Inhibition of Marek’s Disease Virus Replication and Spread by 25-hydroxycholesterol and 27-hydroxycholesterol In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. CEF Culture and Virus Preparations
2.2. Cells and MDV Infection
2.2.1. MDV Titre
2.2.2. Measurement of Virus Plaque Sizes
2.2.3. Quantification of Gene Expression by RTqPCR
2.2.4. CEF Cell IFN-α-Luc Reporter Assay
2.2.5. Statistical Analysis
3. Results
3.1. MDV Infection Transiently induces IFN-α
3.2. IFN-α Upregulates CH25H and CYP27A1
3.3. MDV Infection Induces Moderate and Transient Upregulation of CH25H and CYP27A1
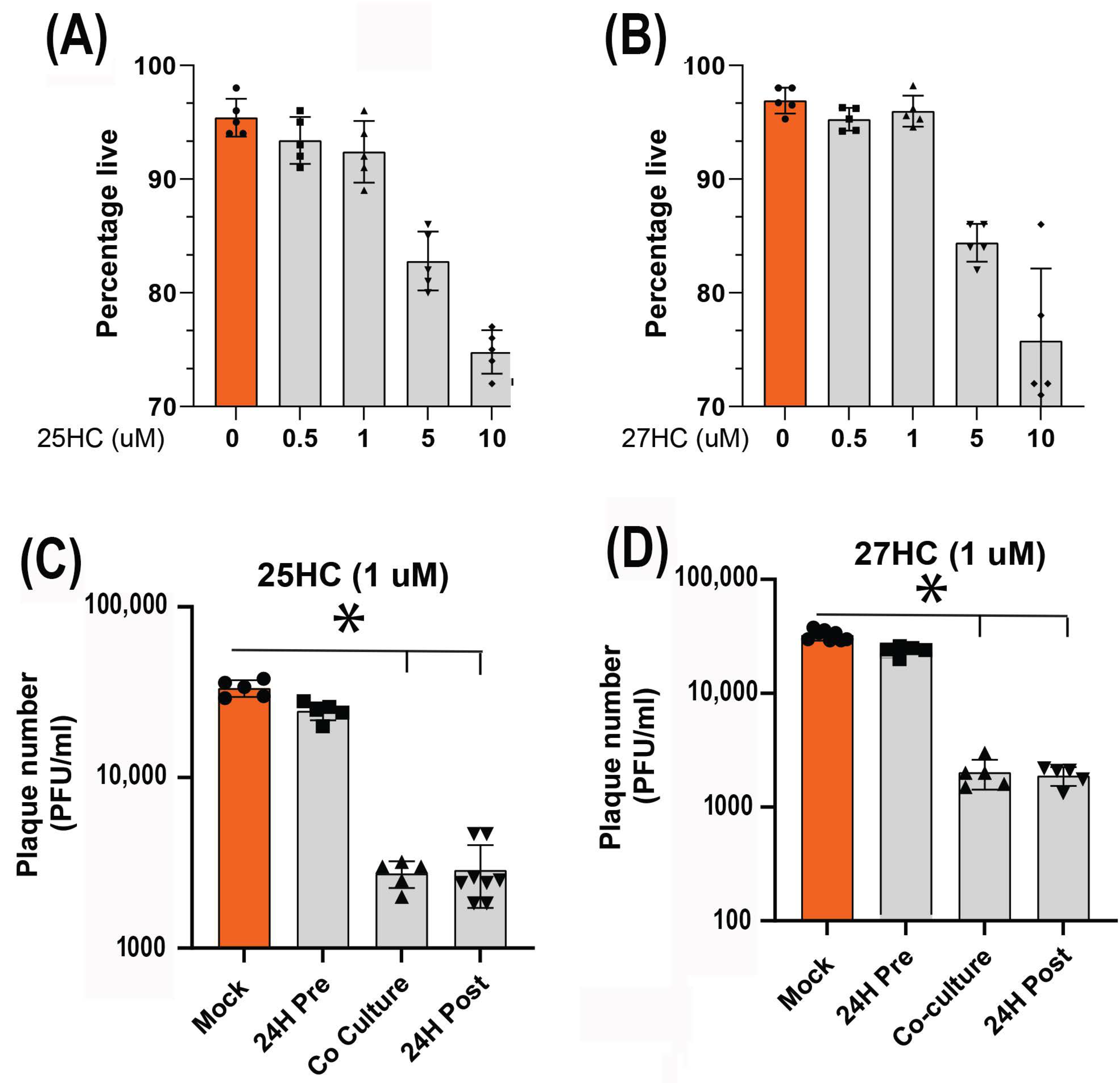
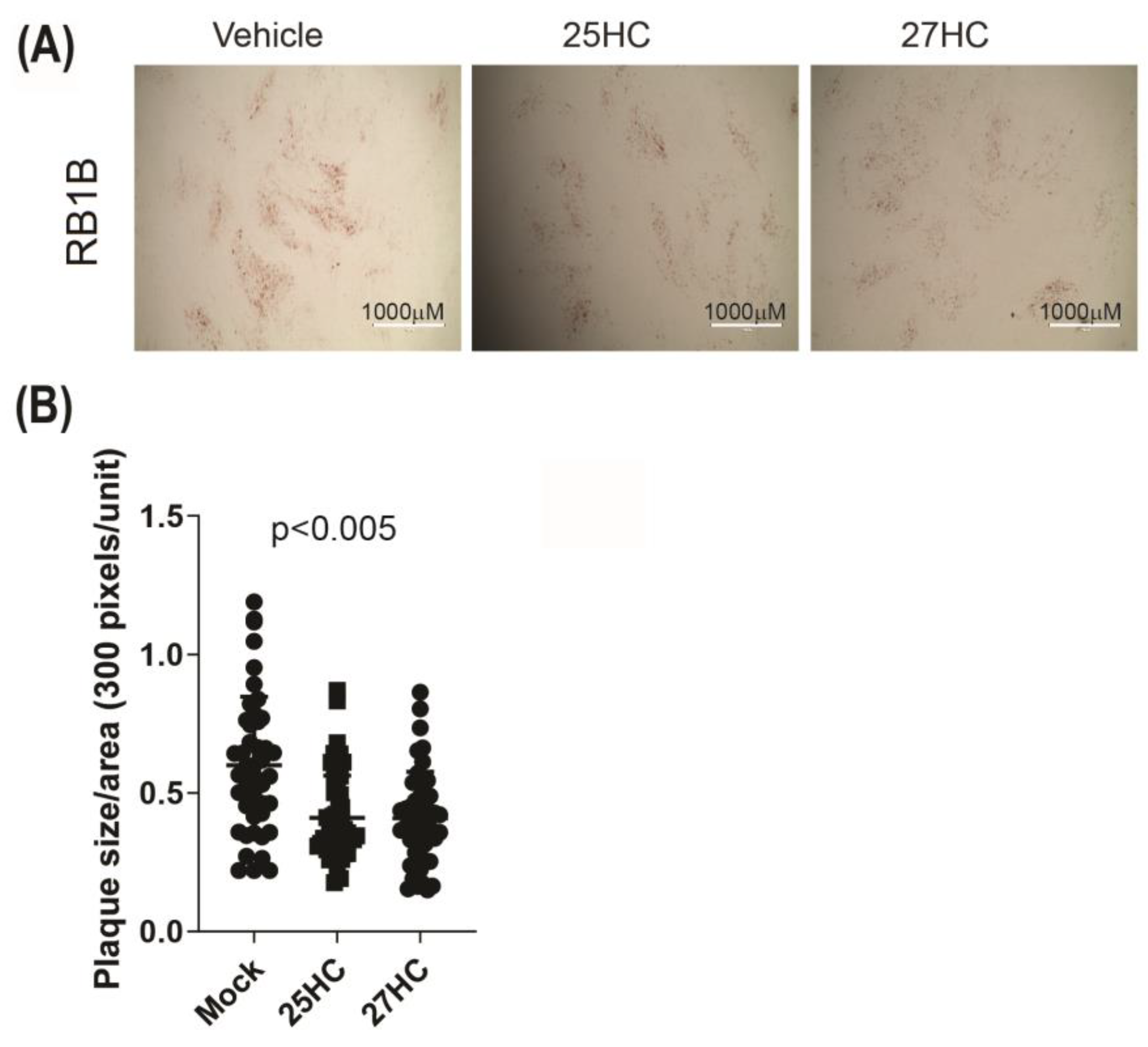
3.4. 25-HC and 27-HC Significantly Reduce MDV Replication and Spread In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boodhoo, N.; Gurung, A.; Sharif, S.; Behboudi, S. Marek’s disease in chickens: A review with focus on immunology. Vet. Res. 2016, 47, 119. [Google Scholar] [CrossRef] [PubMed]
- Kamble, N.; Gurung, A.; Kaufer, B.B.; Pathan, A.A.; Behboudi, S. Marek’s Disease Virus Modulates T Cell Proliferation via Activation of Cyclooxygenase 2-Dependent Prostaglandin E2. Front. Immunol. 2021, 12, 801781. [Google Scholar] [CrossRef] [PubMed]
- Osterrieder, N.; Kamil, J.P.; Schumacher, D.; Tischer, B.K.; Trapp, S. Marek’s disease virus: From miasma to model. Nat. Rev. Microbiol. 2006, 4, 283–294. [Google Scholar] [CrossRef]
- Nair, V. Latency and tumorigenesis in Marek’s disease. Avian Dis. 2013, 57 (Suppl. 2), 360–365. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Kamble, N.; Sharif, S.; Behboudi, S. Glutaminolysis and Glycolysis Are Essential for Optimal Replication of Marek’s Disease Virus. J. Virol. 2020, 94, e01680-19. [Google Scholar] [CrossRef]
- Boodhoo, N.; Kamble, N.; Kaufer, B.B.; Behboudi, S. Replication of Marek’s Disease Virus Is Dependent on Synthesis of De Novo Fatty Acid and Prostaglandin E(2). J. Virol. 2019, 93, e00352-19. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Kamble, N.; Behboudi, S. De Novo Cholesterol Biosynthesis and Its Trafficking in LAMP-1-Positive Vesicles Are Involved in Replication and Spread of Marek’s Disease Virus. J. Virol. 2020, 94, e01001-20. [Google Scholar] [CrossRef] [PubMed]
- Hennig, H.; Osterrieder, N.; Muller-Steinhardt, M.; Teichert, H.M.; Kirchner, H.; Wandinger, K.P. Detection of Marek’s disease virus DNA in chicken but not in human plasma. J. Clin. Microbiol. 2003, 41, 2428–2432. [Google Scholar] [CrossRef]
- Lantier, I.; Mallet, C.; Souci, L.; Larcher, T.; Conradie, A.M.; Courvoisier, K.; Trapp, S.; Pasdeloup, D.; Kaufer, B.B.; Denesvre, C. In vivo imaging reveals novel replication sites of a highly oncogenic avian herpesvirus in chickens. PLoS Pathog. 2022, 18, e1010745. [Google Scholar] [CrossRef]
- Boodhoo, N.; Behboudi, S. Marek’s disease virus-specific T cells proliferate, express antiviral cytokines but have impaired degranulation response. Front. Immunol. 2022, 13, 973762. [Google Scholar] [CrossRef]
- Boodhoo, N.; Behboudi, S. Differential Virus-Specific IFN-Gamma Producing T Cell Responses to Marek’s Disease Virus in Chickens With B19 and B21 MHC Haplotypes. Front. Immunol. 2021, 12, 784359. [Google Scholar] [CrossRef]
- Read, A.F.; Baigent, S.J.; Powers, C.; Kgosana, L.B.; Blackwell, L.; Smith, L.P.; Kennedy, D.A.; Walkden-Brown, S.W.; Nair, V.K. Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLoS Biol. 2015, 13, e1002198. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R. Type I interferons in viral control and immune regulation. Curr. Opin. Virol. 2016, 16, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Sanchez, D.J.; Aliyari, R.; Lu, S.; Cheng, G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl. Acad. Sci. USA 2012, 109, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.S.; Vandeberg, J.L.; Cox, L.A. Genomics and proteomics of vertebrate cholesterol ester lipase (LIPA) and cholesterol 25-hydroxylase (CH25H). 3 Biotech. 2011, 1, 99–109. [Google Scholar] [CrossRef]
- Cyster, J.G.; Dang, E.V.; Reboldi, A.; Yi, T. 25-Hydroxycholesterols in innate and adaptive immunity. Nat. Rev. Immunol. 2014, 14, 731–743. [Google Scholar] [CrossRef]
- Reiss, A.B.; Awadallah, N.W.; Malhotra, S.; Montesinos, M.C.; Chan, E.S.; Javitt, N.B.; Cronstein, B.N. Immune complexes and IFN-gamma decrease cholesterol 27-hydroxylase in human arterial endothelium and macrophages. J. Lipid Res. 2001, 42, 1913–1922. [Google Scholar] [CrossRef]
- Ahmed, D.; Jaworski, A.; Roy, D.; Willmore, W.; Golshani, A.; Cassol, E. Transcriptional Profiling Suggests Extensive Metabolic Rewiring of Human and Mouse Macrophages during Early Interferon Alpha Responses. Mediat. Inflamm. 2018, 2018, 5906819. [Google Scholar] [CrossRef]
- Giotis, E.S.; Robey, R.C.; Skinner, N.G.; Tomlinson, C.D.; Goodbourn, S.; Skinner, M.A. Chicken interferome: Avian interferon-stimulated genes identified by microarray and RNA-seq of primary chick embryo fibroblasts treated with a chicken type I interferon (IFN-alpha). Vet. Res. 2016, 47, 75. [Google Scholar] [CrossRef]
- Xie, T.; Feng, M.; Dai, M.; Mo, G.; Ruan, Z.; Wang, G.; Shi, M.; Zhang, X. Cholesterol-25-hydroxylase Is a Chicken ISG That Restricts ALV-J Infection by Producing 25-hydroxycholesterol. Viruses 2019, 11, 498. [Google Scholar] [CrossRef]
- Staines, K.; Batra, A.; Mwangi, W.; Maier, H.J.; Van Borm, S.; Young, J.R.; Fife, M.; Butter, C. A Versatile Panel of Reference Gene Assays for the Measurement of Chicken mRNA by Quantitative PCR. PLoS ONE 2016, 11, e0160173. [Google Scholar] [CrossRef]
- Civra, A.; Francese, R.; Gamba, P.; Testa, G.; Cagno, V.; Poli, G.; Lembo, D. 25-Hydroxycholesterol and 27-hydroxycholesterol inhibit human rotavirus infection by sequestering viral particles into late endosomes. Redox Biol. 2018, 19, 318–330. [Google Scholar] [CrossRef]
- Bertzbach, L.D.; Harlin, O.; Härtle, S.; Fehler, F.; Vychodil, T.; Kaufer, B.B.; Kaspers, B. IFNα and IFNγ Impede Marek’s Disease Progression. Viruses 2019, 11, 1103. [Google Scholar] [CrossRef]
- Jarosinski, K.W.; Jia, W.; Sekellick, M.J.; Marcus, P.I.; Schat, K.A. Cellular responses in chickens treated with IFN-alpha orally or inoculated with recombinant Marek’s disease virus expressing IFN-alpha. J. Interferon Cytokine Res. 2001, 21, 287–296. [Google Scholar] [CrossRef]
- Mao, S.; Ren, J.; Xu, Y.; Lin, J.; Pan, C.; Meng, Y.; Xu, N. Studies in the antiviral molecular mechanisms of 25-hydroxycholesterol: Disturbing cholesterol homeostasis and post-translational modification of proteins. Eur. J. Pharmacol. 2022, 926, 175033. [Google Scholar] [CrossRef]
- Campbell, S.M.; Crowe, S.M.; Mak, J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J. Clin. Virol. 2001, 22, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Civra, A.; Colzani, M.; Cagno, V.; Francese, R.; Leoni, V.; Aldini, G.; Lembo, D.; Poli, G. Modulation of cell proteome by 25-hydroxycholesterol and 27-hydroxycholesterol: A link between cholesterol metabolism and antiviral defense. Free Radic. Biol. Med. 2020, 149, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Bai, J.; Nauwynck, H.; Lin, L.; Liu, X.; Yu, J.; Jiang, P. 25-Hydroxycholesterol provides antiviral protection against highly pathogenic porcine reproductive and respiratory syndrome virus in swine. Vet. Microbiol. 2019, 231, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Boodhoo, N.; Sharif, S.; Behboudi, S. 1alpha,25(OH)2 Vitamin D3 Modulates Avian T Lymphocyte Functions without Inducing CTL Unresponsiveness. PLoS ONE 2016, 11, e0150134. [Google Scholar] [CrossRef]
- Gao, L.; Li, K.; Zhang, Y.; Liu, Y.; Liu, C.; Zhang, Y.; Gao, Y.; Qi, X.; Cui, H.; Wang, Y.; et al. Inhibition of DNA-Sensing Pathway by Marek’s Disease Virus VP23 Protein through Suppression of Interferon Regulatory Factor 7 Activation. J. Virol. 2019, 93, e01934-18. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, L.; Xu, Z.; Luo, D.; Zhang, Y.; Gao, Y.; Liu, C.; Zhang, Y.; Qi, X.; Cui, H.; et al. Marek’s Disease Virus RLORF4 Inhibits Type I Interferon Production by Antagonizing NF-κB Activation. J. Virol. 2019, 93, e01037-19. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Careem, M.F.; Hunter, B.D.; Lee, L.F.; Fairbrother, J.H.; Haghighi, H.R.; Read, L.; Parvizi, P.; Heidari, M.; Sharif, S. Host responses in the bursa of Fabricius of chickens infected with virulent Marek’s disease virus. Virology 2008, 379, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Jarosinski, K.W.; Njaa, B.L.; O’Connell, P.H.; Schat, K.A. Pro-inflammatory responses in chicken spleen and brain tissues after infection with very virulent plus Marek’s disease virus. Viral Immunol. 2005, 18, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Magoro, T.; Dandekar, A.; Jennelle, L.T.; Bajaj, R.; Lipkowitz, G.; Angelucci, A.R.; Bessong, P.O.; Hahn, Y.S. IL-1β/TNF-α/IL-6 inflammatory cytokines promote STAT1-dependent induction of CH25H in Zika virus-infected human macrophages. J. Biol. Chem. 2019, 294, 14591–14602. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Tang, J.J.; Tao, W.; Cao, X.; Song, B.L.; Zhong, J. Identification of Cholesterol 25-Hydroxylase as a Novel Host Restriction Factor and a Part of the Primary Innate Immune Responses against Hepatitis C Virus Infection. J. Virol. 2015, 89, 6805–6816. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.W.; Sofer, L.; Anderson, A.S.; Bernberg, E.L.; Cui, J.; Burnside, J. Induction of host gene expression following infection of chicken embryo fibroblasts with oncogenic Marek’s disease virus. J. Virol. 2001, 75, 533–539. [Google Scholar] [CrossRef]
- Qu, H.; Yang, L.; Meng, S.; Xu, L.; Bi, Y.; Jia, X.; Li, J.; Sun, L.; Liu, W. The differential antiviral activities of chicken interferon alpha (ChIFN-alpha) and ChIFN-beta are related to distinct interferon-stimulated gene expression. PLoS ONE 2013, 8, e59307. [Google Scholar] [CrossRef]
- Reno, J.M.; Lee, L.F.; Boezi, J.A. Inhibition of herpesvirus replication and herpesvirus-induced deoxyribonucleic acid polymerase by phosphonoformate. Antimicrob. Agents Chemother. 1978, 13, 188–192. [Google Scholar] [CrossRef]
- Eidson, C.S.; Than, V.T.; Kleven, S.H. The in vitro and in vivo effect of chemotherapeutic agents on the Marek’s disease herpesvirus of chickens. Poult. Sci. 1974, 53, 1533–1538. [Google Scholar] [CrossRef]
- Collins, P. The spectrum of antiviral activities of acyclovir in vitro and in vivo. J. Antimicrob. Chemother. 1983, 12 (Suppl. B), 19–27. [Google Scholar] [CrossRef]
- Schat, K.A.; Schinazi, R.F.; Calnek, B.W. Cell-specific antiviral activity of 1-(2-fluoro-2-deoxy-beta-D-arabinofuranosyl)-5-iodocytosine (FIAC) against Marek’s disease herpesvirus and turkey herpesvirus. Antivir. Res. 1984, 4, 259–270. [Google Scholar] [CrossRef]
- Chang, T.S. AUS in the prevention of Marek’s disease. Avian Dis. 1984, 28, 154–159. [Google Scholar] [CrossRef]
- Colmano, G.; Gross, W.B. Effect of metyrapone and DDD on infectious diseases. Poult. Sci. 1971, 50, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Samorek-Salamonowicz, E.; Cakala, A.; Wijaszka, T. Effect of acyclovir on the replication of turkey herpesvirus and Marek’s disease virus. Res. Vet. Sci. 1987, 42, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.S.; Kermani-Arab, V.; Moll, T. Cyclophosphamide-induced amelioration of Marek’s disease in Marek’s disease-susceptible chickens. Am. J. Vet. Res. 1976, 37, 687–692. [Google Scholar] [PubMed]
- Kermani-Arab, V.; Moll, T.; Cho, B.R.; Davis, W.C.; Lu, Y.S. Effect of cyclophosphamide on the response of chickens to a virulent strain of Marek’s disease virus. Infect. Immun. 1975, 12, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, L.; Song, M.; Zhao, X.; Sun, N.; He, J.; Wu, C.; Jiang, J.; Bai, Y.; Guo, J.; et al. Screening compounds of Chinese medicinal herbs anti-Marek’s disease virus. Pharm. Biol. 2014, 52, 841–847. [Google Scholar] [CrossRef]
- Yang, F.; Feng, C.; Yao, Y.; Qin, A.; Shao, H.; Qian, K. Antiviral effect of baicalin on Marek’s disease virus in CEF cells. BMC Vet. Res. 2020, 16, 371. [Google Scholar] [CrossRef]
- Li, C.; Deng, Y.Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.Y.; Zhang, N.N.; Watanabe, M.; Dong, H.L.; Liu, P.; et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Trindade, B.C.; Ceglia, S.; Berthelette, A.; Raso, F.; Howley, K.; Muppidi, J.R.; Reboldi, A. The cholesterol metabolite 25-hydroxycholesterol restrains the transcriptional regulator SREBP2 and limits intestinal IgA plasma cell differentiation. Immunity 2021, 54, 2273–2287.e6. [Google Scholar] [CrossRef]
- de Freitas, F.A.; Levy, D.; Reichert, C.O.; Cunha-Neto, E.; Kalil, J.; Bydlowski, S.P. Effects of Oxysterols on Immune Cells and Related Diseases. Cells 2022, 11, 1251. [Google Scholar] [CrossRef] [PubMed]



| Gene | GenBank Accession No. | Primer Sequence | Tm (°C) | Product Size (bp) |
|---|---|---|---|---|
| CYP27A1 | XM_040676620.2 | For-ACCGCCTCCAGCTGATGT | 61 | 133 |
| Rev-ATCGGGTATTTGCCCTCCTG | 59 | |||
| CH25H | NM_001277354.1 | For-GACCTTCCGTGGTCAACTCA | 59 | 70 |
| Rev-GGAGATCATGATGCGGTGCT | 60 | |||
| IFIT5 | NM_001320422 | For-CTCCCAAATCCCTCTCAACA | 62 | 146 |
| Rev-CCGGTCATCGTCTGCATATT | 62 | |||
| PKR | AB125660.1 | For-CAGGCGTTGGTAAGAGTAAGAA | 62 | 135 |
| Rev-CATCCGCAGGTAGAGGAGATA | 62 | |||
| Actin-beta | NM_205518.2 | For-CCGTGCTGTGTTCCCATCTA | 59 | 98 |
| Rev-TCTGGGCTTCATCACCAACG | 60 | |||
| RPLP0 | NM_204987 | For-TTGGGCATCACCACAAAGATT | 65 | 82 |
| Rev-CCCACTTTGTCTCCGGTCTTAA | 67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamble, N.; Reddy, V.R.A.P.; Jackson, B.; Anjum, F.R.; Ubachukwu, C.C.; Patil, A.; Behboudi, S. Inhibition of Marek’s Disease Virus Replication and Spread by 25-hydroxycholesterol and 27-hydroxycholesterol In Vitro. Viruses 2023, 15, 1652. https://doi.org/10.3390/v15081652
Kamble N, Reddy VRAP, Jackson B, Anjum FR, Ubachukwu CC, Patil A, Behboudi S. Inhibition of Marek’s Disease Virus Replication and Spread by 25-hydroxycholesterol and 27-hydroxycholesterol In Vitro. Viruses. 2023; 15(8):1652. https://doi.org/10.3390/v15081652
Chicago/Turabian StyleKamble, Nitin, Vishwanatha R. A. P. Reddy, Ben Jackson, Faisal R. Anjum, Chidiebere C. Ubachukwu, Ajit Patil, and Shahriar Behboudi. 2023. "Inhibition of Marek’s Disease Virus Replication and Spread by 25-hydroxycholesterol and 27-hydroxycholesterol In Vitro" Viruses 15, no. 8: 1652. https://doi.org/10.3390/v15081652
APA StyleKamble, N., Reddy, V. R. A. P., Jackson, B., Anjum, F. R., Ubachukwu, C. C., Patil, A., & Behboudi, S. (2023). Inhibition of Marek’s Disease Virus Replication and Spread by 25-hydroxycholesterol and 27-hydroxycholesterol In Vitro. Viruses, 15(8), 1652. https://doi.org/10.3390/v15081652





