Peripheral Blood and Nasopharyngeal Swab MiRNA-155 Expression in Infants with Respiratory Syncytial Virus Infection
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. miR Isolation, cDNA Synthesis, and Real-Time PCR
2.3. Statistical Analysis
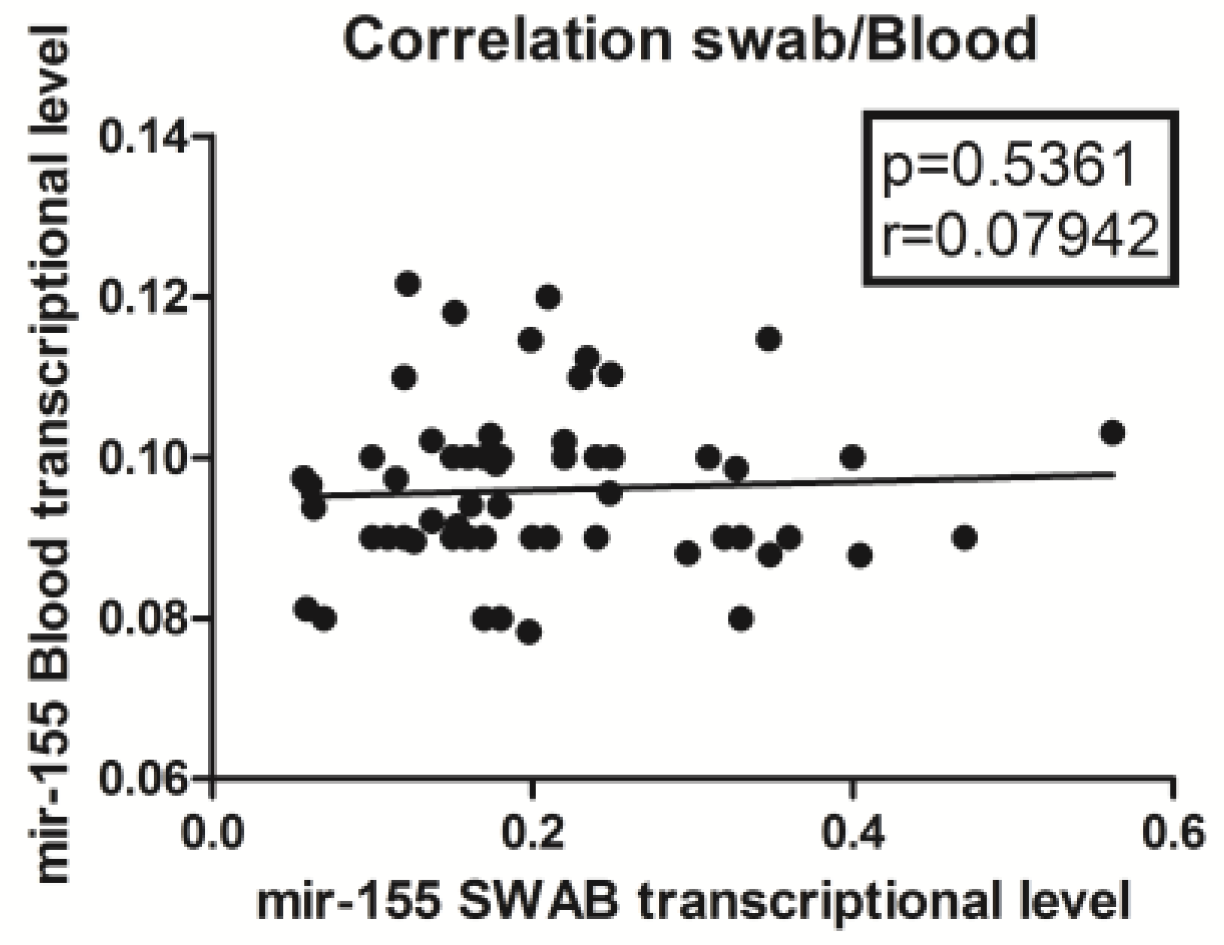
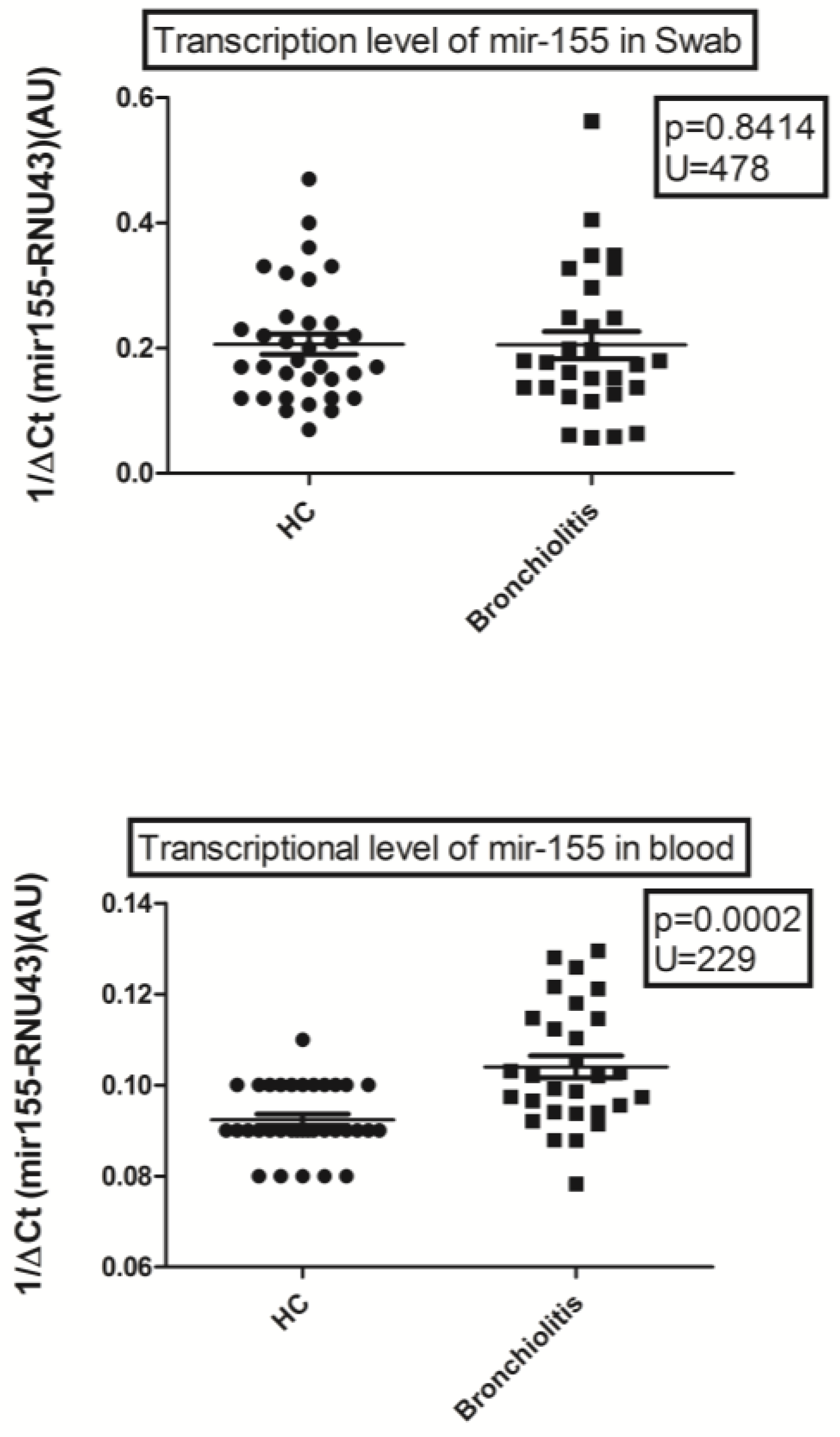
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Vennard, S.; Mahdy, S.; Nair, H.; RESCEU Investigators. Risk Factors for Poor Outcome or Death in Young Children with Respiratory Syncytial Virus-Associated Acute Lower Respiratory Tract Infection: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2022, 226 (Suppl. S1), S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Kuhdari, P.; Brosio, F.; Malaventura, C.; Stefanati, A.; Orsi, A.; Icardi, G.; Gabutti, G. Human respiratory syncytial virus and hospitalization in young children in Italy. Ital. J. Pediatr. 2018, 44, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rima, B.; Collins, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.A.; Lee, B.; Maisner, A.; Rota, P.; Wang, L. Ictv Report Consortium. ICTV virus taxonomy profile: Pneumoviridae. J. Gen. Virol. 2017, 98, 2912–2913. [Google Scholar] [CrossRef]
- Pruccoli, G.; Castagno, E.; Raffaldi, I.; Denina, M.; Barisone, E.; Baroero, L.; Timeus, F.; Rabbone, I.; Monzani, A.; Terragni, G.M.; et al. The Importance of RSV Epidemiological Surveillance: A Multicenter Observational Study of RSV Infection during the COVID-19 Pandemic. Viruses 2023, 15, 280. [Google Scholar] [CrossRef]
- McClure, D.L.; Kieke, B.A.; Sundaram, M.E.; Simpson, M.D.; Meece, J.K.; Sifakis, F.; Gasser, R.A., Jr.; Belongia, E.A. Seasonal Incidence of Medically Attended Respiratory Syncytial Virus Infection in a Community Cohort of Adults ≥50 Years Old. PLoS ONE 2014, 9, e102586. [Google Scholar] [CrossRef]
- Soni, A.; Kabra, S.K.; Lodha, R. Respiratory Syncytial Virus Infection: An Update. Indian J. Pediatr. 2023. [Google Scholar] [CrossRef]
- Ozkan, H.; Celebi, S.; Koksal, N.; Hacımustafaoğlu, M.; Koc, E.; Tezer, H.; Cetinkaya, M.; Cebeci, B.; Erdeve, O.; Ozdemir, H. Turkish Neonatal Society RSV Study Group. Risk Factors for Respiratory Syncytial Virus Infections in Moderate/Late Premature Infants in Turkey: A Prospective Multicenter Epidemiological Study. Am. J. Perinatol. 2021, 38, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- de Fougerolles, A.; Vornlocher, H.P.; Maraganore, J.; Lieberman, J. Interfering with disease: A progress report on siRNA-based therapeutics. Nat. Rev. Drug Discov. 2007, 6, 443–453. [Google Scholar] [CrossRef]
- Barnes, M.V.C.; Openshaw, P.J.M.; Thwaites, R.S. Mucosal Immune Responses to Respiratory Syncytial Virus. Cells 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Othumpangat, S.; Walton, C.; Piedimonte, G. MicroRNA-221 modulates RSV replication in human bronchial epithelium by targeting NGF expression. PLoS ONE 2012, 7, e30030. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Hao, R.; Li, P.; Zhang, X.; Liu, N.; Qiu, S.; Wang, L.; Wang, Y.; Xue, W.; Liu, K.; et al. MicroRNA expression profile of mouse lung infected with 2009 pandemic H1N1 influenza virus. PLoS ONE 2013, 8, e74190. [Google Scholar] [CrossRef] [PubMed]
- Gaytán-Pacheco, N.; Ibáñez-Salazar, A.; Herrera-Van Oostdam, A.S.; Oropeza-Valdez, J.J.; Magaña-Aquino, M.; Adrián López, J.; Monárrez-Espino, J.; López-Hernández, Y. miR-146a, miR-221, and miR-155 are Involved in Inflammatory Immune Response in Severe COVID-19 Patients. Diagnostics 2022, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hou, J.; Lin, L.; Wang, C.; Liu, X.; Li, D.; Ma, F.; Wang, Z.; Cao, X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J. Immunol. 2010, 185, 6226–6233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, S.; Crotta, S.; McCabe, T.M.; Wack, A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat. Commun. 2014, 5, 3864. [Google Scholar] [CrossRef] [Green Version]
- Girardi, E.; López, P.; Pfeffer, S. On the Importance of Host MicroRNAs During Viral Infection. Front. Genet. 2018, 9, 439. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhang, Q.; Gao, L.; Li, N.; Chen, X.; Feng, W. Increasing Expression of MicroRNA 181 Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication and Has Implications for Controlling Virus Infection. J. Virol. 2013, 87, 1159–1171. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Fan, P.; Chen, M.; Xu, Y.; Zhao, D. miRNAs and Leukotrienes in Respiratory Syncytial Virus Infection. Front. Pediatr. 2021, 9, 602195. [Google Scholar] [CrossRef]
- Garg, A.; Seeliger, B.; Derda, A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.; Welte, T.; et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 468–475. [Google Scholar] [CrossRef]
- Arroyo, M.; Salka, K.; Chorvinsky, E.; Xuchen, X.; Abutaleb, K.; Perez, G.F.; Weinstock, J.; Gaviria, S.; Gutierrez, M.J.; Nino, G. Airwaymir-155 responsesare associated with TH1 cytokine polarization in young children with viral respiratory infections. PLoS ONE 2020, 5, e0233352. [Google Scholar]
- Kang, K.; Peng, X.; Luo, J.; Gou, D. Identification of circulating miRNA biomarkers based on global quantitative real-time PCR profiling. J. Anim. Sci. Biotechnol. 2012, 3, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergallo, M.; Gambarino, S.; Montanari, P.; Daprà, V.; Rassu, M.; Galliano, I.; Ravanini, P. miR-155 expression is downregulated in kidney transplant patients with human cytomegalovirus infection. Transpl. Immunol. 2017, 43, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Daprà, V.; Galliano, I.; Rassu, M.; Calvi, C.; Montanari, P.; Bergallo, M. miR-155 expression is downregulated in hematopoietic stem cell transplantation patients with Epstein-Barr virus infection. Minerva Pediatr. 2023, 75, 550–556. [Google Scholar] [CrossRef]
- Bergallo, M.; Gambarino, S.; Martino, S.; Montin, D.; Montanari, P.; Galliano, I.; Tovo, P.A. Comparison of Two Available RNA Extraction Protocols for microRNA Amplification in Serum Samples. J. Clin. Lab. Anal. 2016, 30, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Salazar, C.; Nagadia, R.; Pandit, P.; Cooper-White, J.; Banerjee, N.; Dimitrova, N.; Coman, W.B.; Punyadeera, C.L. A novel saliva-based microRNA biomarker panel to detect head and neck cancers. Cell Oncol. 2014, 37, 331–338. [Google Scholar] [CrossRef]
- Park, N.J.; Zhou, H.; Elashoff, D.; Henson, B.S.; Kastratovic, D.A.; Abemayor, E.; Wong, D.T. Salivary microRNA: Discovery, characterization, and clinical utility for oral cancer detection. Clin. Cancer Res. 2009, 15, 5473–5477. [Google Scholar] [CrossRef] [Green Version]
- Patel, R.S.; Jakymiw, A.; Yao, B.; Pauley, B.A.; Carcamo, W.C.; Katz, J.; Cheng, J.Q.; Chan, E.K. High resolution of microRNA signatures in human whole saliva. Arch. Oral Biol. 2011, 56, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.D.; Hou, W.J.; Wu, Z.F.; Wang, Y.; Yi, Y.; Lin, W. miRNA-144 in the saliva is a genetic marker for early diagnosis of esophageal cancer. J. South Med. Univ. 2013, 33, 1783–1786. [Google Scholar]
- Swaminathan, G.; Rossi, F.; Sierra, L.J.; Gupta, A.; Navas-Martin, S.; Martin-Garcia, J. A role for microRNA-155 modulation in the anti-HIV-1 effects of Toll-like receptor 3 stimulation in macrophages. PLoS Pathog. 2012, 8, e1002937. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Lee, E.J.; Schmittgen, T.D. Increased expression of microRNA-155 in Epstein-Barr virus transformed lymphoblastoid cell lines. Genes Chromosomes Cancer 2006, 45, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Zhai, A.; Qian, J.; Kao, W.; Li, A.; Li, Y.; He, J.; Zhang, Q.; Song, W.; Fu, Y.; Wu, J.; et al. Borna disease virus encoded phosphoprotein inhibits host innate immunity by regulating miR-155. Antivir. Res. 2013, 98, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, M.T.; Dy, G.; Tam, W.; Beemon, K.L. Reticuloendotheliosis virus strain T induces miR-155, which targets JARID2 and promotes cell survival. J. Virol. 2009, 83, 12009–12017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Vasoya, D.; Kgosana, L.; Smith, L.P.; Gao, Y.; Wang, X.; Watson, M.; Nair, V. Activation of gga-miR-155 by reticuloendotheliosis virus T strain and its contribution to transformation. J. Gen. Virol. 2017, 98, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Thounaojam, M.C.; Kaushik, D.K.; Kundu, K.; Basu, A. MicroRNA-29b modulates Japanese encephalitis virus-induced microglia activation by targeting tumor necrosis factor alpha-induced protein 3. J. Neurochem. 2014, 129, 143–154. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Kundu, K.; Kaushik, D.K.; Swaroop, S.; Mahadevan, A.; Shankar, S.K.; Basu, A. MicroRNA 155 regulates Japanese encephalitis virus-induced inflammatory response by targeting Src homology 2-containing inositol phosphatase 1. J. Virol. 2014, 88, 4798–4810. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, M.J.; Gomez, J.L.; Perez, G.F.; Pancham, K.; Val, S.; Pillai, D.K.; Giri, M.; Ferrante, S.; Freishtat, R.; Rose, M.C.; et al. Airway Secretory microRNAome Changes during Rhinovirus Infection in Early Childhood. PLoS ONE 2016, 11, e0162244. [Google Scholar] [CrossRef] [Green Version]
- Inchley, C.S.; Sonerud, T.; Fjærli, H.O.; Nakstad, B. Nasal mucosal microRNA expression in children with respiratory syncytial virus infection. BMC Infect. Dis. 2015, 25, 15–150. [Google Scholar] [CrossRef] [Green Version]
- Asadpour-Behzadi, A.; Kariminik, A.; Kheirkhah, B. MicroRNA-155 is a main part of proinflammatory puzzle during severe coronavirus disease 2019 (COVID-19). Allergol. Immunopathol. 2023, 51, 115–119. [Google Scholar] [CrossRef]
- Mirzaei, R.; Mahdavi, F.; Badrzadeh, F.; Hosseini-Fard, S.R.; Heidary, M.; Jeda, A.S.; Mohammadi, T.; Roshani, M.; Yousefimashouf, R.; Keyvani, H.; et al. The emerging role of microRNAs in the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Int. Immunopharmacol. 2021, 90, 107204. [Google Scholar] [CrossRef]
- Montagner, S.; Orlandi, E.M.; Merante, S.; Monticelli, S. The role of miRNAs in mast cells and other innate immune cells. Immunol. Rev. 2013, 253, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; van Dongen, S.; Grocock, R.J.; Das, P.P.; Miska, E.A.; et al. Requirement of bic/microRNA-155 for normal immune function. Science 2007, 316, 608–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connell, R.M.; Chaudhuri, A.A.; Rao, D.S.; Baltimore, D. Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. USA 2009, 106, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lee, H.H.; Bell, J.J.; Gregg, R.K.; Ellis, J.S.; Gessner, A.; Zaghouani, H. IL-4 utilizes an alternative receptor to drive apoptosis of Th1 cells and skews neonatal immunity toward Th2. Immunity 2004, 20, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Jafarzadeh, A.; Naseri, A.; Shojaie, L.; Nemati, M.; Jafarzadeh, S.; Bannazadeh Baghi, H.; Hamblin, M.R.; Akhlagh, S.A.; Mirzaei, H. MicroRNA-155 and antiviral immune responses. Int. Immunopharmacol. 2021, 101, 108188. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucl Acids Res. 2018, 47, D155–D162. [Google Scholar] [CrossRef]
- Woods, P.S.; Doolittle, L.M.; Rosas, L.E.; Nana-Sinkam, S.P.; Tili, E.; Davis, I.C. Increased expression of microRNA-155-5p by alveolar type II cells contributes to development of lethal ARDS in H1N1 influenza A virus-infected mice. Virology 2020, 545, 40–52. [Google Scholar] [CrossRef]
| Infected by RSV (n = 29) | Healthy Controls (n = 37) | p-Value | |
|---|---|---|---|
| Type of delivery: | |||
| vaginal/caesarean | 18/11 | 22/15 | N.S. |
| Age at enrolment (mean days, range) | 85 (9–146) | 93 (9–48) | N.S. * |
| Gender | |||
| Male, n (%) | 13 (46) | 19 (51) | N.S. # |
| Female n (%) | 16 (54) | 18 (49) | N.S. # |
| Birth weight (g, range.) | 3500 (2780–3730) | 3020 (2560–3980) | N.S. * |
| Gestational age (wks ± s.d.) | 38 ± 2 | 37 ± 1.5 | N.S. * |
| Target | RNU43 |
| sequence | GAACUUAUUGACGGGCGGACAGAAACUGUGUGCUGAUUGUCACGUUCUGAUU |
| SLP | GGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAATCAG |
| Forward | TGACGGGCGGACAGAAA |
| Probe MGB fam | TGTGTGCTGATTGTCA |
| Target | miR-155 |
| sequence | UUAAUGCUAAUCGUGAUAGGGGU |
| SLP | GGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCACCCCT |
| Forward | CGCAGTTAATGCTAATCGTGATA |
| Probe MGB fam | GGGGTGGCTCTGG |
| Universal reverse primer | TGCAGGGTCCGAGGTATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savino, F.; Gambarino, S.; Dini, M.; Savino, A.; Clemente, A.; Calvi, C.; Galliano, I.; Bergallo, M. Peripheral Blood and Nasopharyngeal Swab MiRNA-155 Expression in Infants with Respiratory Syncytial Virus Infection. Viruses 2023, 15, 1668. https://doi.org/10.3390/v15081668
Savino F, Gambarino S, Dini M, Savino A, Clemente A, Calvi C, Galliano I, Bergallo M. Peripheral Blood and Nasopharyngeal Swab MiRNA-155 Expression in Infants with Respiratory Syncytial Virus Infection. Viruses. 2023; 15(8):1668. https://doi.org/10.3390/v15081668
Chicago/Turabian StyleSavino, Francesco, Stefano Gambarino, Maddalena Dini, Andrea Savino, Anna Clemente, Cristina Calvi, Ilaria Galliano, and Massimiliano Bergallo. 2023. "Peripheral Blood and Nasopharyngeal Swab MiRNA-155 Expression in Infants with Respiratory Syncytial Virus Infection" Viruses 15, no. 8: 1668. https://doi.org/10.3390/v15081668
APA StyleSavino, F., Gambarino, S., Dini, M., Savino, A., Clemente, A., Calvi, C., Galliano, I., & Bergallo, M. (2023). Peripheral Blood and Nasopharyngeal Swab MiRNA-155 Expression in Infants with Respiratory Syncytial Virus Infection. Viruses, 15(8), 1668. https://doi.org/10.3390/v15081668








