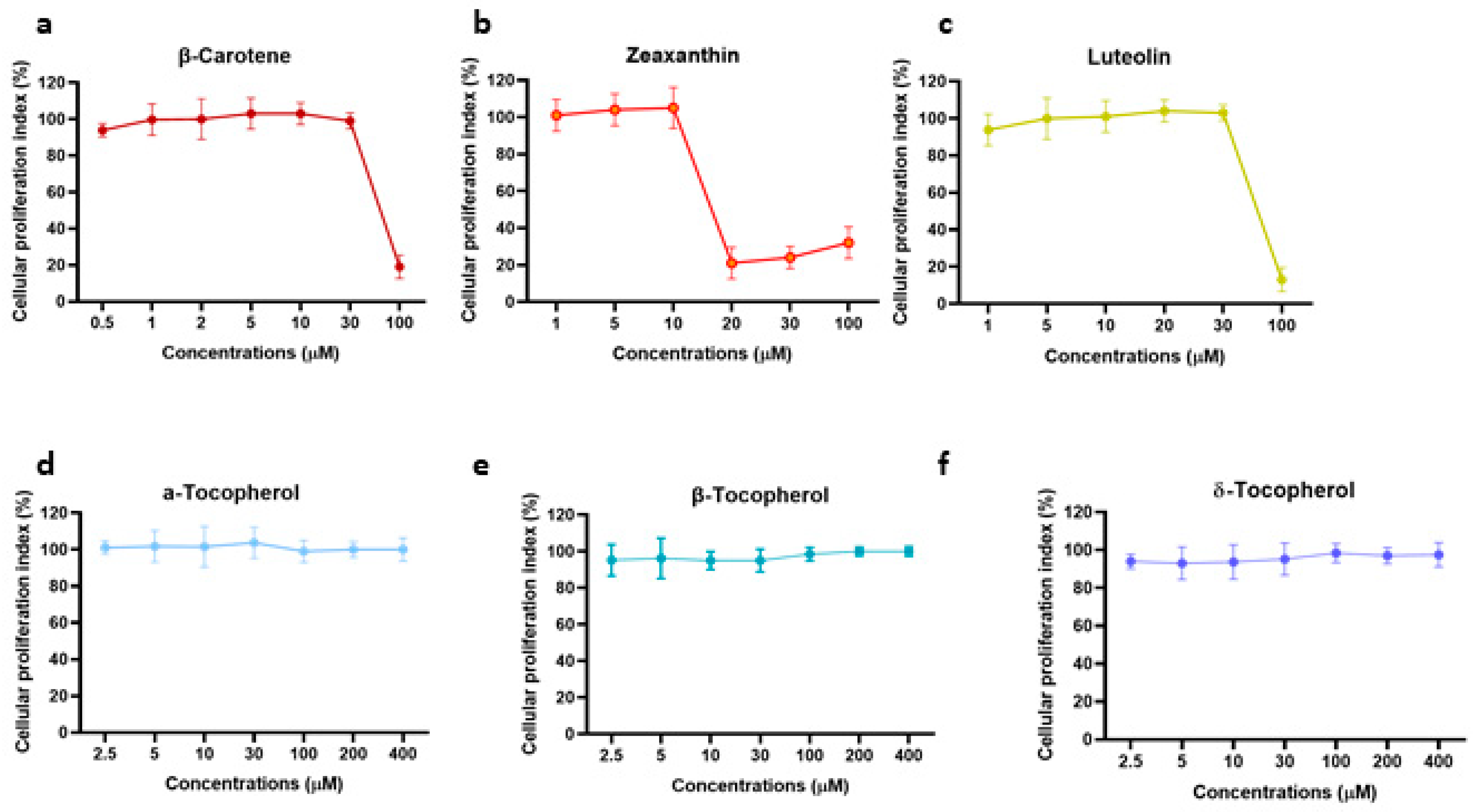

| Extract | CC50 (mg/mL) | EC50 (mg/mL) | SI |
|---|---|---|---|
| NRRE n-hexane | 4.6 | 0.50 | 9.2 |
| RURE n-hexane | 3.29 | 0.66 | 4.98 |
| NRRE | 2.5 | 0.77 | 3.2 |
| RURE | 4.5 | 0.65 | 6.9 |
| mg/100 g | ||||
|---|---|---|---|---|
| Compound | RURE n-Hexane | NRRE n-Hexane | RURE | NRRE |
| Extraction Method 1 | Extraction Method 2 | |||
| Tocopherols | ||||
| a-tocopherol | trace | trace | - | - |
| β-tocopherol | 0.17 ± 0.02 | 0.12 ± 0.02 | - | - |
| δ-tocopherol | 0.92 ± 0.08 | 0.62 ± 0.08 | - | - |
| Carotenoids | ||||
| β-carotene | 0.10 ± 0.01 | 0.11 ± 0.01 | - | - |
| Xanthophylls | ||||
| Zeaxanthin | 0.08 ± 0.01 | 0.07 ± 0.01 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennisi, R.; Trischitta, P.; Tamburello, M.P.; Barreca, D.; Mandalari, G.; Sciortino, M.T. Mechanistic Understanding of the Antiviral Properties of Pistachios and Zeaxanthin against HSV-1. Viruses 2023, 15, 1651. https://doi.org/10.3390/v15081651
Pennisi R, Trischitta P, Tamburello MP, Barreca D, Mandalari G, Sciortino MT. Mechanistic Understanding of the Antiviral Properties of Pistachios and Zeaxanthin against HSV-1. Viruses. 2023; 15(8):1651. https://doi.org/10.3390/v15081651
Chicago/Turabian StylePennisi, Rosamaria, Paola Trischitta, Maria Pia Tamburello, Davide Barreca, Giuseppina Mandalari, and Maria Teresa Sciortino. 2023. "Mechanistic Understanding of the Antiviral Properties of Pistachios and Zeaxanthin against HSV-1" Viruses 15, no. 8: 1651. https://doi.org/10.3390/v15081651
APA StylePennisi, R., Trischitta, P., Tamburello, M. P., Barreca, D., Mandalari, G., & Sciortino, M. T. (2023). Mechanistic Understanding of the Antiviral Properties of Pistachios and Zeaxanthin against HSV-1. Viruses, 15(8), 1651. https://doi.org/10.3390/v15081651










