First Identification and Pathogenicity Evaluation of an EV-G17 Strain Carrying a Torovirus Papain-like Cysteine Protease (PLCP) Gene in China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Virus Isolation
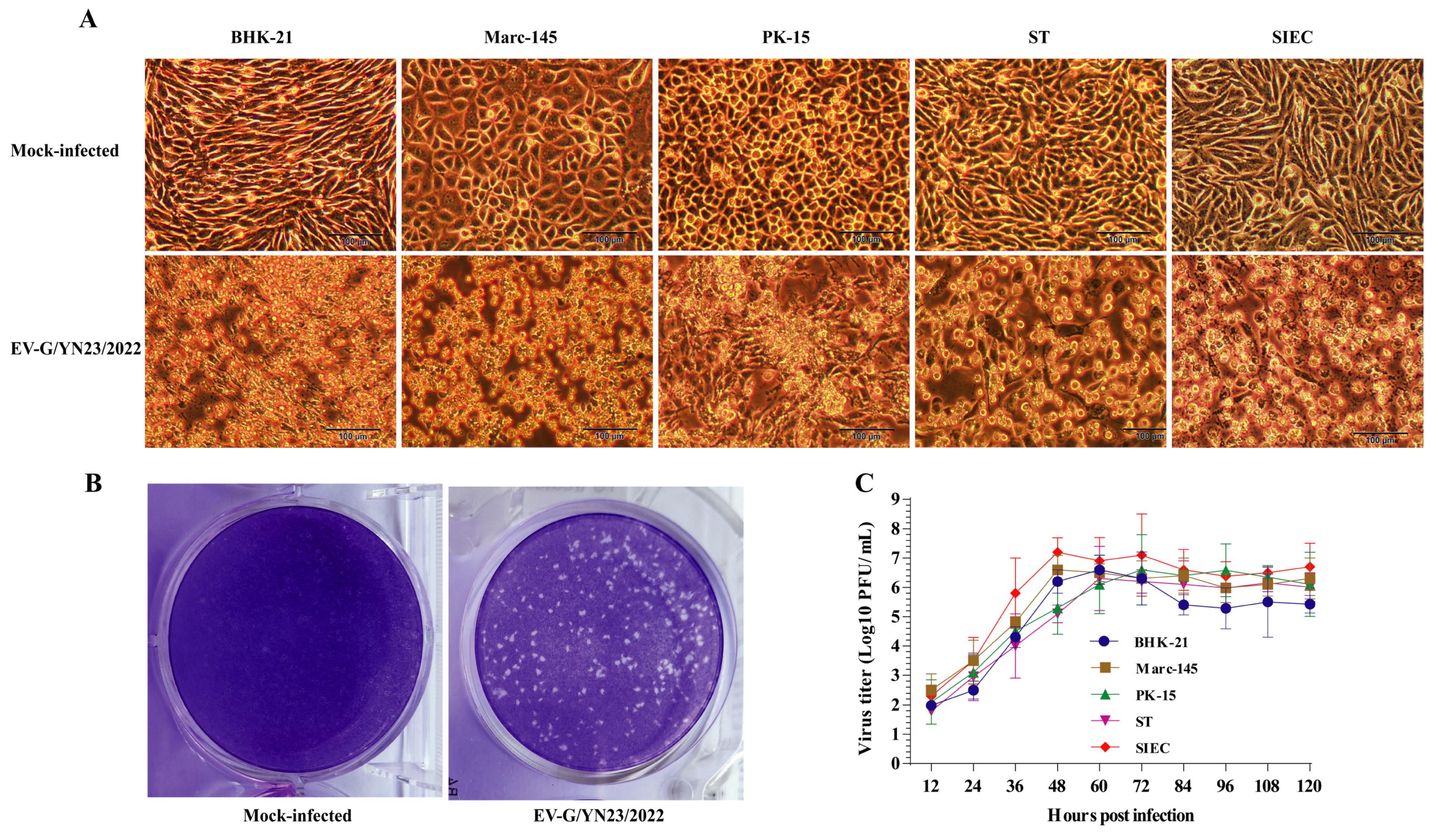
2.2. Virus Purification and Replication Kinetics Analysis
2.3. Virus Genome Amplification and Sequencing
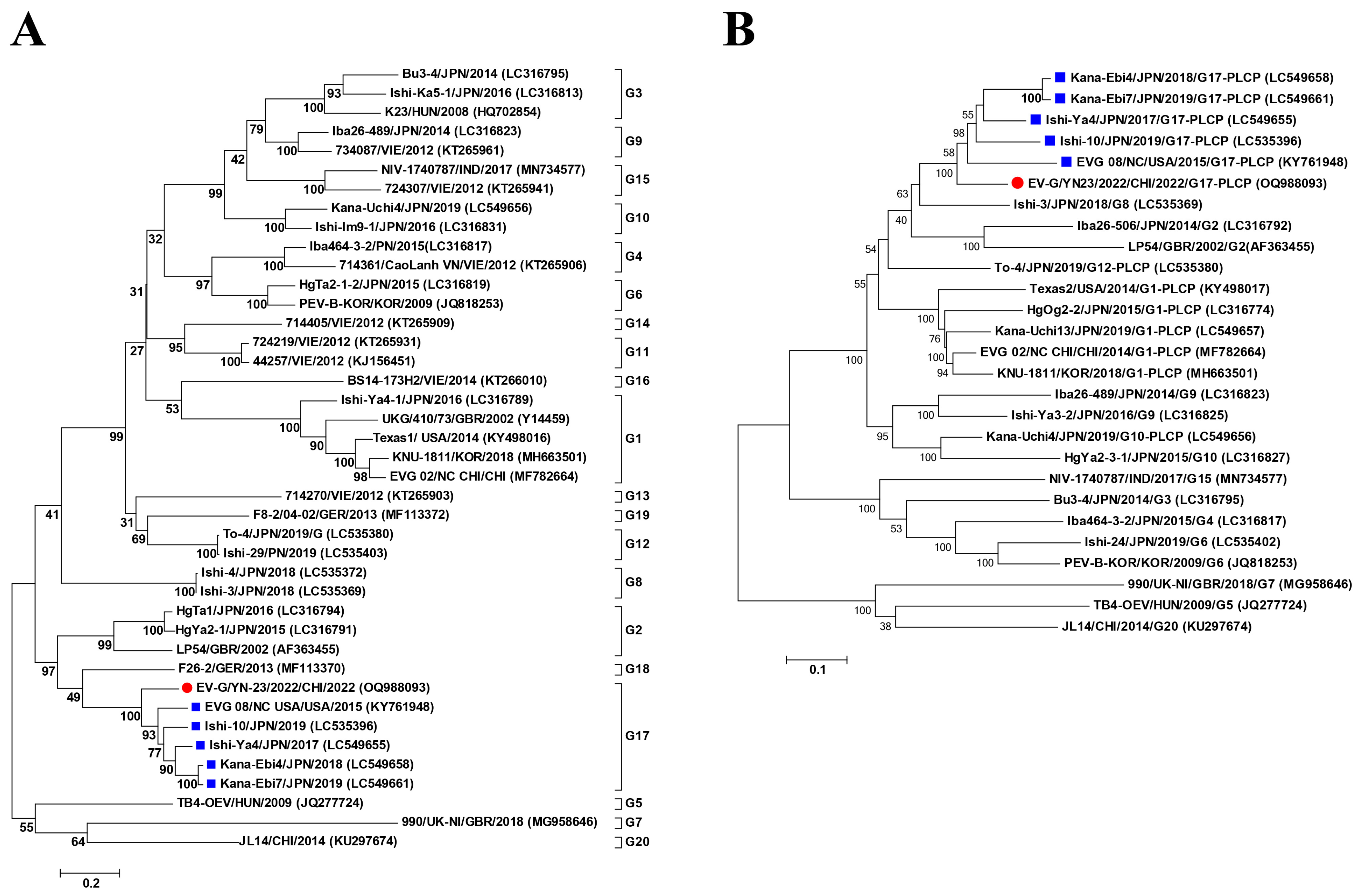
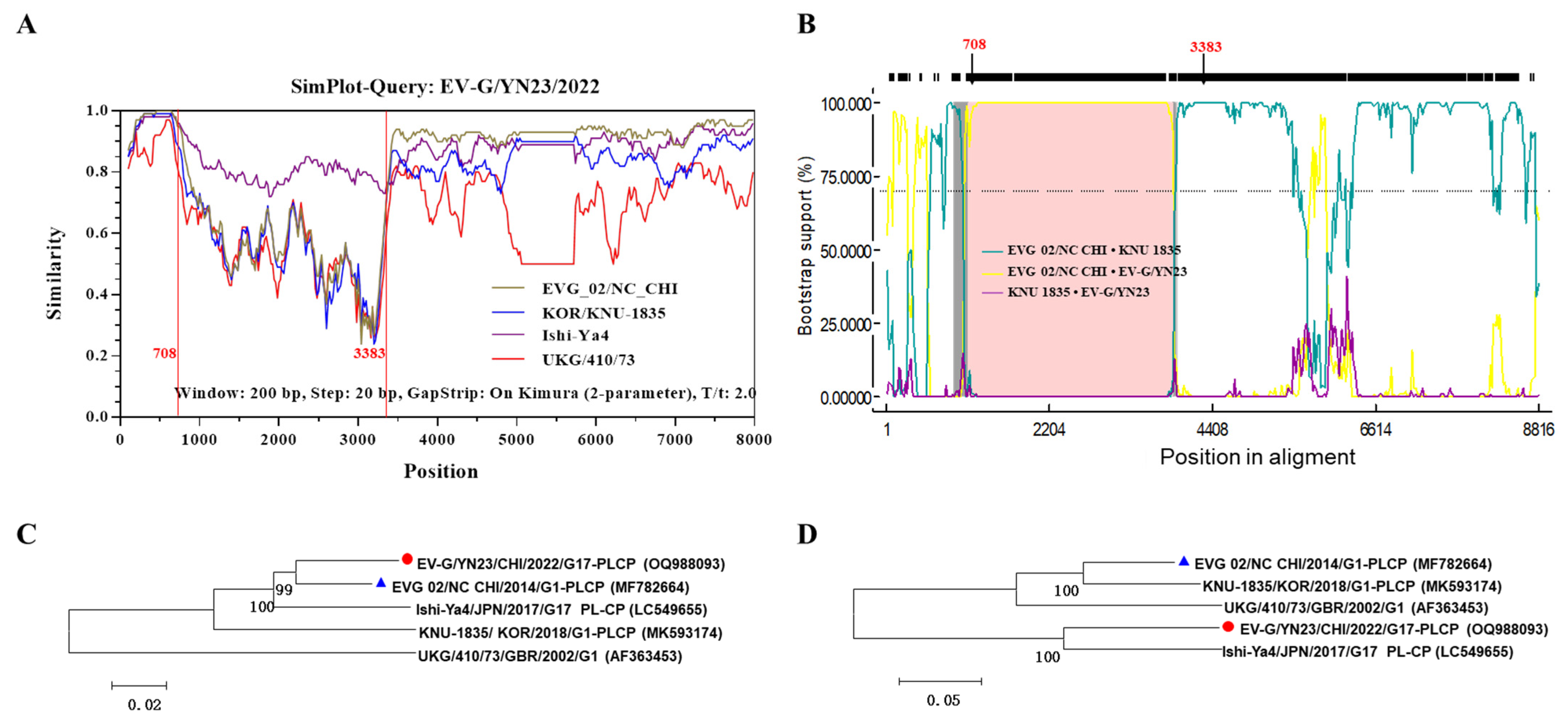
2.4. Sequence and Phylogenetic Analysis
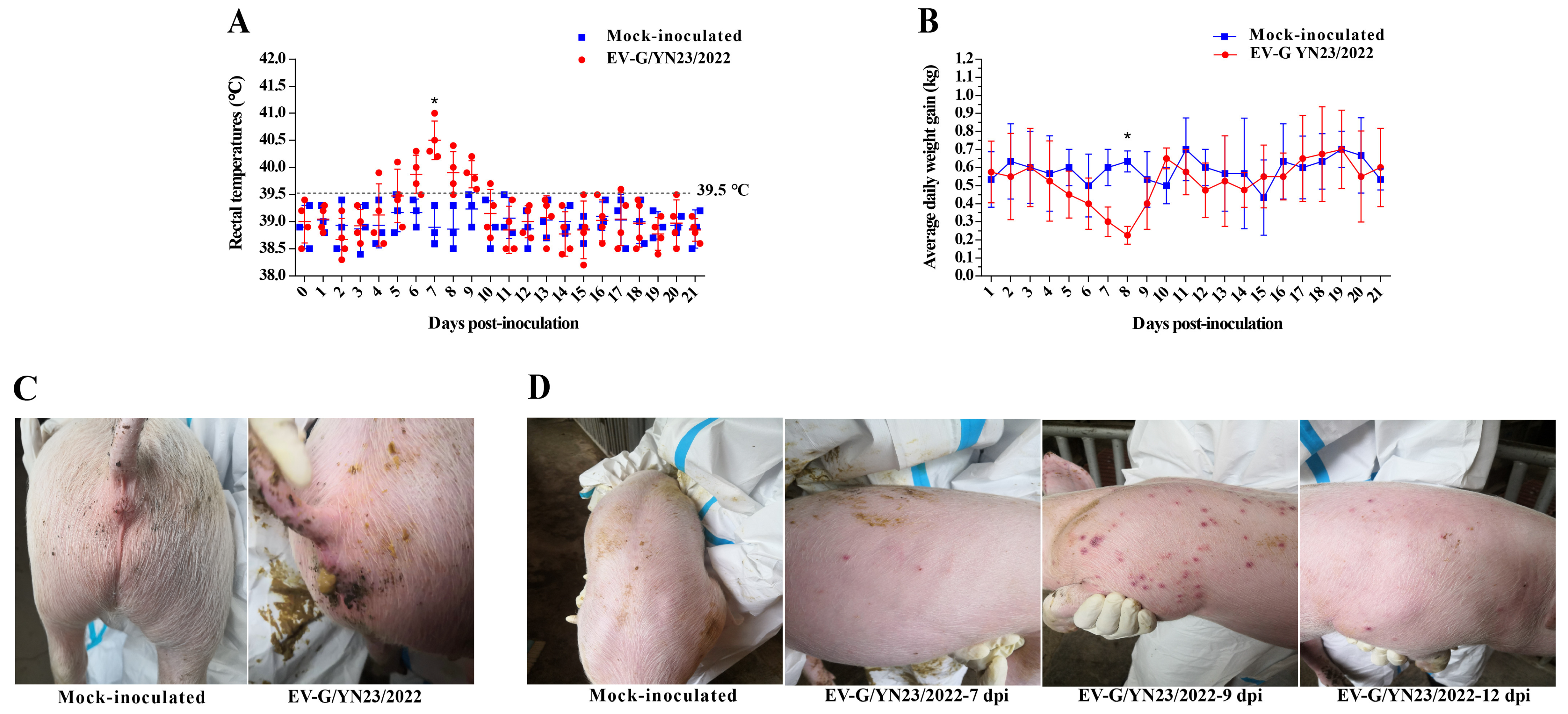
2.5. Experimental Infection of Piglets
2.6. Detection of Cytokine mRNA Levels and Concentrations
2.7. Virus Shedding and Tissue Virus Load Detection
2.8. Serum Neutralization Antibodies Detection
2.9. Histopathological Examination
2.10. Statistical Analysis
2.11. Ethics Statement
3. Results
3.1. EV-G Isolation and Identification
3.2. Analysis of Genomic Sequences
3.3. Phylogenetic Analysis
3.4. Recombination Analysis
3.5. Clinical Symptoms Observation
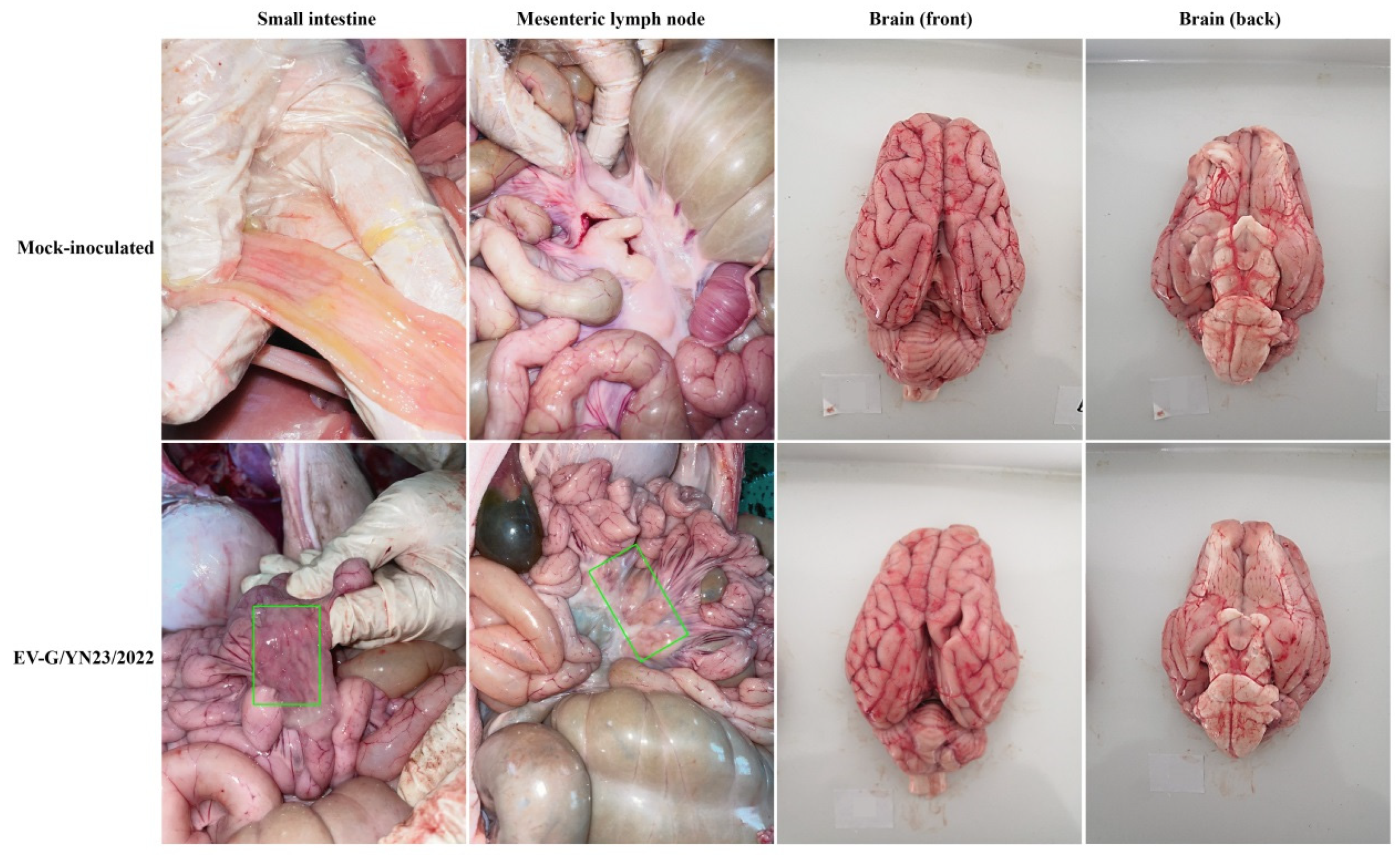
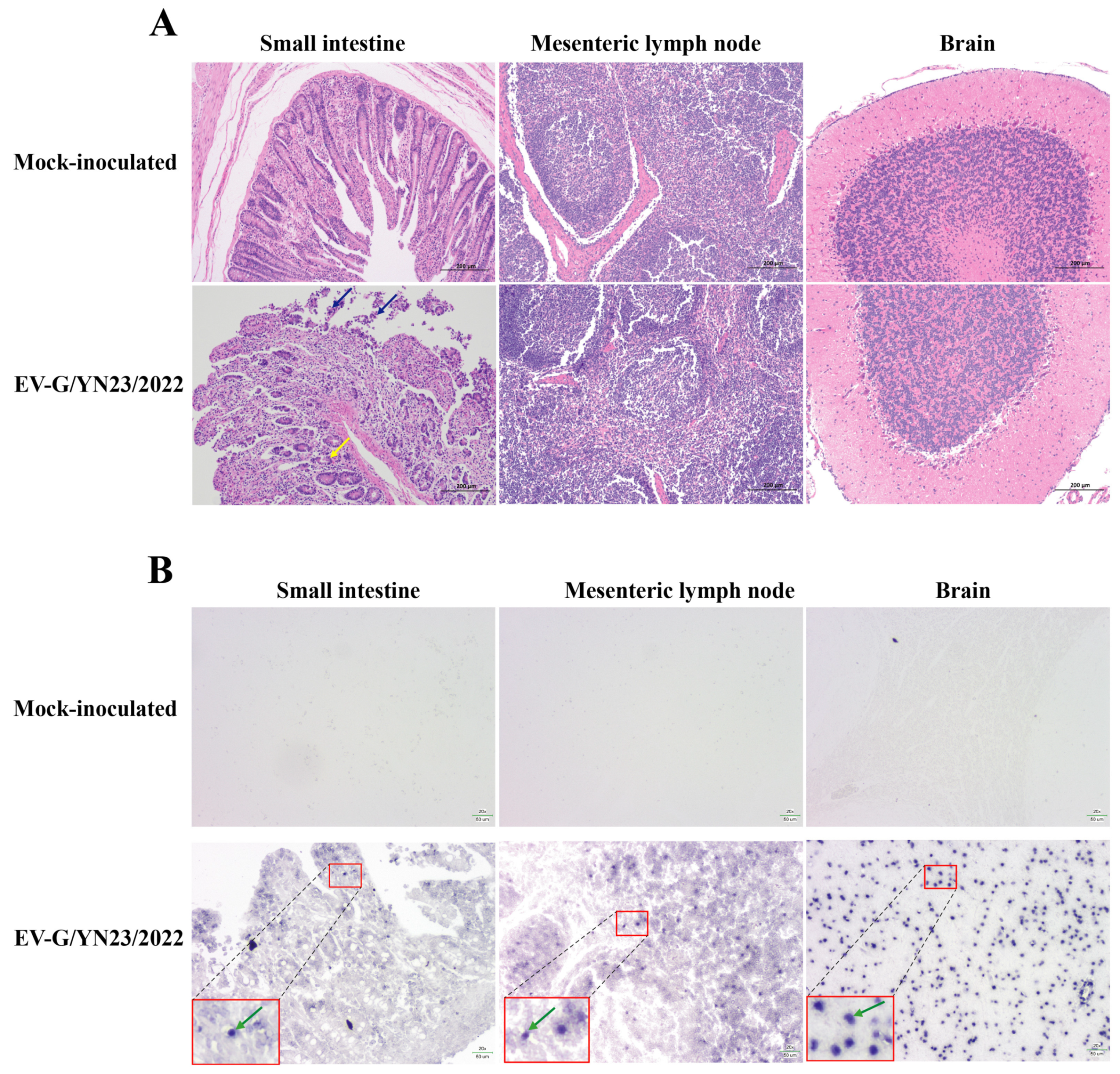
3.6. Gross Pathology and Histopathological Analysis
3.7. Fecal Virus Shedding and Virus Load in Tissues
3.8. Antibody Responses of Challenged Piglets
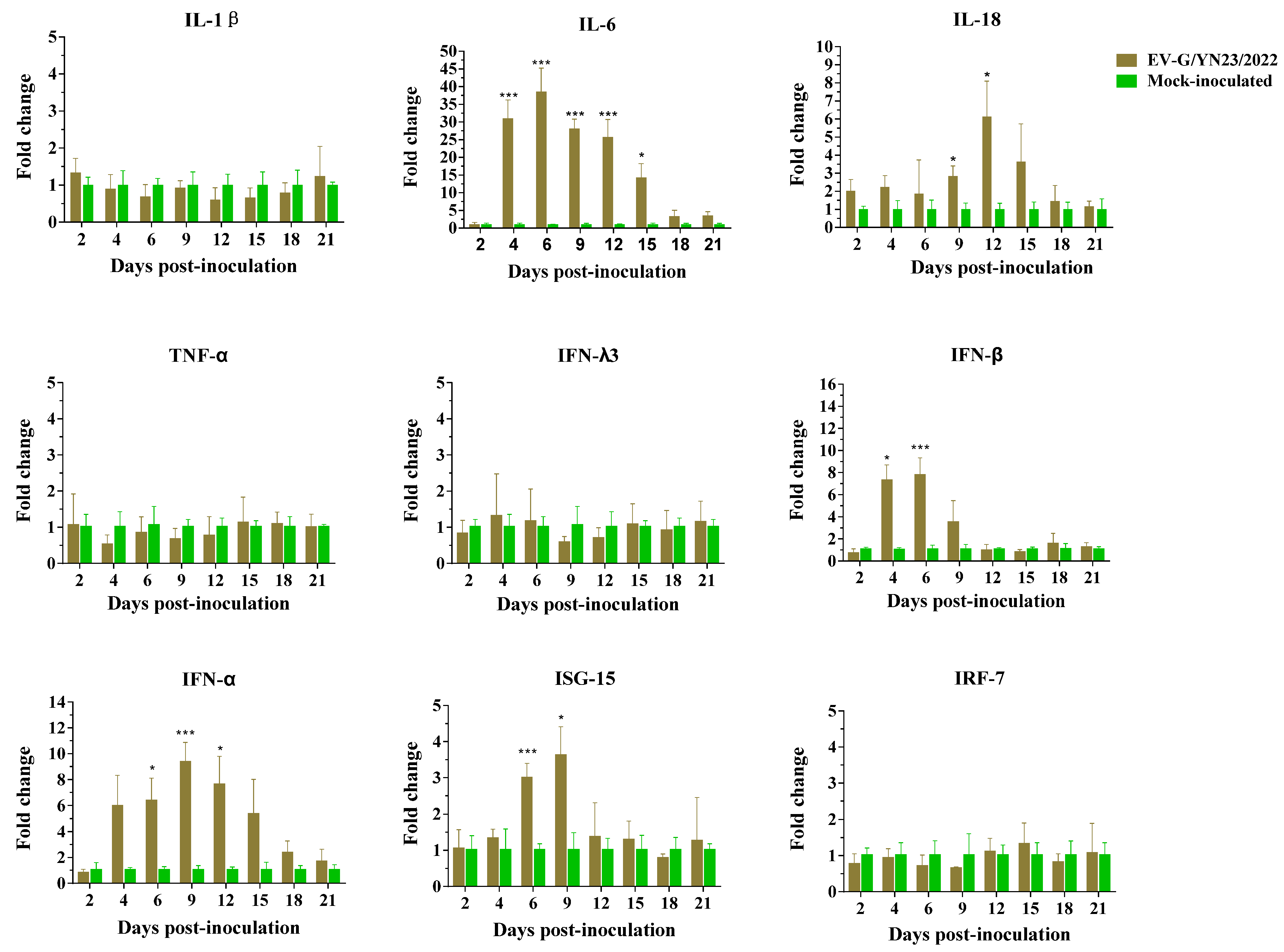
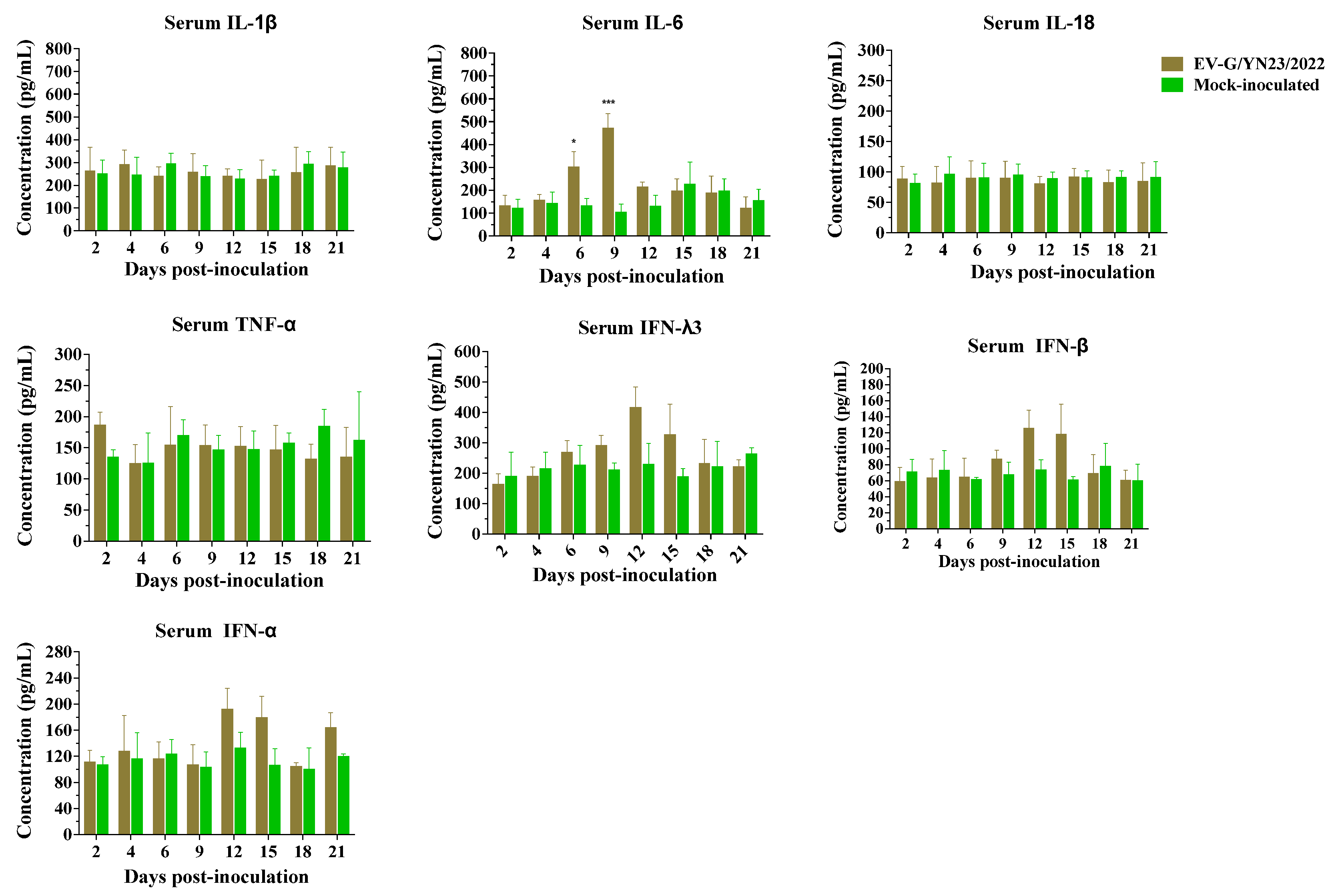
3.9. Cytokine Gene mRNA Levels in PBMCs and Concentrations in the Serum of EV-G/YN23/2022-Infected Piglets
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anbalagan, S.; Hesse, R.A.; Hause, B.M. First identification and characterization of porcine enterovirus G in the United States. PLoS ONE 2014, 9, e97517. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiaka, S.; Naoi, Y.; Imai, R.; Masuda, T.; Ito, M.; Akagami, M.; Ouchi, Y.; Ishii, K.; Sakaguchi, S.; Omatsu, T.; et al. Genetic diversity and recombination of enterovirus G strains in Japanese pigs: High prevalence of strains carrying a papain-like cysteine protease sequence in the enterovirus G population. PLoS ONE 2018, 13, e0190819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dung, N.; Anh, P.H.; Van Cuong, N.; Hoa, N.T.; Carrique-Mas, J.; Hien, V.B.; Campbell, J.; Baker, S.; Farrar, J.; Woolhouse, M.E.; et al. Prevalence, genetic diversity and recombination of species G enteroviruses infecting pigs in Vietnam. J. Gen. Virol. 2014, 95, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Knowles, N.J. Porcine enteric picornaviruses. In Diseases of Swine, 9th ed.; Straw, B.E., Zimmermann, J.J., D’Allaire, S.D., Taylor, D.J., Eds.; Blackwell Publishing Inc.: Ames, IA, USA, 2006; pp. 337–345. [Google Scholar]
- Boros, A.; Pankovics, P.; Reuter, G. Characterization of a novel porcine enterovirus in domestic pig in Hungary. Infect. Genet. Evol. 2011, 11, 1096–1102. [Google Scholar] [CrossRef]
- Oberste, M.S.; Maher, K.; Kilpatrick, D.R.; Pallansch, M.A. Molecular evolution of the human enteroviruses: Correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 1999, 73, 1941–1948. [Google Scholar] [CrossRef]
- Van Dung, N.; Anh, P.H.; Van Cuong, N.; Hoa, N.T.; Carrique-Mas, J.; Hien, V.B.; Sharp, C.; Rabaa, M.; Berto, A.; Campbell, J.; et al. Large-scale screening and characterization of enteroviruses and kobuviruses infecting pigs in Vietnam. J. Gen. Virol. 2016, 97, 378–388. [Google Scholar] [CrossRef]
- Perez-Losada, M.; Arenas, M.; Galan, J.C.; Palero, F.; Gonzalez-Candelas, F. Recombination in viruses: Mechanisms, methods of study, and evolutionary consequences. Infect. Genet. Evol. 2015, 30, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Simon-Loriere, E.; Holmes, E.C. Why do RNA viruses recombine? Nat. Rev. Microbiol. 2011, 9, 617–626. [Google Scholar] [CrossRef]
- Lukashev, A.N. Role of recombination in evolution of enteroviruses. Rev. Med. Virol. 2005, 15, 157–167. [Google Scholar] [CrossRef]
- Simmonds, P.; Welch, J. Frequency and dynamics of recombination within different species of human enteroviruses. J. Virol. 2006, 80, 483–493. [Google Scholar] [CrossRef] [Green Version]
- Boros, A.; Pankovics, P.; Knowles, N.J.; Reuter, G. Natural interspecies recombinant bovine/porcine enterovirus in sheep. J. Gen. Virol. 2012, 93, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Holmblat, B.; Jegouic, S.; Muslin, C.; Blondel, B.; Joffret, M.L.; Delpeyroux, F. Nonhomologous recombination between defective poliovirus and coxsackievirus genomes suggests a new model of genetic plasticity for picornaviruses. mBio 2014, 5, e01119-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muslin, C.; Joffret, M.L.; Pelletier, I.; Blondel, B.; Delpeyroux, F. Evolution and emergence of enteroviruses through intra- and inter-species recombination: Plasticity and phenotypic impact of modular genetic exchanges in the 5′ untranslated region. PLoS Pathog. 2015, 11, e1005266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkegaard, K.; Baltimore, D. The mechanism of RNA recombination in poliovirus. Cell 1986, 47, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Liu, Z.; Fu, X.; Yuan, J.; Zhao, J.; Lin, Y.; Shen, Q.; Wang, X.; Deng, X.; et al. Full-length and defective enterovirus G genomes with distinct torovirus protease insertions are highly prevalent on a Chinese pig farm. Arch. Virol. 2018, 163, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Imai, R.; Nagai, M.; Oba, M.; Sakaguchi, S.; Ujike, M.; Kimura, R.; Kida, M.; Masuda, T.; Kuroda, M.; Wen, R.; et al. A novel defective recombinant porcine enterovirus G virus carrying a porcine torovirus papain-like cysteine protease gene and a putative anti-apoptosis gene in place of viral structural protein genes. Infect. Genet. Evol. 2019, 75, 103975. [Google Scholar] [CrossRef]
- Nagata, A.; Sekiguchi, Y.; Oi, T.; Sunaga, F.; Madarame, H.; Imai, R.; Sano, K.; Katayama, Y.; Omatsu, T.; Oba, M.; et al. Genetic diversity of enterovirus G detected in faecal samples of wild boars in Japan: Identification of novel genotypes carrying a papain-like cysteine protease sequence. J. Gen. Virol. 2020, 101, 840–852. [Google Scholar] [CrossRef]
- Bunke, J.; Receveur, K.; Oeser, A.C.; Fickenscher, H.; Zell, R.; Krumbholz, A. High genetic diversity of porcine enterovirus G in Schleswig-Holstein, Germany. Arch. Virol. 2018, 163, 489–493. [Google Scholar] [CrossRef]
- Janetanakit, T.; Chaiyawong, S.; Charoenkul, K.; Tangwangvivat, R.; Chamsai, E.; Udom, K.; Jairak, W.; Amonsin, A. Distribution and genetic diversity of enterovirus G (EV-G) on pig farms in Thailand. BMC Vet. Res. 2021, 17, 277. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Shen, Q.; Zhang, W.; Hua, X. Prevalence of porcine enterovirus 9 in pigs in middle and eastern China. Virol. J. 2013, 10, 99. [Google Scholar] [CrossRef] [Green Version]
- Mi, X.; Yang, C.; Lu, Y.; Wang, H.; Qin, Q.; Chen, R.; Chen, Z.; Luo, Y.; Chen, Y.; Wei, Z.; et al. Isolation, identification, and evaluation of the pathogenicity of a porcine enterovirus G isolated from China. Front. Vet. Sci. 2021, 8, 712679. [Google Scholar] [CrossRef]
- Knowles, N.J. The association of group III porcine enteroviruses with epithelial tissue. Vet. Rec. 1988, 122, 441–442. [Google Scholar] [CrossRef]
- Shang, P.; Misra, S.; Hause, B.; Fang, Y. A naturally occurring recombinant enterovirus expresses a torovirus deubiquitinase. J. Virol. 2017, 91, 10–128. [Google Scholar] [CrossRef] [Green Version]
- Zell, R.; Krumbholz, A.; Henke, A.; Birch-Hirschfeld, E.; Stelzner, A.; Doherty, M.; Hoey, E.; Dauber, M.; Prager, D.; Wurm, R. Detection of porcine enteroviruses by nRT-PCR: Differentiation of CPE groups I-III with specific primer sets. J. Virol. Methods 2000, 88, 205–218. [Google Scholar] [CrossRef]
- Ding, G.; Fu, Y.; Li, B.; Chen, J.; Wang, J.; Yin, B.; Sha, W.; Liu, G. Development of a multiplex RT-PCR for the detection of major diarrhoeal viruses in pig herds in China. Transbound. Emerg. Dis. 2020, 67, 678–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Asimenos, G.; Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 2009, 537, 39–64. [Google Scholar] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nuclc. Acids Symp. 1999, 41, 95–98. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. Rdp4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [Green Version]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef]
- Zhou, P.; Li, L.F.; Zhang, K.; Wang, B.; Tang, L.; Li, M.; Wang, T.; Sun, Y.; Li, S.; Qiu, H.J. Deletion of the H240R gene of African swine fever virus decreases infectious progeny virus production due to aberrant virion morphogenesis and enhances inflammatory cytokine expression in porcine macrophages. J. Virol. 2022, 96, e0166721. [Google Scholar] [CrossRef]
- Razzuoli, E.; Mignone, G.; Lazzara, F.; Vencia, W.; Ferraris, M.; Masiello, L.; Vivaldi, B.; Ferrari, A.; Bozzetta, E.; Amadori, M. Impact of cadmium exposure on swine enterocytes. Toxicol. Lett. 2018, 287, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Temeeyasen, G.; Sinha, A.; Gimenez-Lirola, L.G.; Zhang, J.Q.; Pineyro, P.E. Differential gene modulation of pattern-recognition receptor TLR and RIG-I-like and downstream mediators on intestinal mucosa of pigs infected with PEDV non S-INDEL and PEDV S-INDEL strains. Virology 2018, 517, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; van Geelen, A.; Buckley, A.C.; O’Brien, A.; Pillatzki, A.; Lager, K.M.; Faaberg, K.S.; Baker, S.C. Coronavirus endoribonuclease activity in porcine epidemic diarrhea virus suppresses type I and type III interferon responses. J. Virol. 2019, 93, e02000-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Xue, Y.; Niu, H.; Shi, C.; Cheng, M.; Wang, J.; Zou, B.; Wang, J.; Niu, T.; Bao, M.; et al. African swine fever virus MGF360-11L negatively regulates cGAS-STING-mediated inhibition of type I interferon production. Vet. Res. 2022, 53, 7. [Google Scholar] [CrossRef]
- Xue, Q.; Liu, H.; Zhu, Z.; Yang, F.; Ma, L.; Cai, X.; Xue, Q.; Zheng, H. Seneca valley virus 3Cpro abrogates the IRF3- and IRF7-mediated innate immune response by degrading IRF 3 and IRF 7. Virology 2018, 518, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Yang, Z.; Li, L.; Gao, L.; Xie, J.; Liao, D.; Gao, X.; Hu, Z.; Niu, B.; et al. Isolation and characterization of two novel serotypes of Tibet orbivirus from Culicoides and sentinel cattle in Yunnan Province of China. Transbound. Emerg. Dis. 2022, 69, 3371–3387. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Z.; Wang, E.; Yang, R.; Xiao, Y.; Han, H.; Lang, F.; Li, X.; Xia, Y.; Gao, F.; et al. Herpes simplex virus 1 infection of tree shrews differs from that of mice in the severity of acute infection and viral transcription in the peripheral nervous system. J. Virol. 2016, 90, 790–804. [Google Scholar] [CrossRef] [Green Version]
- Income, N.; Kosoltanapiwat, N.; Taksinoros, S.; Leaungwutiwong, P.; Maneekan, P.; Chavez, I.F. Molecular identification of enteroviruses from cattle and goat feces and environment in Thailand. Appl. Environ. Microbiol. 2019, 85, e02420-18. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, Y.; Nagata, A.; Sunaga, F.; Oi, T.; Imai, R.; Madarame, H.; Katayama, Y.; Oba, M.; Okabayashi, T.; Misawa, N.; et al. Multiple genotypes of enterovirus G carrying a papain-like cysteine protease (PL-CP) sequence circulating on two pig farms in Japan: First identification of enterovirus G10 carrying a PL-CP sequence. Arch. Virol. 2020, 165, 2909–2914. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, C. First detection of novel enterovirus G recombining a torovirus papain-like protease gene associated with diarrhoea in swine in South Korea. Transbound. Emerg. Dis. 2019, 66, 1023–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conceicao-Neto, N.; Theuns, S.; Cui, T.; Zeller, M.; Yinda, C.K.; Christiaens, I.; Heylen, E.; Van Ranst, M.; Carpentier, S.; Nauwynck, H.J.; et al. Identification of an enterovirus recombinant with a torovirus-like gene insertion during a diarrhea outbreak in fattening pigs. Virus Evol. 2017, 3, vex024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilar, M.J.; Peralta, B.; Garcia-Bocanegra, I.; Simon-Grife, M.; Bensaid, A.; Casal, J.; Segales, J.; Pina-Pedrero, S. Distribution and genetic characterization of Enterovirus G and Sapelovirus A in six Spanish swine herds. Virus Res. 2016, 215, 42–49. [Google Scholar] [CrossRef] [PubMed]
- LaRue, R.; Myers, S.; Brewer, L.; Shaw, D.P.; Brown, C.; Seal, B.S.; Njenga, M.K. A wild-type porcine encephalomyocarditis virus containing a short poly(C) tract is pathogenic to mice, pigs, and cynomolgus macaques. J. Virol. 2003, 77, 9136–9146. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Miyazaki, A.; Yamamoto, Y.; Nakamura, K.; Ito, M.; Tsunemitsu, H.; Narita, M. Experimental teschovirus encephalomyelitis in gnotobiotic pigs. J. Comp. Pathol. 2014, 150, 276–286. [Google Scholar] [CrossRef]
- Schock, A.; Gurrala, R.; Fuller, H.; Foyle, L.; Dauber, M.; Martelli, F.; Scholes, S.; Roberts, L.; Steinbach, F.; Dastjerdi, A. Investigation into an outbreak of encephalomyelitis caused by a neuroinvasive porcine sapelovirus in the United Kingdom. Vet. Microbiol. 2014, 172, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xu, H.; Zhao, J.; Leng, C.; Xiang, L.; Li, C.; Fu, J.; Tang, Y.D.; Peng, J.; Wang, Q.; et al. Pathogenicity of NADC34-like PRRSV HLJDZD32-1901 isolated in China. Vet. Microbiol. 2020, 246, 108727. [Google Scholar] [CrossRef]
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.; Xiao, C.T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef]
- Yang, Q.Y.; Sun, Z.; Tan, F.F.; Guo, L.H.; Wang, Y.Z.; Wang, J.; Wang, Z.Y.; Wang, L.L.; Li, X.D.; Xiao, Y.; et al. Pathogenicity of a currently circulating Chinese variant pseudorabies virus in pigs. World J. Virol. 2016, 5, 23–30. [Google Scholar] [CrossRef]
- Lin, C.M.; Saif, L.J.; Marthaler, D.; Wang, Q. Evolution, antigenicity and pathogenicity of global porcine epidemic diarrhea virus strains. Virus Res. 2016, 226, 20–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annamalai, T.; Saif, L.J.; Lu, Z.; Jung, K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immunopathol. 2015, 168, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Knowles, N.J.; Buckley, L.S.; Pereira, H.G. Classification of porcine enteroviruses by antigenic analysis and cytopathic effects in tissue culture: Description of 3 new serotypes. Arch. Virol. 1979, 62, 201–208. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, B.; Wang, T.; Gao, L.; Yang, Z.J.; Wang, F.F.; Shang, H.W.; Hua, R.; Xu, J.D. Biological characteristics of IL-6 and related intestinal diseases. Int. J. Biol. Sci. 2021, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Kruse, R.; Essén-Gustavsson, B.; Fossum, C.; Jensen-Waern, M. Blood concentration of the cytokines IL-1beta, IL-6, IL-10, TNF-alpha and IFN-gamma during experimentally induced swine dysentery. Acta Vet. Scand. 2008, 50, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, B.; Zhong, R.; Wu, W.; Luo, C.; Meng, Q.; Gao, Q.; Zhao, Y.; Chen, L.; Zhang, S.; Zhao, X.; et al. Mucin O-glycan-microbiota axis orchestrates gut homeostasis in a diarrheal pig model. Microbiome 2022, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xue, M.; Fu, F.; Yin, L.; Feng, L.; Liu, P. IFN-lambda 3 mediates antiviral protection against porcine epidemic diarrhea virus by inducing a distinct antiviral transcript profile in porcine intestinal epithelia. Front. Immunol. 2019, 10, 2394. [Google Scholar] [CrossRef] [Green Version]
- Morales, D.J.; Lenschow, D.J. The antiviral activities of ISG15. J. Mol. Biol. 2013, 13, 4995–5008. [Google Scholar] [CrossRef]

| Primer | Sequence (5′⟶3′) | Position a | Product Length (bp) |
|---|---|---|---|
| EV-G-F1 | TTAAAACAGCCTGTGGGTTG | 1–20 | 2058 |
| EV-G-R1 | TCCACACAGGGTTTTGGACAT | 2038–2058 | |
| EV-G-F2 | AGATGAGGATAGCAGCGCATGT | 1806–1827 | 3016 |
| EV-G-R2 | CTCAGCCACTTCAATATCCACA | 4800–4821 | |
| EV-G-F3 | TGGTGATGGATGACCTTAATCAG | 4583–4605 | 1908 |
| EV-G-R3 | AGAACGACTCCACCACACTGTCC | 6469–6491 | |
| EV-G-F4 | CGACCGTTCTATCCAGCTTAGT | 5888–5909 | 2159 |
| EV-G-R4 | GTACACCCCATCCGGTGGGTGTAT | 8023–8046 | |
| EV-G-SG-F | CTGAATGCGGCTAATCCTAAC | 492–512 | 107 |
| EV-G-SG-R | AACACGGACACCCAAAGTA | 580–598 |
| EV-G/YN23/2022 Gene or Protein Region (nt/aa) | G17-PLCP (LC535396) | G17-PLCP (LC549655) | G17-PLCP (LC549658) | G17-PLCP (LC549661) | G17-PLCP (KY761948) | G1-PLCP (MF782664) | G1-PLCP (KY498017) | G8 (LC535369) | G7 (MG958646) |
|---|---|---|---|---|---|---|---|---|---|
| 5′UTR (812) | NA a | 92.5 | 91.8 | 92 | 94.9 | 95.6 b | 84.5 | 84.7 | 50.4 |
| 3′UTR (66) | 89.1 | 90.4 | NA a | 92.4 | 89.1 | 87.8 | NA a | 87.8 | 78.6 |
| Complete genome/ polyprotein (8033/2385) | NA a/95.8 | 87.5/96.6 | NA a/95.3 | 85.7/95.4 | 85.5/95.3 | 84.5/88.5 | NA a/87.4 | 76.8/82.5 | 62.5/73.1 |
| 1A/VP4 (207/69) | 82.6/100.0 | 89.8/100.0 | 87.9/98.5 | 87.9/98.5 | 87.4/97.1 | 79.7/91.3 | 75.3/85.5 | 79.2/86.9 | 74.8/85.5 |
| 1B/VP2 (738/246) | 80.8/96.7 | 81.8/98.3 | 81.0/97.5 | 82.1/97.5 | 82.9/98.7 | 69.1/83.3 | 70.8/84.5 | 74.9/88.2 | 66.1/76.0 |
| 1C/VP3 (840/280) | 81.7/93.5 | 82.2/95.3 | 80.5/95.3 | 81.1/95.7 | 84.1/95.7 | 67.9/71.7 | 66.6/71.4 | 71.1/76.7 | 59.2/64.6 |
| 1D/VP1 (729/243) | 82.5/97.5 | 82.8/97.9 | 82.8/97.9 | 83.1/98.3 | 82.5/96.7 | 60.5/62.5 | 60.6/62.5 | 66.1/70.3 | 60.5/58.6 |
| 2A/Pro (450/150) | 84.0/96.0 | 84.4/94.6 | 84.8/95.3 | 84.8/95.3 | 84.6/95.3 | 92.6/96.6 | 85.1/94.6 | 92.2/95.3 | 73.5/78.6 |
| 2B (297/98) | 83.8/96.9 | 87.5/94.8 | 82.1/96.9 | 82.4/96.9 | 88.2/95.9 | 91.5/97.9 | 83.1/94.8 | 89.8/96.9 | 69.3/81.6 |
| 2C/ATPase (987/329) | 87.8/97.5 | 88.7/98.4 | 83.7/95.4 | 84.4/95.7 | 86.0/96.9 | 91.9/97.8 | 85.7/96.6 | 90.3/98.1 | 75.8/88.7 |
| PLCP (642/214) | 82.9/83.4 | 85.0/86.9 | 76.8/76.6 | 77.0/77.1 | 76.5/79.0 | 85.8/85.2 | 80.2/79.8 | NA c | NA c |
| 3A (267/89) | 89.5/97.7 | 88.0/96.6 | 85.0/100.0 | 86.8/100.0 | 85.0/98.8 | 94.0/100.0 | 86.8/96.6 | 91.0/100.0 | 74.1/85.3 |
| 3B/VPg (66/22) | 83.3/100.0 | 86.3/100.0 | 92.4/100.0 | 83.3/100.0 | 93.9/100.0 | 89.3/100.0 | 89.3/100.0 | 95.4/100.0 | 75.7/90.9 |
| 3C/Pro (549/183) | 88.8/97.8 | 90.8/98.9 | 89.7/98.9 | 89.4/98.9 | 85.6/97.2 | 92.5/98.3 | 89.0/98.9 | 92.1/98.9 | 77.7/90.7 |
| 3D/Pol (1383/461) | 91.9/98.4 | 91.7/98.2 | 92.2/98.0 | 91.7/97.8 | 86.8/96.7 | 94.2/98.4 | 90.6/98.2 | 90.0/97.6 | 79.5/91.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-H.; Li, Z.-R.; Zhu, P.; Zhang, Z.-X.; Song, J.-L. First Identification and Pathogenicity Evaluation of an EV-G17 Strain Carrying a Torovirus Papain-like Cysteine Protease (PLCP) Gene in China. Viruses 2023, 15, 1747. https://doi.org/10.3390/v15081747
Li Z-H, Li Z-R, Zhu P, Zhang Z-X, Song J-L. First Identification and Pathogenicity Evaluation of an EV-G17 Strain Carrying a Torovirus Papain-like Cysteine Protease (PLCP) Gene in China. Viruses. 2023; 15(8):1747. https://doi.org/10.3390/v15081747
Chicago/Turabian StyleLi, Zhan-Hong, Zhuo-Ran Li, Pei Zhu, Zhen-Xing Zhang, and Jian-Ling Song. 2023. "First Identification and Pathogenicity Evaluation of an EV-G17 Strain Carrying a Torovirus Papain-like Cysteine Protease (PLCP) Gene in China" Viruses 15, no. 8: 1747. https://doi.org/10.3390/v15081747





