Survival of Bacteriophage T4 in Quasi-Pure Ionic Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Preparation of T4 Virions
2.3. Preparation of Suspension of T4 Virions in Quasi-Pure Solution
2.4. Ionic Solutions
2.5. Plaque Forming Unit (pfu)
3. Results
3.1. Time Course of Viral Activity
3.2. H+: HCl and H2SO4
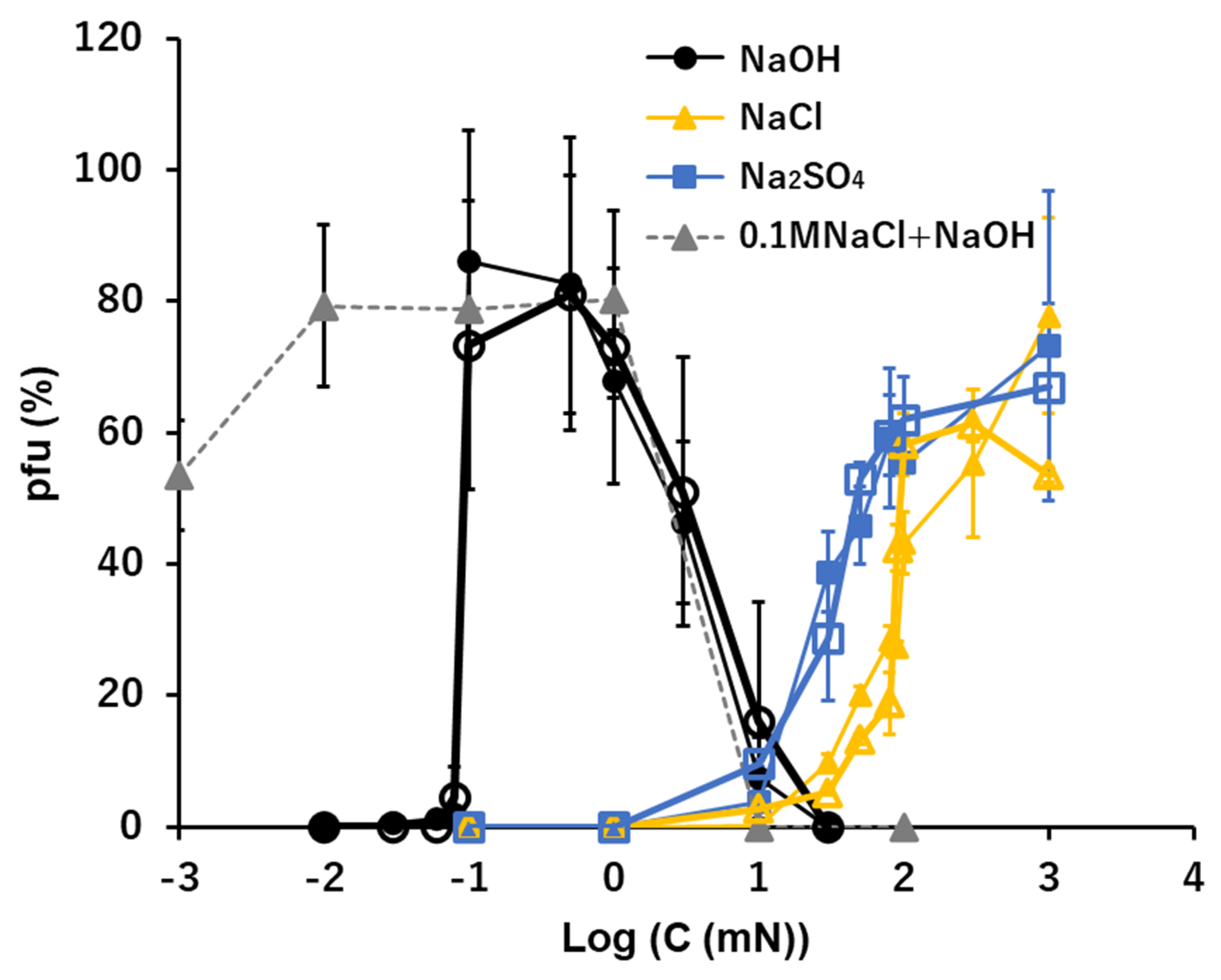
3.3. Na+: NaOH, NaCl, and Na2SO4
3.4. K+: KOH, KCl, and K2SO4
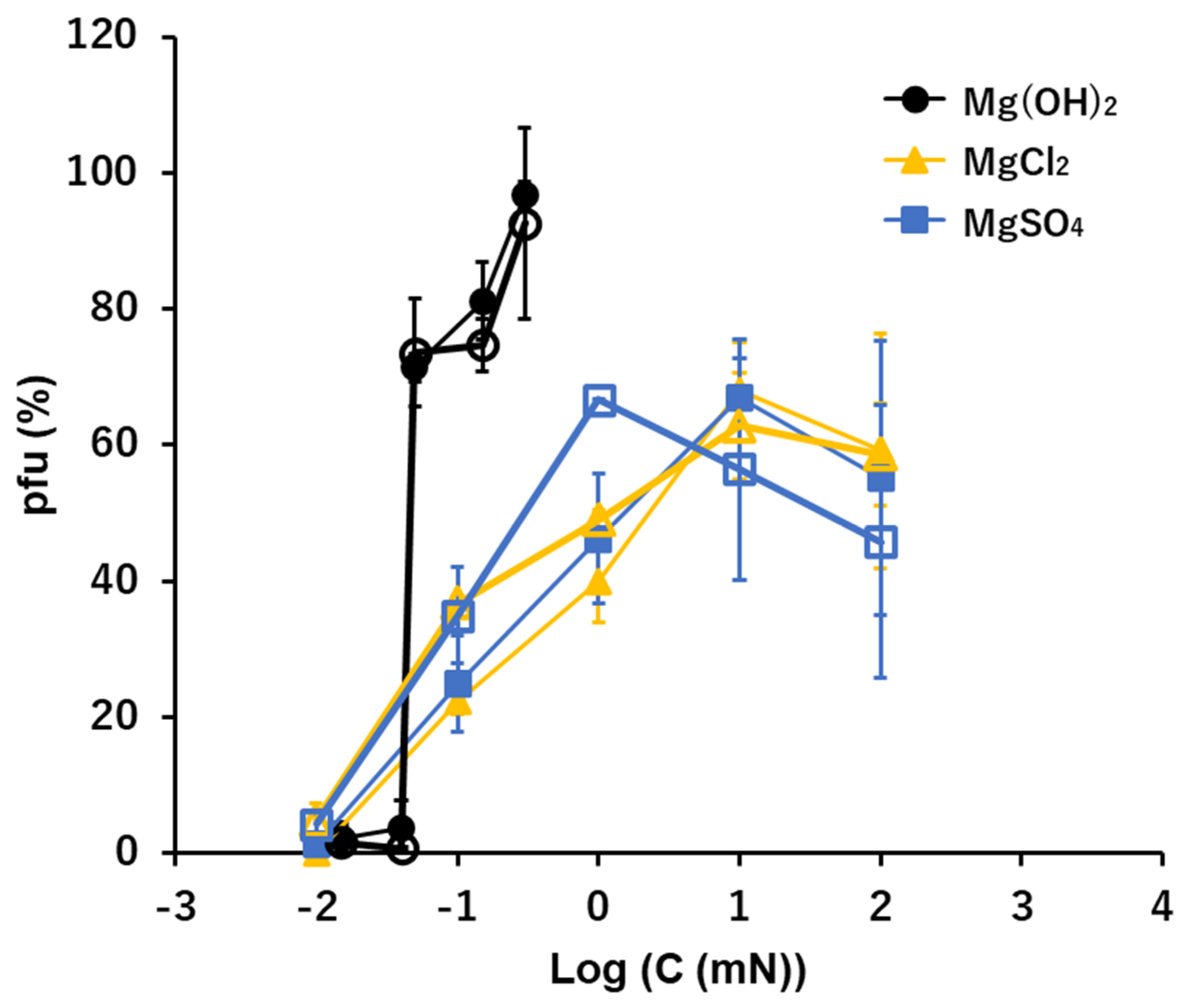
3.5. Mg2+: Mg(OH)2, MgCl2, and MgSO4
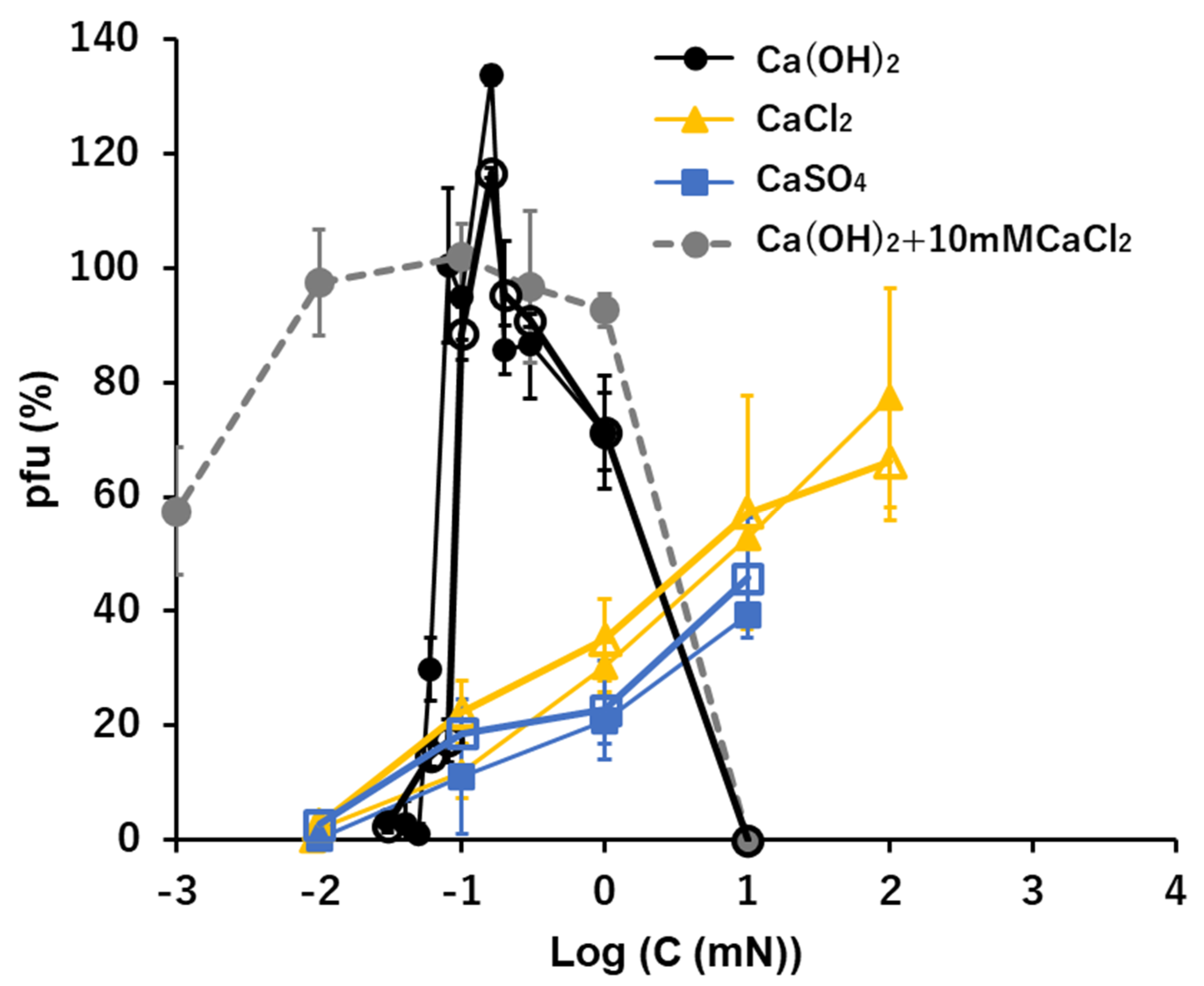
3.6. Ca2+: Ca(OH)2, CaCl2, and CaSO4
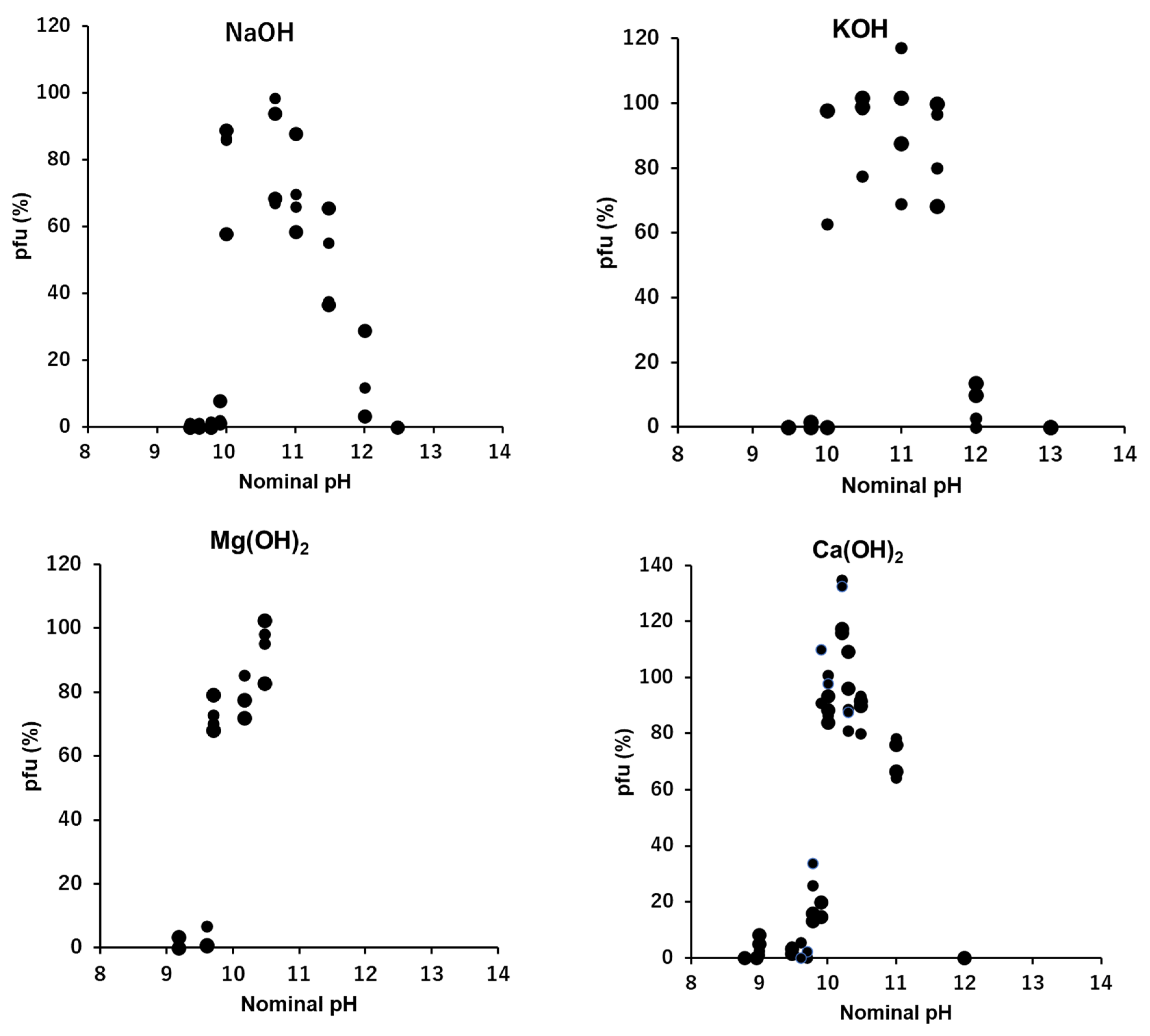
3.7. OH−: NaOH, KOH, Mg(OH)2 and Ca(OH)2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christi, K.; Elliman, J.; Owens, L.A. Synthesis of the divalent cation requirements for efficient adsorption of bacteriophage on to bacterial cells. In Bacteriophages: An Overview and Synthesis of a Re-Emerging Field; Harrington, D., Ed.; Bacteriology Research Developments; Nova Science Publishers Inc.: New York, NY, USA, 2017; pp. 43–69. [Google Scholar]
- Adams, M.H. The stability of bacterial viruses in solutions of salts. J. Gen. Physiol. 1949, 32, 579–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puck, T.T.; Garen, A.; Cline, J. The mechanism of virus attachment to host cells: I. The role of ions in the primary reaction. J. Exp. Medic. 1951, 93, 65–88. [Google Scholar] [CrossRef]
- Rountree, P.M. The Role of Certain Electrolytes in the Adsorption of Staphylococcal Bacteriophages. J. Gen. Microbiol. 1951, 5, 673–680. [Google Scholar] [CrossRef] [Green Version]
- Landry, E.F.; Zsigray, R.M. Effects of Calcium on the Lytic Cycle of Bacillus subtilis Phage 41c. J. Gen. Virol. 1980, 51, 125–135. [Google Scholar] [CrossRef]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages—Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemb, O.; Manefield, M.; Thomas, F.; Jacquet, S. Phage adsorption to bacteria in the light of the electrostatics: A case study using E. coli, T2 and flow cytometry. J. Virol. Meth. 2013, 189, 283–289. [Google Scholar] [CrossRef]
- Luria, S.E.; Steiner, D.I. The role of calcium in the penetration of bacteriophage t5 into its host. J. Bacteriol. 1954, 67, 635. [Google Scholar] [CrossRef]
- Cvirkaitė-Krupovič, V.; Krupovič, M.; Daugelavičius, R.; Bamford, D.H. Calcium ion-dependent entry of the membrane-containing bacteriophage PM2 into its Pseudoalteromonas host. Virology 2010, 405, 120–128. [Google Scholar] [CrossRef]
- Jakutytė, L.; Lurz, R.; Baptista, C.; Carballido-Lopez, R.; São-José, C.; Tavares, P.; Daugelavičius, R. First steps of bacteriophage SPP1 entry into Bacillus subtilis. Virology 2012, 422, 425–434. [Google Scholar] [CrossRef]
- Fang, C.; Wang, X.; Wang, L.; Li, Z.; Che, J.; Wang, L.; Li, X.; Cao, Z.; Zhang, J.; Jin, L.; et al. Evaluation of the Efficacy of a Bacteriophage in the Treatment of Pneumonia Induced by Multidrug Resistance Klebsiella pneumoniae in Mice. BioMed Res. Int. 2015, 2015, 752930. [Google Scholar]
- Ahmadi, M.; Torshizi, M.A.K.; Rahimi, S.; Dennehy, J.J. Prophylactic Bacteriophage Administration More Effective than Post-infection Administration in Reducing Salmonella enterica serovar Enteritidis Shedding in Quail. Front. Microbiol. 2016, 7, 1253. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, M.M.; Marmo, P.; De Angelis, L.H.; Palmieri, M.; Ciacci, N.; Di Lallo, G.; Demattè, E.; Vannuccini, E.; Lupetti, P.; Rossolini, G.M.; et al. φBO1E, a newly discovered lytic bacteriophage targeting carbapenemase-producing Klebsiella pneumoniae of the pandemic Clonal Group 258 clade II lineage. Sci. Rep. 2017, 7, 2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Cai, L.; Jiao, N.; Zhang, R. Isolation and characterization of the first phage infecting ecologically important marine bacteria. Erythrobacter. Virol. J. 2017, 14, 104. [Google Scholar] [CrossRef] [Green Version]
- Phothichaisri, W.; Ounjai, P.; Phetruen, T.; Janvilisri, T.; Khunrae, P.; Singhakaew, S.; Wangroongsarb, P.; Chankhamhaengdecha, S. Characterization of Bacteriophages Infecting Clinical Isolates of Clostridium difficile. Front. Microbiol. 2018, 9, 1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.H.; Sridhar, S.; Zhang, R.R.; Chu, H.; Fung, A.Y.F.; Chan, G.; Chan, J.F.W.; To, K.K.W.; Hung, I.F.N.; Cheng, V.C.C.; et al. Factors affecting stability and infectivity of SARS-CoV-2. J. Hosp. Infect 2020, 106, 226–231. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Brodetsky, A.M.; Romig, W.R. Characterization of Bacillus subtilis bacteriophages. J. Bacteriol. 1965, 90, 1655–1663. [Google Scholar] [CrossRef]
- Tran, X.T.T.; Tam, L.D.; Hoang, H.A. Stability and activity of TG25P phage in control of Aeromonas hydrophila in striped catfish pond water. Sci. Tech. Dev. J. 2018, 21, 64–70. [Google Scholar]
- Dawson, R.M.C.; Elliott, D.C.; Elliott, W.H.; Jones, K.M. Data for Biochemical Research, 2nd ed.; Clarendon Press: Oxford, UK, 1969. [Google Scholar]
- Ageno, M.; Dore, E.; Frontali, C. The Alkaline Denaturation of DNA. Biophys. J. 1969, 9, 1281–1311. [Google Scholar] [CrossRef] [Green Version]
- Sato, S.; Matsumoto, N.; Hisaie, K.; Uematsu, S. Alcohol abrogates human norovirus infectivity in a pH-dependent manner. Sci. Rep. 2020, 10, 15878. [Google Scholar] [CrossRef]
- Park, G.W.; Barclay, L.; Macinga, D.; Charbonneau, D.; Pettigrew, C.A.; Vinje, J. Comparative Efficacy of Seven Hand Sanitizers against Murine Norovirus, Feline Calicivirus, and GII.4 Norovirus. J. Food Prot. 2010, 73, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Alam, M.S.; Suzuki, M.; Takahashi, S.; Komura, M.; Sangsriratakul, N.; Shoham, D.; Takehara, K. Virucidal activity of a quaternary ammonium compound associated with calcium hydroxide on avian influenza virus, Newcastle disease virus and infectious bursal disease virus. J. Vet. Med. Sci. 2018, 80, 574–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sangsriratanakul, N.; Toyofuku, C.; Suzuki, M.; Komura, M.; Yamada, M.; Alam, M.S.; Ruenphet, S.; Shoham, D.; Sakai, K.; Takehara, K. Virucidal efficacy of food additive grade calcium hydroxide against surrogate of human norovirus. J. Virol. Meth. 2018, 251, 83–87. [Google Scholar] [CrossRef]
- Kuo, T.T.; Huang, T.C.; Wu, R.Y.; Chen, C.P. Specific Dissociation of Phage Xpl2 by Sodium Citrate. J. Gen. Virol. 1971, 10, 199–202. [Google Scholar] [CrossRef]
- Evilevitch, A.; Fang, L.T.; Yoffe, A.M.; Castelnovo, M.; Rau, D.C.; Parsegian, V.A.; Gelbart, W.M.; Knobler, C.M. Effects of Salt Concentrations and Bending Energy on the Extent of Ejection of Phage Genomes. Biophys. J. 2008, 94, 1110–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singer, M.; Berg, P. Genes & Genomes, a Changing Perspective; University Science Books: Mill Valley, CA, USA, 1991. [Google Scholar]
- Marmur, J.; Doty, P. Determination of the Base Composition of Deoxyribonucleic Acid from its Thermal Denaturation Temperature. J. Mol. Biol. 1962, 5, 109–118. [Google Scholar] [CrossRef]
- Rao, V.B.; Black, L.W. Structure and assembly of bacteriophage T4 head. Virol. J. 2010, 7, 356. [Google Scholar] [CrossRef] [Green Version]


| −k (min−1) ± S.E. | V0 ± S.E. | p Value of −k | R2 | n | |
|---|---|---|---|---|---|
| 0.0001 T-buffer | † | † | † | † | 5 (5) |
| 0.001 T-buffer | −3.8 × 10−1 ± 2.7 × 10−3 | 311 ± 1.0 | 4.4 × 10−3 | 1.00 | 5 (2) |
| 0.01 T-buffer | −1.6 × 10−2 ± 5.9 × 10−4 | 78 ± 1.0 | 1.1 × 10−4 | 1.00 | 5 |
| 1 T-buffer | −4.5 × 10−5 ± 3.2 × 10−6 | 89 ± 1.1 | 3.1 × 10−5 | 0.98 | 7 |
| 100 mM NaCl | −1.4 × 10−3 ± 1.0 × 10−4 | 430 ± 1.2 | 5.2 × 10−3 | 0.99 | 5 |
| 10 mM CaCl2 | −2.2 × 10−3 ± 1.8 × 10−4 | 68 ± 1.1 | 2.2 × 10−4 | 0.98 | 6 |
| 0.2 mN NaOH | −1.5 × 10−3 ± 2.5 × 10−4 | 58 ± 1.2 | 4.1 × 10−3 | 0.90 | 7 |
| 0.2 mN Ca(OH)2 | −4.9 × 10−5 ± 5.5 × 10−6 | 52 ± 1.1 | 1.1 × 10−4 | 0.92 | 8 |
| 0.2 mN Mg(OH)2 | −3.6 × 10−6 ± 4.1 × 10−6 | 82 ± 1.1 | 4.4 × 10−1 | 0.21 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hara, S.; Koike, I. Survival of Bacteriophage T4 in Quasi-Pure Ionic Solutions. Viruses 2023, 15, 1737. https://doi.org/10.3390/v15081737
Hara S, Koike I. Survival of Bacteriophage T4 in Quasi-Pure Ionic Solutions. Viruses. 2023; 15(8):1737. https://doi.org/10.3390/v15081737
Chicago/Turabian StyleHara, Seiko, and Isao Koike. 2023. "Survival of Bacteriophage T4 in Quasi-Pure Ionic Solutions" Viruses 15, no. 8: 1737. https://doi.org/10.3390/v15081737




