The Additional 15 nt of 5′ UTR in a Novel Recombinant Isolate of Chilli Veinal Mottle Virus in Solanum nigrum L. Is Crucial for Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection, De Novo Assembly and RACE
2.2. Viral Recombination Analysis
2.3. Phylogenetic and Sequence Analysis
2.4. Plasmid Construction and Agroinfiltration
2.5. Virion’s Purification
2.6. Sap Inoculation and Western Blot
3. Results
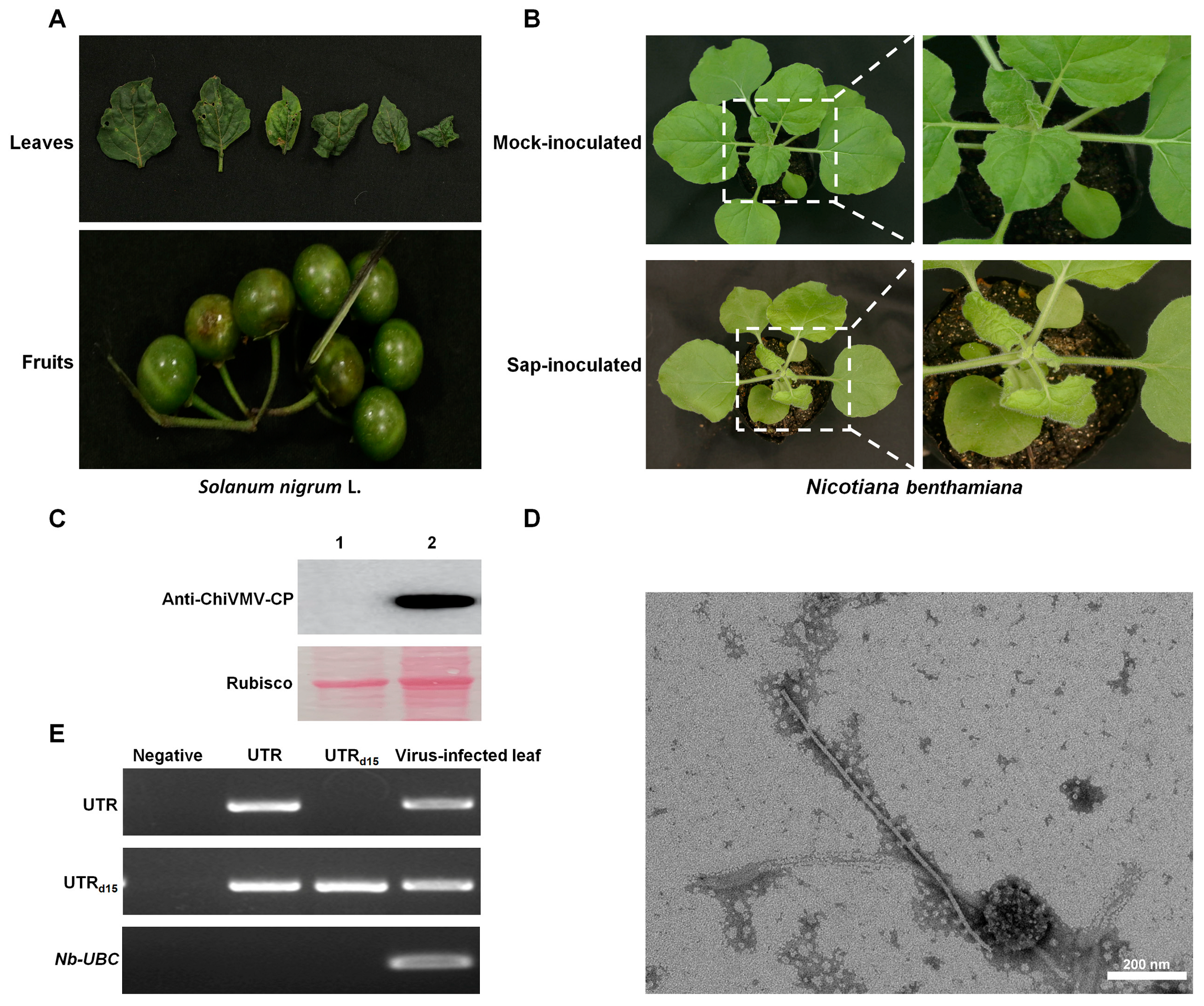
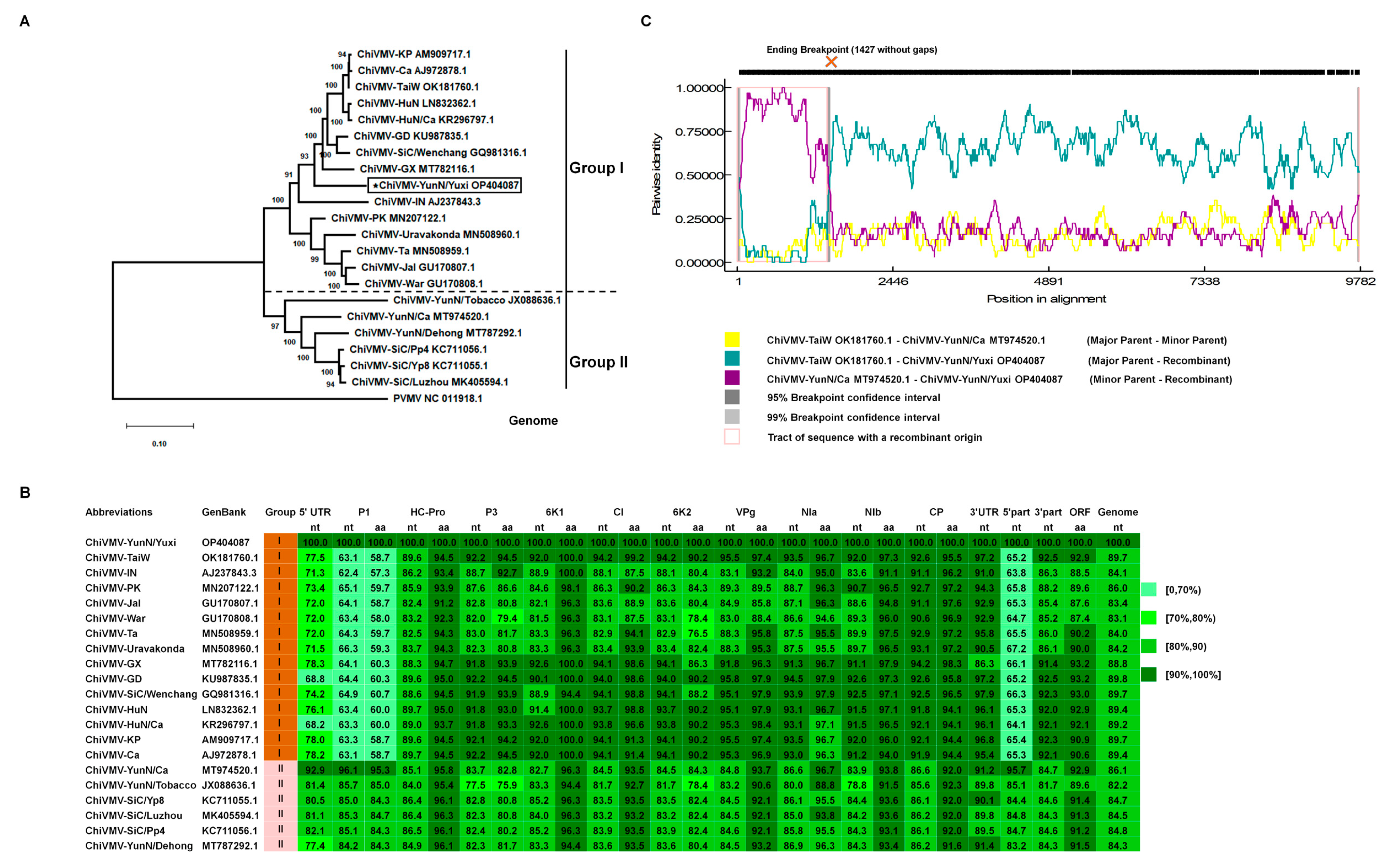
3.1. Sequence, Phylogenetic and Recombinant Analysis of ChiVMV-YunN/Yuxi from Solanum nigrum L. in Yuxi City
3.2. ChiVMV-YunN/Yuxi Has a Longer 5′ UTR Than Other Isolates That Is Stable in Subculture
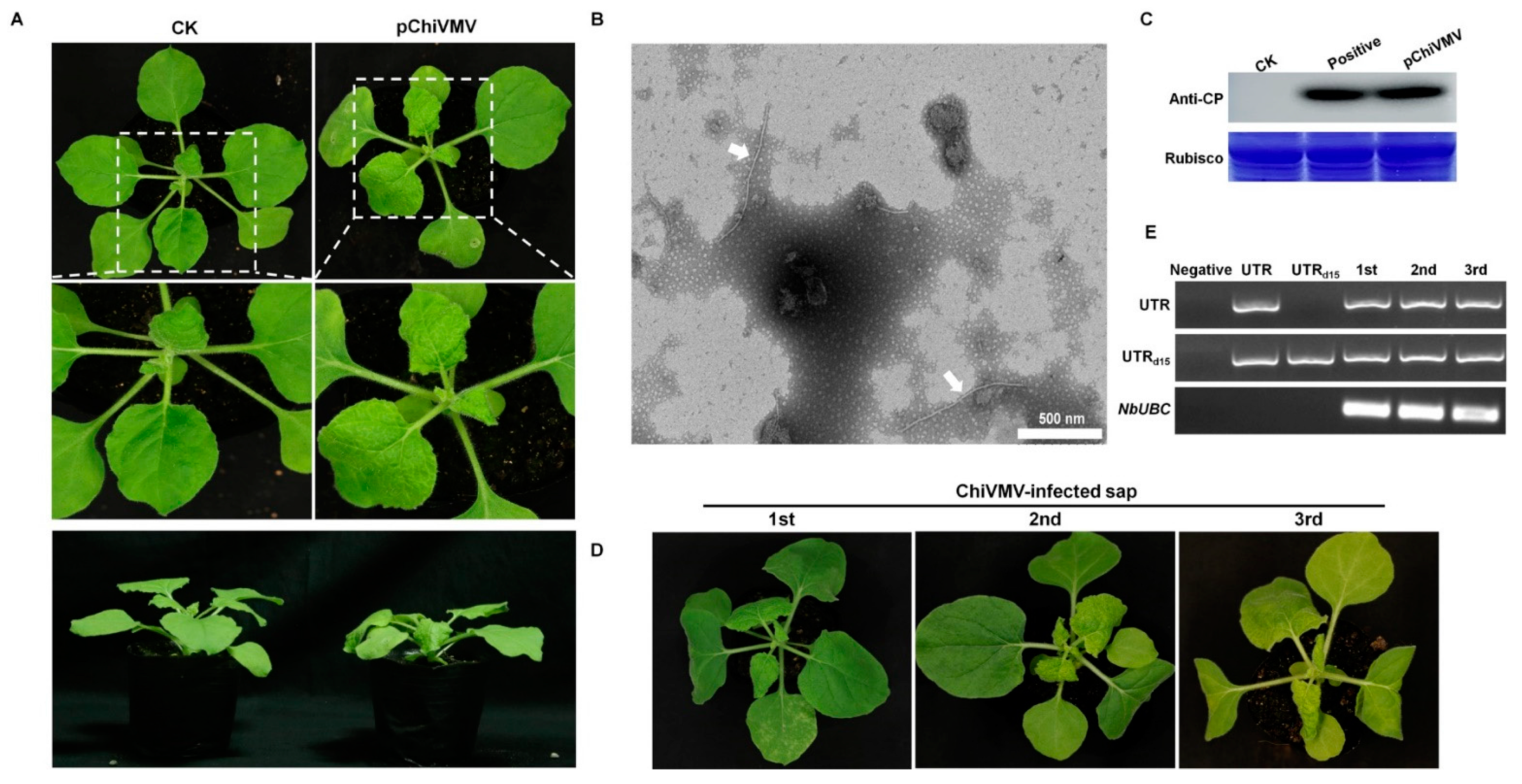
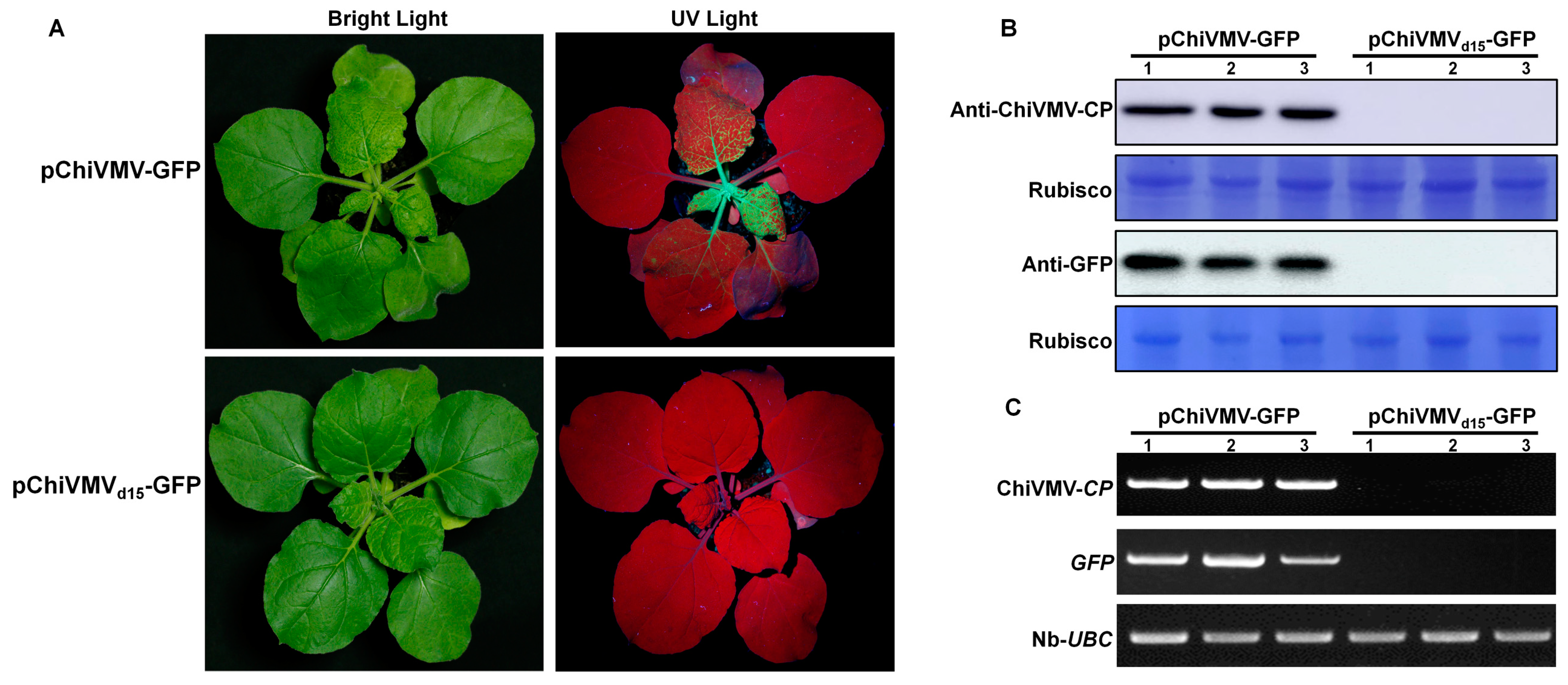
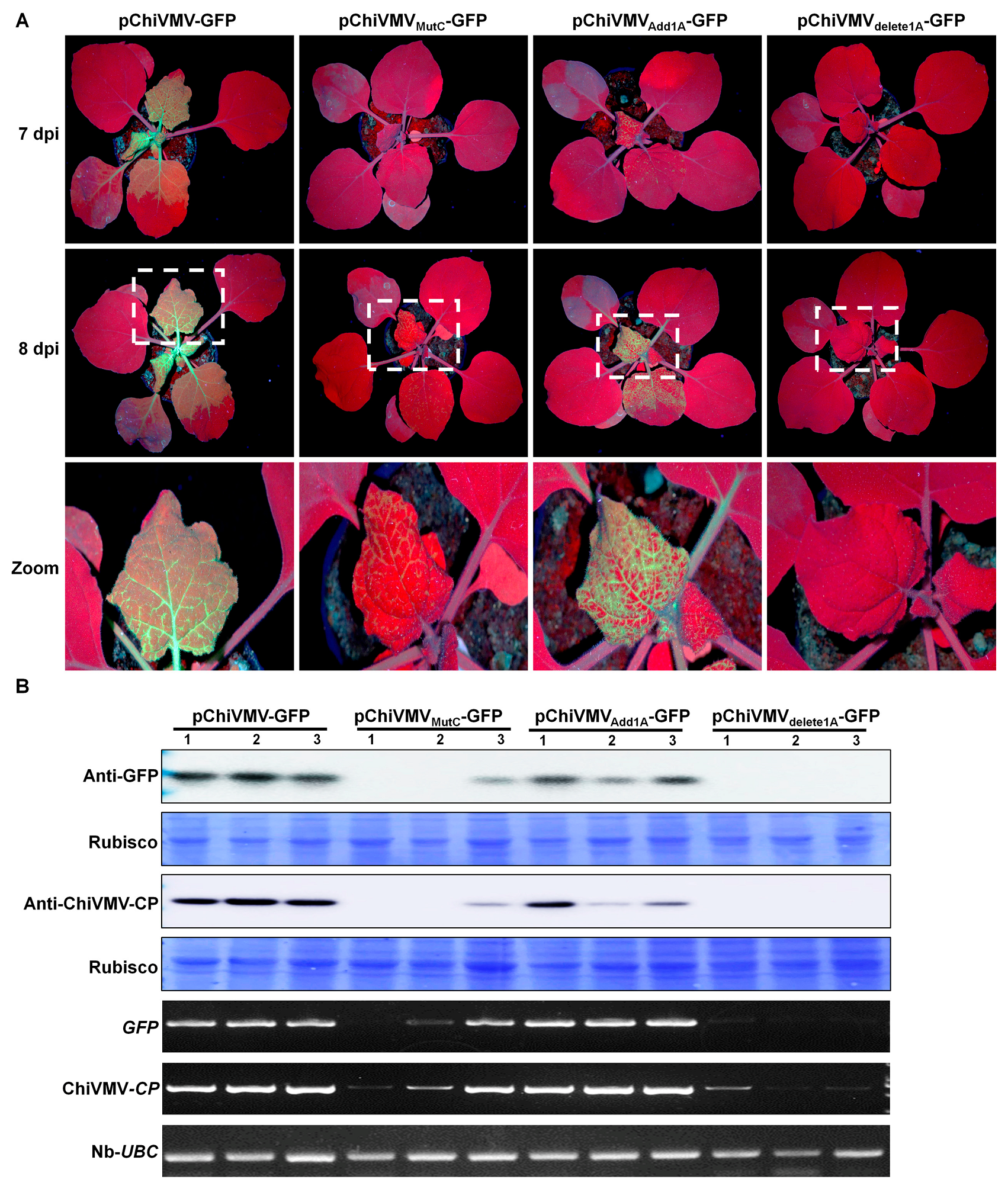
3.3. The Additional 15 nt in the 5′ UTR Are Crucial in Viral Infection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, C.A.; Varghese, G.; Ting, W.P. The effect of Chilli veinal mottle virus on yield of Chilli (Capsicum annuum L.). MARDI Res. Bull. 1980, 8, 74–78. [Google Scholar]
- Ravi, K.S.; Joseph, J.; Nagaraju, N.; Prasad, S.K.; Reddy, H.R.; Savithri, H.S. Characterization of a Pepper Vein Banding Virus from Chili Pepper in India. Plant Dis. 1997, 81, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.F.; Xi, D.H.; Liu, J.; Deng, X.G.; Lin, H.H. First Report of Chilli veinal mottle virus Infecting Tomato (Solanum lycopersicum) in China. Plant Dis. 2014, 98, 1589. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Choi, S.; Ryu, K. Complete genomic RNA sequence of pepper-infecting Chilli veinal mottle virus Korean isolate. J. Plant Dis. Prot. 2013, 120, 153–159. [Google Scholar] [CrossRef]
- Tan, Z.; Wada, Y.; Chen, J.; Ohshima, K. Inter- and intralineage recombinants are common in natural populations of Turnip mosaic virus. J. Gen. Virol. 2004, 85, 2683–2696. [Google Scholar] [CrossRef]
- Gao, F.; Jin, J.; Zou, W.; Liao, F.; Shen, J. Geographically driven adaptation of Chilli veinal mottle virus revealed by genetic diversity analysis of the coat protein gene. Arch. Virol. 2016, 161, 1329–1333. [Google Scholar] [CrossRef]
- Rao, S.; Chen, X.; Qiu, S.; Peng, J.; Zheng, H.; Lu, Y.; Wu, G.; Chen, J.; Jiang, W.; Zhang, Y.; et al. Identification of Two New Isolates of Chilli veinal mottle virus from Different Regions in China: Molecular Diversity, Phylogenetic and Recombination Analysis. Front. Microbiol. 2020, 11, 616171. [Google Scholar] [CrossRef]
- Ahmad, A.; Ashfaq, M. Genetic diversity and recombination analysis based on capsid protein gene of Chilli veinal mottle virus isolates from Pakistan. Eur. J. Plant Pathol. 2018, 151, 891–900. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- Shatkin, A.J. Capping of eucaryotic mRNAs. Cell 1977, 9, 645–653. [Google Scholar] [CrossRef]
- Pelletier, J.; Schmeing, T.M.; Sonenberg, N. The multifaceted eukaryotic cap structure. Wiley Interdiscip. Rev. RNA 2021, 12, e1636. [Google Scholar] [CrossRef]
- Firth, A.E.; Brierley, I. Non-canonical translation in RNA viruses. J. Gen. Virol. 2012, 93, 1385–1409. [Google Scholar] [CrossRef]
- Dreher, T.W.; Miller, W.A. Translational control in positive strand RNA plant viruses. Virology 2006, 344, 185–197. [Google Scholar] [CrossRef]
- Niepel, M.; Gallie, D.R. Identification and characterization of the functional elements within the tobacco etch virus 5′ leader required for cap-independent translation. J. Virol. 1999, 73, 9080–9088. [Google Scholar] [CrossRef]
- Nicolaisen, M.; Johansen, E.; Poulsen, G.B.; Borkhardt, B. The 5′ untranslated region from pea seedborne mosaic potyvirus RNA as a translational enhancer in pea and tobacco protoplasts. FEBS Lett. 1992, 303, 169–172. [Google Scholar]
- Yang, L.J.; Hidaka, M.; Sonoda, J.; Masaki, H.; Uozumi, T. Mutational analysis of the potato virus Y 5′ untranslated region for alteration in translational enhancement in tobacco protoplasts. Biosci. Biotechnol. Biochem. 1997, 61, 2131–2133. [Google Scholar] [CrossRef]
- Zhao, K.; Yin, Y.; Hua, M.; Wang, S.; Mo, X.; Yuan, E.; Zheng, H.; Lin, L.; Chen, H.; Lu, Y.; et al. Pod pepper vein yellows virus, a new recombinant polerovirus infecting Capsicum frutescens in Yunnan province, China. Virol. J. 2021, 18, 42. [Google Scholar] [CrossRef]
- Peng, J.; Bu, S.; Yin, Y.; Hua, M.; Zhao, K.; Lu, Y.; Zheng, H.; Wan, Q.; Zhang, S.; Chen, H.; et al. Biological and Genetic Characterization of Pod Pepper Vein Yellows Virus-Associated RNA From Capsicum frutescens in Wenshan, China. Front. Microbiol. 2021, 12, 662352. [Google Scholar] [CrossRef]
- Zhang, H.M.; Yang, J.; Xin, X.; Chen, J.P.; Adams, M.J. Molecular characterization of the largest and smallest genome segments, S1 and S12, of Rice gall dwarf virus. Virus Genes 2007, 35, 815–823. [Google Scholar] [CrossRef]
- Martin, D.P.; Varsani, A.; Roumagnac, P.; Botha, G.; Maslamoney, S.; Schwab, T.; Kelz, Z.; Kumar, V.; Murrell, B. RDP5: A computer program for analyzing recombination in, and removing signals of recombination from, nucleotide sequence datasets. Virus Evol. 2021, 7, veaa087. [Google Scholar] [CrossRef]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, D.; Tang, W.; Wang, L.; Li, Q.; Lu, Z.; Liu, H.; Zhong, Y.; He, T.; Guo, S. Phytoremediation of cadmium-polluted soil assisted by D-gluconate-enhanced Enterobacter cloacae colonization in the Solanum nigrum L. rhizosphere. Sci. Total Environ. 2020, 732, 139265. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.J.; Antoniw, J.F.; Fauquet, C.M. Molecular criteria for genus and species discrimination within the family Potyviridae. Arch. Virol. 2005, 150, 459–479. [Google Scholar] [CrossRef]
- Inoue-Nagata, A.K.; Jordan, R.; Kreuze, J.; Li, F.; López-Moya, J.J.; Mäkinen, K.; Ohshima, K.; Wylie, S.J. Ictv Report ICTV Virus Taxonomy Profile_ Potyviridae 2022. J. Gen. Virol. 2022, 103, 001738. [Google Scholar] [CrossRef]
- Adrian, G.; Kazusato, O. Potyviruses and the Digital Revolution. Annu. Rev. Phytopathol. 2010, 48, 205–223. [Google Scholar]
- Léonard, S.; Plante, D.; Wittmann, S.; Daigneault, N.; Fortin, M.G.; Laliberté, J.F. Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J. Virol. 2000, 74, 7730–7737. [Google Scholar] [CrossRef]
- Hwang, J.; Li, J.; Liu, W.Y.; An, S.J.; Cho, H.; Her, N.H.; Yeam, I.; Kim, D.; Kang, B.C. Double mutations in eIF4E and eIFiso4E confer recessive resistance to Chilli veinal mottle virus in pepper. Mol. Cells 2009, 27, 329–336. [Google Scholar] [CrossRef]
- Levis, C.; Tronchet, M.; Meyer, M.; Albouy, J.; Astier-Manifacier, S. Effects of antisense oligodeoxynucleotide hybridization on in vitro translation of potato virus Y RNA. Virus Genes 1992, 6, 33–46. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, Q.; Zheng, K.; Wu, J.; Bu, S.; Jiao, M.; Zhou, H.; Lu, Y.; Zheng, H.; Wu, G.; Rao, S.; et al. The Additional 15 nt of 5′ UTR in a Novel Recombinant Isolate of Chilli Veinal Mottle Virus in Solanum nigrum L. Is Crucial for Infection. Viruses 2023, 15, 1428. https://doi.org/10.3390/v15071428
Wan Q, Zheng K, Wu J, Bu S, Jiao M, Zhou H, Lu Y, Zheng H, Wu G, Rao S, et al. The Additional 15 nt of 5′ UTR in a Novel Recombinant Isolate of Chilli Veinal Mottle Virus in Solanum nigrum L. Is Crucial for Infection. Viruses. 2023; 15(7):1428. https://doi.org/10.3390/v15071428
Chicago/Turabian StyleWan, Qionglian, Kaiyue Zheng, Jian Wu, Shan Bu, Mengting Jiao, Huijie Zhou, Yuwen Lu, Hongying Zheng, Guanwei Wu, Shaofei Rao, and et al. 2023. "The Additional 15 nt of 5′ UTR in a Novel Recombinant Isolate of Chilli Veinal Mottle Virus in Solanum nigrum L. Is Crucial for Infection" Viruses 15, no. 7: 1428. https://doi.org/10.3390/v15071428
APA StyleWan, Q., Zheng, K., Wu, J., Bu, S., Jiao, M., Zhou, H., Lu, Y., Zheng, H., Wu, G., Rao, S., Chen, H., Yan, F., & Peng, J. (2023). The Additional 15 nt of 5′ UTR in a Novel Recombinant Isolate of Chilli Veinal Mottle Virus in Solanum nigrum L. Is Crucial for Infection. Viruses, 15(7), 1428. https://doi.org/10.3390/v15071428





