Elimination of Solanum nigrum ilarvirus 1 and Apple Hammerhead Viroid from Apple Cultivars Using Antivirals Ribavirin, Rimantadine, and Zidovudine
Abstract
:1. Introduction
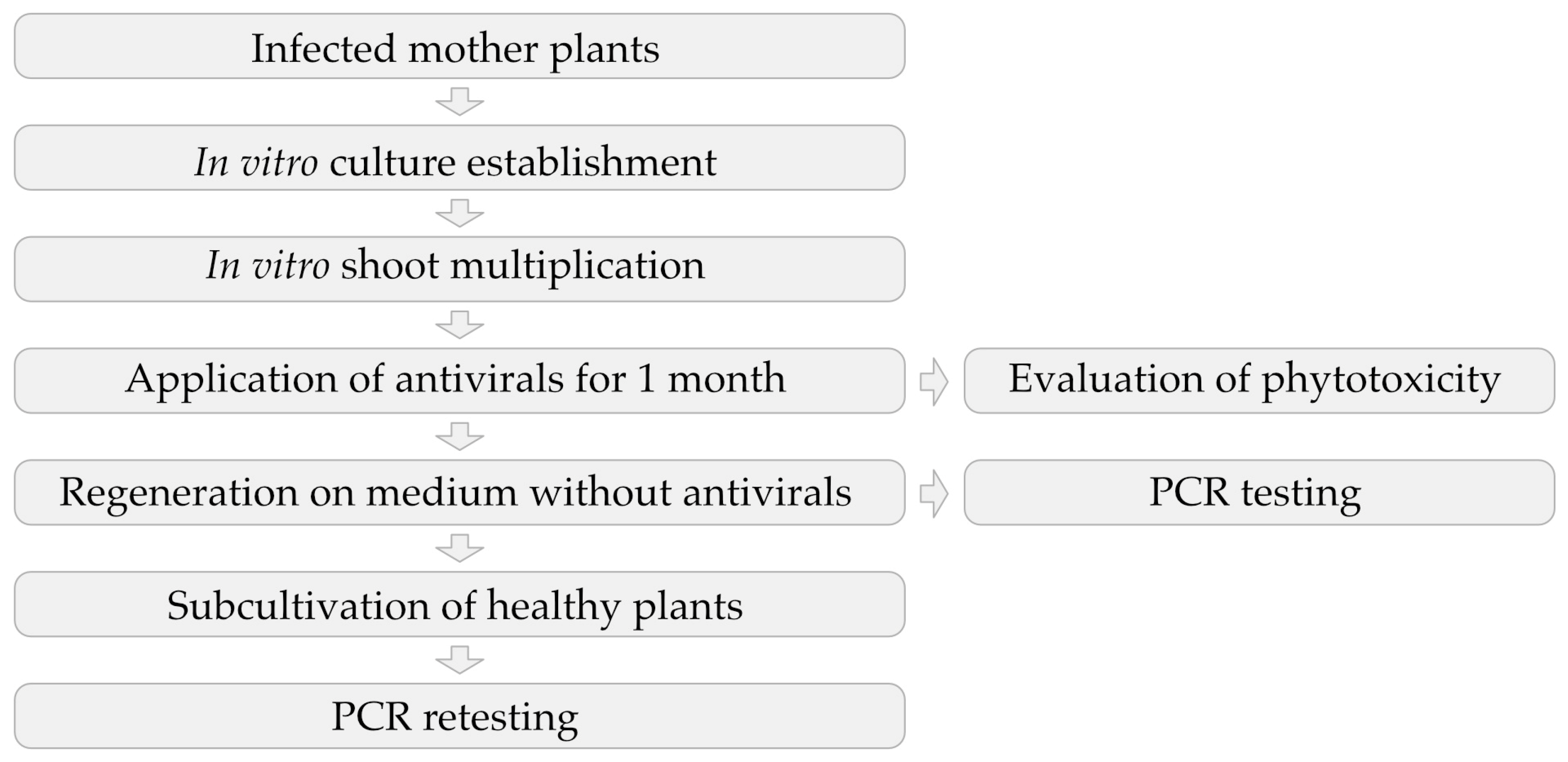
2. Materials and Methods
2.1. In Vitro Cultures
2.2. Antivirals
2.3. Application of Antivirals
2.4. Plant Material & RNA Extraction
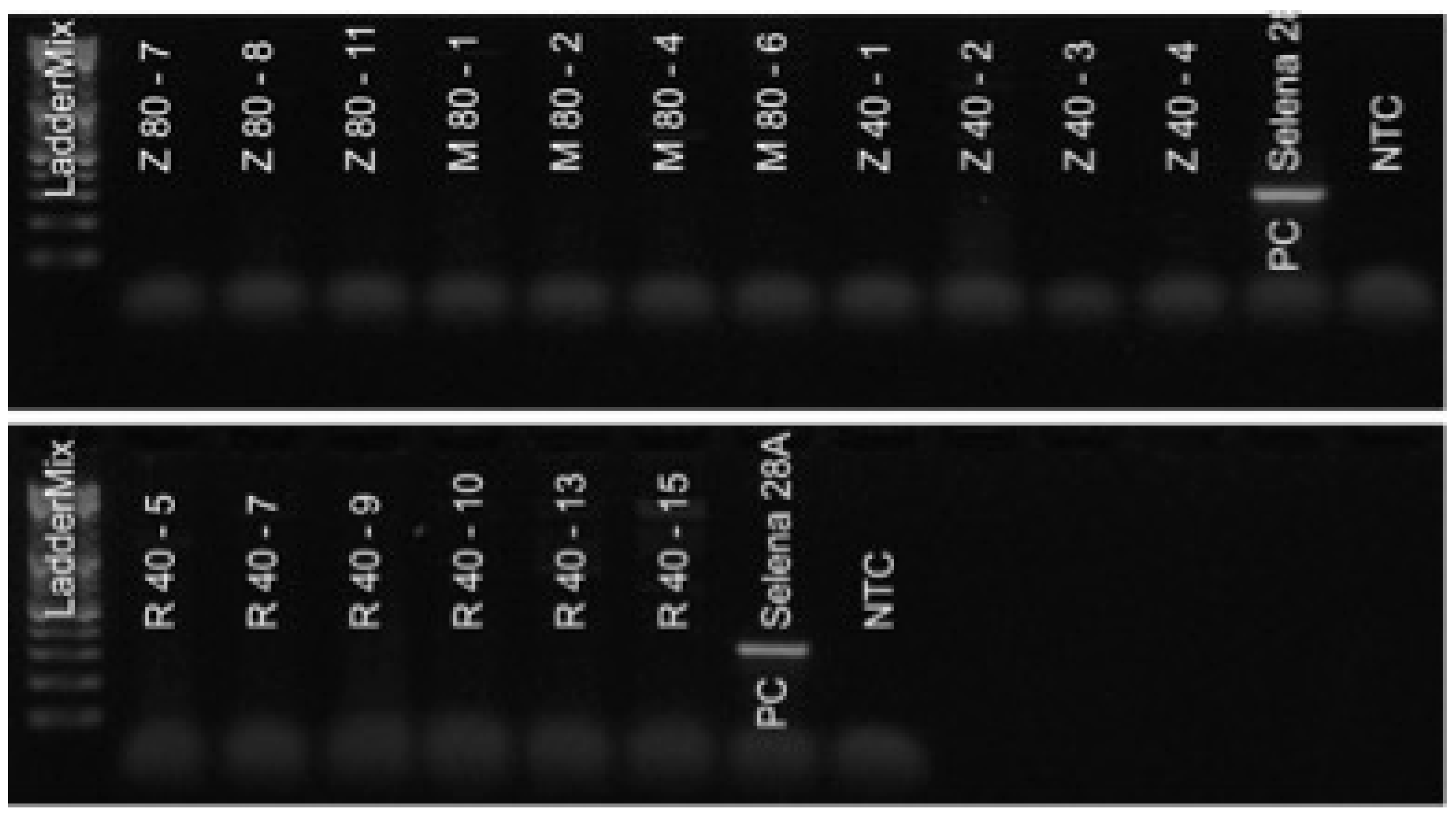
2.5. Diagnostics of SnIV-1 and AHVd by RT-PCR
2.6. Virus and Viroid Diagnostics by RT-qPCR
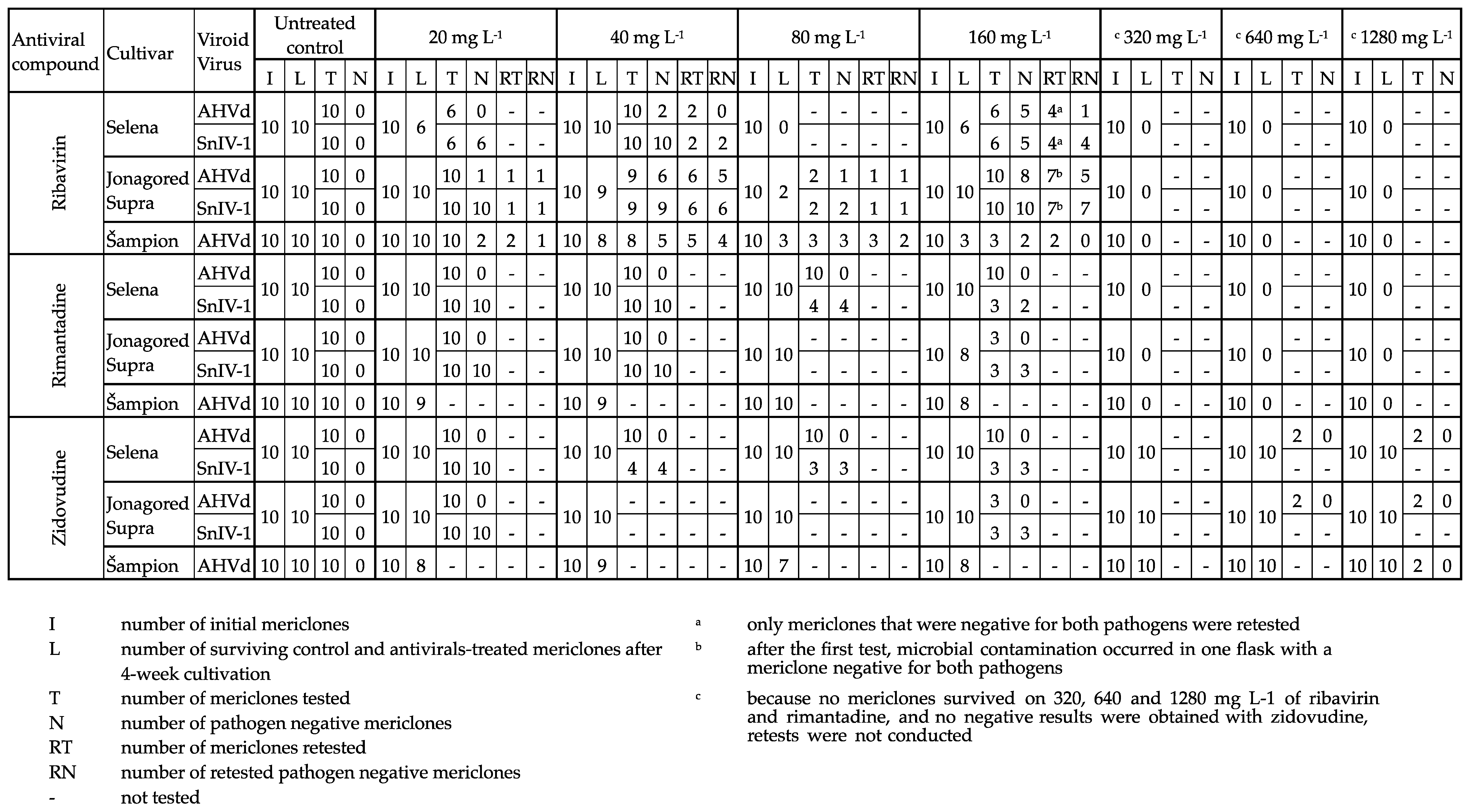
3. Results
3.1. Elimination of SnIV-1
3.2. Elimination of AHVd
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loebenstein, G. Plant virus diseases: Economic aspects. In Desk Encyclopedia of Plant and Fungal Virology, 1st ed.; van Regenmortel, M., Mahy, B., Eds.; Academic Press: Oxford, UK, 2009; pp. 426–430. [Google Scholar]
- Jones, R.A.C.; Naidu, R.A. Global Dimensions of Plant Virus Diseases: Current Status and Future Perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Hilaire, J.; Tindale, S.; Jones, G.; Pingarron-Cardenas, G.; Bačnik, K.; Ojo, M.; Frewer, L.J. Risk perception associated with an emerging agri-food risk in Europe: Plant viruses in agriculture. Agric. Food Secur. 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Pautasso, M.; Petter, F.; Rortais, A.; Roy, A.-S. Emerging risks to plant health: A European perspective. CAB Rev. 2015, 10, 1–16. [Google Scholar] [CrossRef]
- Bettoni, J.C.; Fazio, G.; Costa, L.C.; Hurtado-Gonzales, O.P.P.; Al Rwahnih, M.; Nedrow, A.; Volk, G.M.M. Thermotherapy Followed by Shoot Tip Cryotherapy Eradicates Latent Viruses and Apple Hammerhead Viroid from In Vitro Apple Rootstocks. Plants 2022, 11, 18. [Google Scholar] [CrossRef]
- Xiao, H.; Hao, W.; Storoschuk, G.; MacDonald, J.L.; Sanfaçon, H. Characterizing the Virome of Apple Orchards Affected by Rapid Decline in the Okanagan and Similkameen Valleys of British Columbia (Canada). Pathogens 2022, 11, 1231. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
- Patel, R.; Mitra, B.; Vinchurkar, M.; Adami, A.; Patkar, R.; Giacomozzi, F.; Lorenzelli, L.; Baghini, M.S. A review of recent advances in plant-pathogen detection systems. Heliyon 2022, 8, 12. [Google Scholar] [CrossRef]
- Massart, S.; Candresse, T.; Gil, J.; Lacomme, C.; Predajna, L.; Ravnikar, M.; Reynard, J.S.; Rumbou, A.; Saldarelli, P.; Skoric, D.; et al. A Framework for the Evaluation of Biosecurity, Commercial, Regulatory, and Scientific Impacts of Plant Viruses and Viroids Identified by NGS Technologies. Front. Microbiol. 2017, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Varallyay, E.; Pribylova, J.; Galbacs, Z.N.; Jahan, A.; Varga, T.; Spak, J.; Lenz, O.; Franova, J.; Sedlak, J.; Koloniuk, I. Detection of Apple Hammerhead Viroid, Apple Luteovirus 1 and Citrus Concave Gum-Associated Virus in Apple Propagation Materials and Orchards in the Czech Republic and Hungary. Viruses 2022, 14, 20. [Google Scholar] [CrossRef]
- Serra, P.; Messmer, A.; Sanderson, D.; James, D.; Flores, R. Apple hammerhead viroid-like RNA is a bona fide viroid: Autonomous replication and structural features support its inclusion as a new member in the genus Pelamoviroid. Virus Res. 2018, 249, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.X.; Marais, A.; Lefebvre, M.; Faure, C.; Candresse, T. Metagenomic analysis of virome cross-talk between cultivated Solanum lycopersicum and wild Solanum nigrum. Virology 2020, 540, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Messmer, A.; Sanderson, D.; Braun, G.; Serra, P.; Flores, R.; James, D. Molecular and phylogenetic identification of unique isolates of hammerhead viroid-like RNA from ‘Pacific Gala’ apple (Malus domestica) in Canada. Can. J. Plant Pathol. 2017, 39, 342–353. [Google Scholar] [CrossRef]
- Graci, J.D.; Cameron, C.E. Mechanisms of action of ribavirin against distinct viruses. Rev. Med. Virol. 2006, 16, 37–48. [Google Scholar] [CrossRef]
- Hansen, A.J. Antiviral chemicals for plant-disease control. Crit. Rev. Plant Sci. 1989, 8, 45–88. [Google Scholar] [CrossRef]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Ren, F.; Zhou, J. Virus elimination from in vitro apple by thermotherapy combined with chemotherapy. Plant Cell Tissue Organ Cult. 2015, 121, 435–443. [Google Scholar] [CrossRef]
- Kudelkova, M.; Pavelkova, R.; Ondrusikova, E. Virus elimination in peach using chemotherapy. Acta Hortic. 2017, 1155, 431–437. [Google Scholar] [CrossRef]
- Pavelkova, R.; Kudelkova, M.; Ondrusikova, E.; Eichmeier, A. Virus Elimination in Peach cv. ‘Red Haven’ by Chemotherapy. Agric. Commun. 2015, 3, 16–20. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Witkowski, J.T.; Allen, L.B.; Robins, R.K.; Khare, G.P.; Huffman, J.H. Broad-spectrum antiviral activity of Virazole: 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide. Science 1972, 177, 705–706. [Google Scholar] [CrossRef]
- Huffman, J.H.; Sidwell, R.W.; Khare, G.P.; Witkowski, J.T.; Allen, L.B.; Robins, R.K. In vitro effect of 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole, ICN 1229) on deoxyribonucleic acid and ribonucleic-acid viruses. Antimicrob. Agents Chemother. 1973, 3, 235–241. [Google Scholar] [CrossRef] [Green Version]
- James, D. Long term assessment of the effects of in vitro chemotherapy as a tool for apple stem grooving virus elimination. Acta Hortic. 2001, 550, 459–462. [Google Scholar] [CrossRef]
- Wright, K. AIDS therapy. First tentative signs of therapeutic promise. Nature 1986, 323, 283. [Google Scholar] [CrossRef] [Green Version]
- Tochikura, T.S.; Nakashima, H.; Yamamoto, N. Antiviral agents with activity against human retroviruses. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1989, 2, 441–447. [Google Scholar]
- Hannoun. Rimantadine in the prevention and treatment of influenza A. Rev. Médecine Interne 1988, 9, 554–558. [Google Scholar]
- Thomaston, J.L.; Samways, M.L.; Konstantinidi, A.; Ma, C.; Hu, Y.; Macdonald, H.E.B.; Wang, J.; Essex, J.W.; DeGrado, W.F.; Kolocouris, A. Rimantadine binds to and inhibits the influenza A M2 proton channel without enantiomeric specificity. Biochemistry 2021, 60, 2471–2482. [Google Scholar] [CrossRef]
- Sasaki-Tanaka, R.; Shibata, T.; Moriyama, M.; Okamoto, H.; Kogure, H.; Kanda, T. Amantadine and Rimantadine Inhibit Hepatitis A Virus Replication through the Induction of Autophagy. J. Virol. 2022, 96, e00646-22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.X.; Qi, S.S.; Tang, N.; Zhang, X.X.; Chen, S.S.; Zhu, P.F.; Ma, L.; Cheng, J.P.; Xu, Y.; Lu, M.G.; et al. Discovery of Replicating Circular RNAs by RNA-Seq and Computational Algorithms. PLoS Pathog. 2014, 10, 14. [Google Scholar] [CrossRef]
- Szostek, S.A.; Wright, A.A.; Harper, S.J. First Report of Apple Hammerhead Viroid in the United States, Japan, Italy, Spain, and New Zealand. Plant Dis. 2018, 102, 2670. [Google Scholar] [CrossRef]
- Wright, A.A.; Cross, A.R.; Harper, S.J. A bushel of viruses: Identification of seventeen novel putative viruses by RNA-seq in six apple trees. PLoS ONE 2020, 15, e0227669. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Moon, J.S.; Cho, I.S.; Kim, H.R.; Lee, S.H. First Report of Apple Hammerhead Viroid Infecting Apple Trees in South Korea. Plant Dis. 2019, 103, 2700. [Google Scholar] [CrossRef]
- Nabi, S.U.; Baranwal, V.K. First Report of Apple Hammerhead Viroid Infecting Apple Cultivars in India. Plant Dis. 2020, 104, 3086–3087. [Google Scholar] [CrossRef]
- Hamdi, I.; Soltani, R.; Baraket, G.; Varsani, A.; Najar, A. First report of apple hammerhead viroid infecting ‘Richared Delicious’ apple (Malus domestica) in Tunisia. J. Plant Pathol. 2022, 104, 811–812. [Google Scholar] [CrossRef]
- Barba, M.; Hosakawa, M.; Wang, Q.-C.; Taglienti, A.; Hamborg, Z. Viroid Elimination by Thermotherapy, Cold Therapy, Tissue Culture, In Vitro Micrografting, or Cryotherapy. In Viroids and Satellites, 1st ed.; Hadidi, A., Flores, R., Randles, J.W., Palukaitis, P., Eds.; Academic Press: Oxford, UK, 2017; pp. 425–435. [Google Scholar]
- Desvignes, J.C.; Grasseau, N.; Boye, R.; Cornaggia, D.; Aparicio, F.; Di Serio, F.; Flores, R. Biological properties of apple scar skin viroid: Isolates, host range, different sensitivity of apple cultivars, elimination, and natural transmission. Plant Dis. 1999, 83, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.J.; Zhang, Z.P.; Dong, Y.F.; Fan, X.D.; Ren, F.; Zhu, H.J. Efficiency of virus elimination from potted apple plants by thermotherapy coupled with shoot-tip grafting. Austral. Plant Pathol. 2015, 44, 167–173. [Google Scholar] [CrossRef]
- Wang, M.-R.; Bi, W.-L.; Bettoni, J.C.; Zhang, D.; Volk, G.M.; Wang, Q.-C. Shoot tip cryotherapy for plant pathogen eradication. Plant Pathol. 2022, 71, 1241–1254. [Google Scholar] [CrossRef]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Ren, F. Inefficiency of ribavirin to eliminate apple scar skin viroid from apple plants. Plant Cell Tissue Organ Cult. 2022, 151, 189–197. [Google Scholar] [CrossRef]
- Hu, G.J.; Dong, Y.F.; Zhang, Z.P.; Fan, X.D.; Ren, F.; Li, Z.N. Efficacy of virus elimination from apple by thermotherapy coupled with in vivo shoot-tip grafting and in vitro meristem culture. J. Phytopathol. 2017, 165, 701–706. [Google Scholar] [CrossRef]
- Bhat, A.I.; Rao, G.P. Virus Elimination by Meristem-Tip Culture. In Characterization of Plant Viruses; Springer Protocols Handbooks; Humana: New York, NY, USA, 2020. [Google Scholar] [CrossRef]
| Target | Primer Name | Primer Sequence (5’-3’) | Usage; Region | Reference |
|---|---|---|---|---|
| Malus domestica, NADH a | 2277 | TGCTCCATGGATCTCATCGG | RT-qPCR, RT-PCR, RNA extraction and PCR amplification control; protein-coding region | [10] |
| 2278 | AATCGAGGGCTATGCGGATC | |||
| AHVd b | 2115 | CTGCCGAAACAGAGGTTGGA | RT-qPCR, RT-PCR; genome | |
| 2116 | GAGAAGTCGCTCTCTCTCGC | |||
| SnIV-1 c | 2696 | TTTGGGTTTGTAGCCGAATC | RT-qPCR; gene for coat protein | this work |
| 2697 | TTACCTCCGAGATCAACGTC | |||
| 2365 | GCCCTATCTCTACCCGAGGT | RT-PCR; gene for polymerase | ||
| 2363 | TGTCGAAGGACAGCCGAAAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlák, J.; Přibylová, J.; Koloňuk, I.; Špak, J.; Lenz, O.; Semerák, M. Elimination of Solanum nigrum ilarvirus 1 and Apple Hammerhead Viroid from Apple Cultivars Using Antivirals Ribavirin, Rimantadine, and Zidovudine. Viruses 2023, 15, 1684. https://doi.org/10.3390/v15081684
Sedlák J, Přibylová J, Koloňuk I, Špak J, Lenz O, Semerák M. Elimination of Solanum nigrum ilarvirus 1 and Apple Hammerhead Viroid from Apple Cultivars Using Antivirals Ribavirin, Rimantadine, and Zidovudine. Viruses. 2023; 15(8):1684. https://doi.org/10.3390/v15081684
Chicago/Turabian StyleSedlák, Jiří, Jaroslava Přibylová, Igor Koloňuk, Josef Špak, Ondřej Lenz, and Matěj Semerák. 2023. "Elimination of Solanum nigrum ilarvirus 1 and Apple Hammerhead Viroid from Apple Cultivars Using Antivirals Ribavirin, Rimantadine, and Zidovudine" Viruses 15, no. 8: 1684. https://doi.org/10.3390/v15081684
APA StyleSedlák, J., Přibylová, J., Koloňuk, I., Špak, J., Lenz, O., & Semerák, M. (2023). Elimination of Solanum nigrum ilarvirus 1 and Apple Hammerhead Viroid from Apple Cultivars Using Antivirals Ribavirin, Rimantadine, and Zidovudine. Viruses, 15(8), 1684. https://doi.org/10.3390/v15081684






